ABSTRACT
The IL-3 alpha chain receptor (CD123) is a cell surface protein that is widely expressed by various subtypes of acute leukemia, including acute myeloid leukemia (AML), acute lymphoblastic leukemia and blastic plasmacytoid dendritic cell neoplasm. Notably, CD123 is preferentially overexpressed in leukemia stem cells (LSC) in contrast to normal hematopoietic stem cells, and this differential expression allows for the selective eradication of LSC and leukemic blasts through therapeutic targeting of CD123, with less impact on hematopoietic cells. The level of CD123 expression in AML correlates with both treatment response and outcomes. Therefore, targeting CD123 represents a promising universal therapeutic target in advanced acute leukemias irrespective of the individual leukemia phenotype. There are currently 31 ongoing clinical trials examining the utility of CD123-based targeted therapies. Here we focus our review on current efforts to target CD123 in acute leukemia through various therapeutic constructs.
KEYWORDS: CD123, Il3, flotetuzumab, bispecific antibody, tagraxofusp, AML, ALL, BPDCN
Introduction
Acute leukemias are characterized by blocked differentiation and increased proliferation of hematopoietic progenitor cells, and present in different phenotypes including acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). While a subset of adult patients with acute leukemia is cured with frontline multi-agent regimens with or without consolidation with allogeneic hematopoietic cell transplantation (alloHCT), a substantial number of patients fail to respond to initial induction therapy or eventually relapse post-remission. The prognosis for patients with acute leukemia who are refractory to chemotherapy or have relapsed is extremely poor and novel treatments are desperately needed. The fields of targeted- and immuno-therapeutics for acute leukemia are advancing rapidly and offer new optimism for the treatment of patients with acute leukemia. However, although promising targets have been identified and validated, with several novel therapies successfully introduced to the clinic, relapse continues to be a limitation of these therapeutics. Relapse after initial response is attributed to a small population of leukemia stem cells (LSC) that survive initial therapy. Therefore, the discovery of novel LSC-specific targets is necessary to achieve cure or extend the survival of these unfortunate patients. Various therapeutic constructs have been utilized to mediate the targeting leukemic cells including monoclonal antibodies, toxin- or drug-conjugated antibodies, bispecific antibodies and chimeric antigen receptor (CAR) T cell therapies.
Each individual leukemia expresses a unique set of potential therapeutic targets including cell surface antigens and proteins within various survival pathways. The IL-3 alpha chain receptor (CD123) is a cell surface protein that is widely expressed across various subtypes of acute leukemia. In normal hematopoietic cells, IL-3 signaling stimulates progression through the cell cycle and promotes both differentiation and resistance to apoptosis. CD123 is highly expressed in AML, B-cell and T-cell ALL, and acute leukemia of ambiguous lineage. Of note, CD123 is universally expressed at high levels in blastic plasmacytoid dendritic cell neoplasm (BPDCN). Therefore, CD123 represents a universal therapeutic target in acute leukemias irrespective of the individual leukemia subtype. There are currently 31 ongoing clinical trials examining the utility of CD123-based targeted therapies. Here we focus our review on current efforts to target CD123 in acute leukemia.
CD123 pathway and expression on hematological malignancies
CD123 is the IL-3 alpha chain receptor (IL-3RA). Upon binding of IL-3, CD123 forms heterodimers with the cytokine receptor common beta chain CD131. This receptor activation induces tyrosine phosphorylation that results in the activation of JAK-STAT signaling and transcription of tightly regulated genes.1,2 The result of this change in gene expression is cell proliferation and induction of anti-apoptotic proteins. Thus, leukemia blasts and LSCs that overexpress CD123 have growth and survival advantages over normal hematopoietic stem cells.3
Normal myeloblasts and monocytes typically have very low levels of CD123 as detected by flow cytometry while plasmacytoid dendritic cells and basophils typically have increased intensity of CD123 expression (moderate to bright). In contrast to this variable expression on normal hematopoietic cells, CD123 is expressed broadly by a variety of hematological malignancies. The majority of AML cases (93%) express CD123 at various intensities, and CD123 expression was observed across all the French-American-British (FAB) subtypes with the exception of the acute megakaryoblastic leukemia (M7) phenotype in one study.3 Similarly, CD123 is expressed by ~90% of cases of B-cell ALL.4,5 In the study of Angelova et al., the median relative fluorescence intensity (RFI) was 21.3 in CD123+ BCR-ABL+ B-ALL with a median MFI ratio of 22.4.4 Interestingly, expression of CD123 by leukemic blasts appears to vary by genetic subtype of B-cell ALL: CD123 expression is more prevalent among Philadelphia chromosome (Ph)-positive cases compared to Ph-negative cases4 and the expression is stronger in cases with hypodiploidy while weaker in cases carrying the ETV6/RUNX1 rearrangement.5 In one study, the expression of CD123 by flow cytometry was noted at higher intensity in B-cell ALL compared to AML.3 Likewise, CD123 is expressed by a subset of immature T-cell ALL (~40%),4,6,7 with the expression of CD123 more frequent in early T-cell precursor (ETP) cases (~85%).4,7
CD123 is also expressed by other less common hematological malignancies. For example, up to 95% of cases of classic hairy cell leukemia express CD123.3,8 CD123 expression is considered the hallmark and one of the characteristic findings in BPDCN, and it is observed in almost all cases.9 Additionally, CD123 is expressed frequently by systemic mastocytosis (SM) (64%), with higher frequency among aggressive subtypes (100%) compared to indolent cases (61%), SM with associated hematological neoplasm (57%) and mast cell leukemia (0%).10 CD123 is also expressed by a large subset of Hodgkin’s lymphoma Reed–Sternberg cells (~60%) but less frequently by non-Hodgkin’s lymphomas.11
Of note, CD123 is preferentially overexpressed in LSC as compared to normal hematopoietic stem cells.12–14 This differential expression allows for the selective eradication of LSC and leukemic blasts through targeting of CD123, with less potential impact on hematopoietic cells.15 Intriguingly, the level of CD123 expression and the percentage of blasts expressing CD34+CD38(low/-)CD123+ in AML was found to correlate with treatment response, relapse and survival.16,17 Likewise, there was a correlation between CD123 expression and inferior relapse-free survival among patients with T-cell ALL in one study,4 likely due to the “stem cell-like” properties of leukemia progenitors in the poor-risk ETP subtype. Lastly, a subset of patients with B-cell ALL treated with CD19-targeted immunotherapies will lose CD19 expression at the time of relapse or progression,18,19 and among these patients with CD19-negative relapsed ALL, CD123 expression is usually maintained and upregulated.14
Together, the growth and survival advantages conferred by expression of CD123, the overexpression of CD123 by different subtypes of acute leukemia, and the differential expression of CD123 by hematological malignancies versus normal hematopoietic stem cell (HSC) have led to therapeutic targeting of CD123. The following discussion highlights the successes and limitations of the major therapeutic modalities that have been thus far tested in clinical trials. Table 1 compares the main therapeutic modalities and Table 2 summarizes the ongoing clinical trials for CD123-targeted therapies.
Table 1.
A comparison of CD123-targeting therapies
| Class | Conjugated antibodies | Bispecific antibodies | CAR T cells |
|---|---|---|---|
| Availability for administration | Immediate, off-the-shelf product and not patient specific | Immediate, off-the-shelf product and not patient specific | Requiring manufacturing, however, off-the-shelf allogeneic CARs are in development |
| Method of administration | IV at repeated doses | Varies from continuous IV infusion to long half-life BiTEs given as repeated IV dosing | Usually single IV infusion |
| Lymphodepletion | Not required | Not required | This is usually required |
| FDA approval | Only tagraxofusp is approved for BPDCN | None yet | None yet |
| Stages in clinical studies | Varied from phase 1 studies to phase 1b/II studies (IMGN632) | Varied from phase 1 (JNJ-63709178, APVO436, XmAb14045) to phase 2/registry studies (flotetuzumab) | Early phases |
| In combination therapy | Actively being tested (tagraxofusp or IMNG632 in combination venetoclax + HMA) | Actively being tested (flotetuzumab + MGA012) | No, but dual “multi-target” CAR T cells are being tested |
| Main toxicity | VOD, CLS | CRS | CRS |
| Product half-life | Intermediate to long | Usually very short, although, there are long half BiTEs (XmAb14045) | Usually long but depends on the co-stimulatory domain and other characteristics |
BiTE, bispecific T cell engager; BPDCN, blastic plasmacytoid dendritic cell neoplasm; CAR, chimeric antigen receptor; CLS, capillary leak syndrome; CRS, cytokine release syndrome; HMA, hypomethylating agent; IV, intravenous; VOD, veno-occlusive disease.
Table 2.
Ongoing immunotherapy studies targeting CD123 in acute leukemia
| Drug/study | NCT; Sponsor |
Disease | Study phase | Status | Prelim results |
|---|---|---|---|---|---|
| Monoclonal antibodies and antibody-drug conjugates | |||||
| Tagraxofusp (STML-401-0114) |
NCT02113982; Stemline Therapeutics, Inc. |
BPDCN, AML | 1/2 | Active, not recruiting | BPDCN cohort: ORR 90%; Pemmaraju et al 2019 N Engl J Med |
| Tagraxofusp with Azacitidine or Azacitidine/Venetoclax |
NCT03113643; Dana-Farber Cancer Institute |
AML, MDS | 1 | Recruiting | NA |
| IMGN623 | NCT03386513; ImmunoGen, Inc. | r/r AML, BPDCN, ALL, Other CD123+ Hem Malignancies | 1/2 | Recruiting | AML cohort: ORR 18% BPDCN cohort: ORR 43%; Daver, Montesinos et al 2019 Blood |
| IMGN623 as monotherapy or with Venetoclax and/or Azacitidine | NCT04086264; ImmunoGen, Inc. | AML | 1b/2 | Recruiting | Daver, Papadantonakis et al 2019 Blood |
| Bispecific antibodies | |||||
| Flotetuzumab | NCT02152956; MacroGenics | AML/hrMDS | 1/2 (Expansion) | Enrolling | Uy et al 2019 Blood |
| Flotetuzumab | NCT03739606; City of Hope Medical Center | CD123+ hematological malignancies | 2 | Not recruiting yet | NA |
| XmAb14045 | NCT02730312; Xencor, Inc. | r/r CD123-expressing hematological malignancies | 1 (Expansion in r/r AML) | Enrolling | Ravandi et al 2018 Blood |
| JNJ-63709178 | NCT02715011; Janssen | r/r AML | 1 | Recruiting | NA |
| Apvo436 | NCT03647800; Aptevo | AML/MDS | 1/1b | Recruiting | NA |
| Chimeric antigen receptor T cells | |||||
| Autologous CD123CAR T cells | NCT02159495; City of Hope Medical Center | AML, BPDCN | 1 | Recruiting | Budde et al 2017 Blood |
| Redirected CD123 Autologous T cells | NCT03766126; University of Pennsylvania | AML | 1 | Recruiting | NA |
| MB-102 (autologous CD123 CAR T cells) | NCT04109482; Mustang Bio | AML, BPDCN, hrMDS | 1/2 | Recruiting | NA |
| UCART123/AMELI-01 (allogeneic CD123 CAR T cells) | NCT03190278; Cellectis S.A. | r/r AML | 1 | Recruiting | NA |
| Donor-derived CD123 CAR T cells | NCT03114670; Affiliated Hospital to Academy of Military Medical Sciences, China | r AML post alloHCT | 1 | Recruiting | NA |
| CD123/CLL1 bispecific CAR T cells | NCT03631576; Fujian Medical University, China | r/r AML | 2/3 | Recruiting | NA |
| CAR-T cells targeting CD123 | NCT03796390; Hebei Senlang Biotechnology Inc., Ltd., China | AML | 1 | Recruiting | NA |
ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; alloHCT: allogeneic hematopoietic cell transplantation; BPDCN: blastic plasmacytoid dendritic cell neoplasm; hrMDS: high-risk myelodysplastic syndrome; MDS: myelodysplastic syndrome; NA: not available; ORR: overall response rate; r: relapsed; r/r: relapsed or refractory.
Monoclonal antibodies and antibody-drug conjugates targeting CD123
Early clinical experience with targeting CD123 to treat acute leukemia utilized unconjugated monoclonal antibodies as well as antibodies or ligands (e.g. IL-3) that are conjugated to cytotoxic drugs (Figure 1A).
Figure 1.
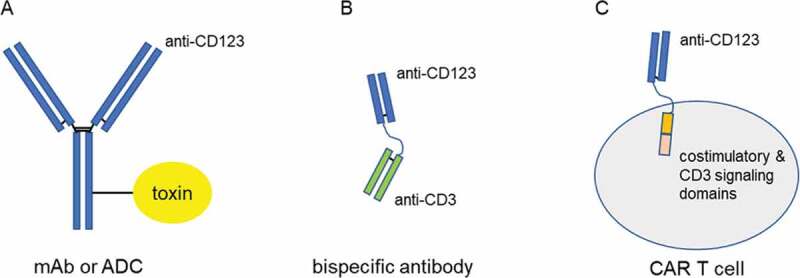
Schematic of the modalities used to target CD123
CD123+ leukemia cells can be targeted and killed by (A) monoclonal antibodies (mAb) or antibody-drug conjugates (ADC) that induce antibody-dependent cytotoxicity and/or apoptosis, (B) bispecific antibodies that link a CD123-targeting domain to a T-cell engaging CD3-targeting domain and (C) Chimeric antigen receptor (CAR) T cells that couple a CD123-targeting domain to the effector function of T cells.
Talacotuzumab (JNJ-56022473) is an IgG1 monoclonal antibody directed toward CD123. Talacotuzumab promotes anti-leukemia activity through both antibody-dependent cellular cytotoxicity (ADCC)20 mediated by natural killer cells and by inhibiting signaling through the IL-3 receptor.21 In a phase 2 study (NCT02992860), talacotuzumab was assessed as a single agent in patients with advanced myelodysplastic syndrome (MDS) or AML who had failed prior hypomethylating agent (HMA) therapy. The study was terminated early after enrolling only 24 of 43 planned patients on the basis of low efficacy and higher than anticipated adverse effects. When used as a single agent, the 4-week mortality rate was 21%. Serious toxicities following treatment with talacotuzumab included infectious complications, cytopenias and multi-organ dysfunction involving cardiac, gastrointestinal and central nervous systems. Only one complete remission (CR) with incomplete count recovery (CRi) was observed in evaluable patients.22 In a separate phase 2 randomized trial (NCT02472145) assessing talacotuzumab in combination with the HMA decitabine for patients with AML who were ineligible for intensive induction therapy, the combination did not show benefit in terms of CR rate compared to decitabine alone (15% vs. 11%, p = .44).23
Tagraxofusp (Stemline Therapeutics), formerly known as SL-401, is a recombinant fusion protein consisting of human IL-3 fused to a truncated diphtheria toxin (DT) payload. Tagraxofusp binds to CD123 and is subsequently internalized to release DT into the leukemia cell, which results in inhibition of protein synthesis and apoptosis of the targeted cells.24 In an open-label multi-center study that enrolled patients with newly diagnosed and relapsed or refractory (r/r) BPDCN, tagraxofusp produced a response rate of 90% (CR = 72%) in newly diagnosed patients (n = 29) and 67% in r/r patients (n = 15). The most common adverse events were transaminitis and hypoalbuminemia, with 19% of patients developing capillary leak syndrome (CLS).25 Tagraxofusp was granted FDA approval for the treatment of BPDCN in adults and children older than 2 y of age on December 21, 2018.24 Tagraxofusp is given by intravenous infusion on d 1 to 5 of every 21-d cycle. For patients who have clinical benefit, tagraxofusp is given indefinitely. In a pivotal cohort (stage III) from the STML-401-0114 study (n = 13), the CR/clinical CR rate of treatment-naïve patients with BPDCN was 54% using the BPDCN response criteria, with a median duration of response not reached (median follow up of 11.5 months). In this cohort, 55% of patients developed CLS of any grade, including 9% with grade ≥3.26 Interestingly, patients with BPDCN who were refractory to or relapsed after tagraxofusp retain CD123 expression. Current evidence suggests that resistance to tagraxofusp is more likely to involve resistance to DT rather than alterations in the IL-3 receptor or IL-3-induced signaling. A recent study evaluated resistance to tagraxofusp and found that resistance was associated with decreased expression of DPH1, which results in deficiency in the diphthamide synthesis pathway and a disruption of the function of DT once it is internalized.25,27
In contrast to its activity in BPDCN, the activity of tagraxofusp as a single agent appears limited in AML, with only 1 CR and 1 partial response (PR) observed among 40 evaluable patients in a phase 1 study. The development of anti-DT antibodies was frequent and occurred in the majority of cases early during therapy.28 In a preclinical study, treatment with HMA restored DPH1 expression, which resulted in re-sensitization of resistant AML and BPDCN cells to tagraxofusp.27 Therefore, the activity of tagraxofusp is currently being evaluated in combination with the HMA 5-azacytidine with or without the Bcl-2 inhibitor venetoclax in patients with MDS or AML (NCT03113643).
SGN-CD123A (Seattle Genetics) is a humanized anti-CD123 antibody-drug conjugate linked to a pyrrolobenzodiazepine dimer (PBD) payload. In preclinical evaluation, SGN-CD123A delivered PBD to CD123+ AML cells, which resulted in DNA damage and apoptosis in vitro and elimination of AML in multiple in vivo xenograft mouse models.29 However, a safety phase 1 study examining SGN-CD123A in patients with r/r AML was terminated early in May 2018 due to grade 3 toxicities.
IMGN632 (ImmunoGen, Inc.) is a humanized anti-CD123 antibody linked to indolinobenzodiazepine pseudodimer, a novel chemical class of DNA alkylator.30 In a phase 1 dose-escalation study, single-agent IMGN632 was administered to patients with r/r AML or BPDCN in one of the two schedules: cohort A was treated on d 1 of a 21-d cycle and cohort B received fractionated dosing on d 1, 4 and 8 of a 21-d cycle. The most frequent treatment toxicity was infusion reaction. Dose-limiting toxicities (DLT) occurred at dose levels of ≥0.18 mg/kg (all in cohort A), including prolonged neutropenia, and two reversible cases of veno-occlusive disease (VOD). Of 71 evaluable patients with AML who had marrow involvement pre-treatment, 13 (18%) achieved response (2 CR, 10 CRi, 1 morphologic leukemia-free state [MLFS]), and there was no difference in efficacy between the two schedules. Three of 9 evaluable patients with BPDCN responded (1 CR, 1 CRi, 1 PR) to single-agent IMGN632.31 Notably, the observed efficacy of IMGN632 in AML was independent of CD123 expression. IMGN632 is also being evaluated in combination with azacytidine with or without venetoclax in patients with AML (NCT04086264).32
To summarize, while several CD123-targeted monoclonal antibody and antibody-drug conjugate-based therapeutics have shown promising results leading in some cases to FDA approval, others have been proven to be too toxic for clinical use. Further, those that have successfully been evaluated in clinical trials may benefit from combination therapies.
Bispecific antibodies targeting CD123
Bispecific antibodies combine two antigen recognition sites from different antibodies into a single construct (Figure 1B). This allows simultaneous binding of both tumor and effector cells, such as T cells, to form an immunologic synapse, thus redirecting the effector cells to kill tumor cells in an MHC-independent fashion. Several anti-CD3 x anti-CD123 bispecific antibodies have been developed that redirect T cells through CD3 to target CD123-positive malignancies.
Flotetuzumab (MacroGenics, Inc.), formerly known as MGD006, is a bispecific CD3 x CD123 dual-affinity re-targeting (DART) protein that engages T cells with CD123-expressing malignant cells. DARTs are heterodimers that combine the variable heavy chain region of one antigen recognition site with the variable light chain region of a second antigen recognition site, stabilized by C-terminal disulfide bonds. In preclinical studies, flotetuzumab mediated T cell activation and expansion and resulted in the eradication of CD123+ leukemic cells in vitro and in vivo.33,34
A phase 1 dose-escalation study that assessed single-agent flotetuzumab to treat patients with advanced AML and high-grade MDS was completed. In this trial, two independent schedules of flotetuzumab administration were compared: continuous infusion versus an intermittent dosing schedule (4 d on; 3 d off). The enrollment in the MDS cohort was halted early due to slow accrual. The continuous schedule of 500 ng/kg/day for 28 d was deemed to be the maximum tolerated dose (MTD) as 2 DLT, including neurotoxicity and grade 3 cytokine release syndrome (CRS), was observed at the 700 ng/kg/day dose. The study is currently enrolling patients to the expansion phase; however, it restricted inclusion to patients with induction failure and early relapse of AML (<6 months) (NCT02152956). Among high-risk patients with induction failure and early relapse who were treated at MTD, the response rate was encouraging with 32% of evaluable patients (N = 28) achieving composite CR/CRi/CR with partial hematological recovery (CRh). Encouraging response to flotetuzumab was observed in patients with AML carrying TP53 mutations (5 of 11 patients). Interestingly, while the study did not require documentation of CD123 expression as part of inclusion criteria, the analysis did not identify a correlation between the intensity of CD123 expression on AML blast cells and response to flotetuzumab.35
The limiting toxicity of flotetuzumab is CRS, which is observed frequently during the first 2 weeks of the first cycle followed by reduced incidence once a steady-state of the drug is reached. While CRS of any grade is common (96%) following treatment with flotetuzumab, grade 3–4 CRS was only observed in 8% of patients.36 The implementation of a multi-step dose-escalation schedule in the first week (i.e. daily dose escalation in the first 7 d of the first cycle) and early utilization of the IL-6 receptor antibody tocilizumab following the onset of CRS resulted in decreased rates of high-grade CRS and an improved ability to deliver the intended dosing of flotetuzumab. Nonetheless, the frequency of CRS episodes, but not the severity, correlated with response to flotetuzumab.35
While flotetuzumab has shown promising efficacy as a single agent, there are several efforts to further improve its response rate through combination therapy. Ruxolitinib, a potent JAK1 and JAK2 inhibitor, has shown the ability to mitigate CRS during immunotherapy without diminishing the anti-tumor effect of T cells in preclinical studies.37 This has motivated the design of a pilot study at the University of Washington in Saint Louis that combines ruxolitinib with flotetuzumab in an attempt to reduce the severity of CRS following flotetuzumab therapy thereby improving drug tolerability and allowing for adequate dosing. Leukemia blasts of patients with AML who failed flotetuzumab showed upregulation of the immune checkpoint protein PD-L1, and combining flotetuzumab with immune checkpoint inhibitors showed synergistic T-cell-mediated cytotoxicity in AML cell lines.38,39 These observations led to the initiation of a clinical study combining flotetuzumab with the anti-PD-1 monoclonal antibody (MGA012) that is currently enrolling patients with r/r AML.
Given the fact that CD123 is expressed frequently in ALL of both B- and T-cell origin, as well as BPDCN and other hematological malignancies, we developed a phase 1 study at City of Hope testing flotetuzumab in two strata. Strata A includes patients with advanced CD123-positive B- or T-cell ALL, while strata B includes other advanced hematological malignancies expressing CD123, including acute leukemia with ambiguous phenotype and BPDCN among others (NCT03739606).
XmAb14045 (Xencor, Inc.) is a CD123 x CD3 bispecific antibody with a unique Fc domain that forms stable heterodimers and contributes to a longer half-life when compared to other bispecific antibodies. XmAb14045 has been evaluated in a phase 1 dose-escalation study of 66 patients with r/r AML (NCT02730312). The median age was 61 y and 30% of patients had had prior alloHCT. The MTD was not reached, and CRS was the most common toxicity (55%), including 6% of patients who experienced grade ≥3 CRS. Five of 18 (28%) evaluable patients with AML achieved CR/CRi at the 2 highest initial dose levels (1.3 and 2.3 mcg/kg/weekly). Two of the responders were successfully bridged to alloHCT and a third, HCT-ineligible responder has remained in remission for over 4 months after discontinuing therapy.40 On February 20, 2019, a partial clinical hold was placed on the study after the deaths of two patients were considered at least possibly related to XmAb14045 (one death due to CRS and one case of pulmonary edema). This hold was subsequently lifted on April 30, 2019, after the study protocol was amended, and the study is currently enrolling.
JNJ-63709178 (Janssen Pharmaceuticals) is a humanized IgG4 CD123 x CD3 bispecific antibody that was generated using the Genmab DuoBody technology. In preclinical studies, JNJ-63709178 has shown potency and specificity in eradicating CD123+ tumor cells in vitro, in vivo and ex vivo.41 A phase 1 study for the treatment of r/r AML was initiated and currently is ongoing (NCT02715011). There were two clinical holds on the study (2016 and 2018) due to toxicities but both were subsequently lifted. No results have been released yet.
APVO436 (Aptevo Therapeutics Inc.) is an anti-CD123/CD3 bispecific monoclonal antibody generated by ADAPTIR platform molecule approach, which has the advantage of maintaining T-cell-mediated activity while reducing cytokine production.42,43 An ongoing phase 1/1b study to treat patients with high-grade MDS and r/r AML is ongoing (NCT03647800). No results have been presented to our knowledge yet.
Overall, while the clinical evaluation of bispecific antibody constructs that bridge CD123+ acute leukemias with cytotoxic effector T cells is preliminary, preclinical data and early clinical data has shown promise for the treatment of acute leukemias.
Chimeric antigen receptor T cell therapy targeting CD123
Chimeric antigen receptor (CAR) T cells are generated by transducing autologous or allogeneic T cells with a vector expressing a chimeric receptor that recognizes the antigen of interest (Figure 1C). Upon engagement with the target antigen, the CAR construct transduces signaling through an intracellular CD3 signaling domain and a co-stimulatory domain, which is typically the intracellular signaling domain of either CD28 or 4–1BB. CD19CAR T cell therapy has proven to be an effective therapy in advanced B-cell ALL and it has produced unprecedented responses in chemo-refractory diseases,19,44,45 which has led to the extension of CAR T cell therapy into other malignancies. Among other targets that are being investigated, CD123-targeting CAR T cells have currently generated an interest in the treatment of AML, ALL and BCDCN. In preclinical studies, CD123CAR T cells have successfully led to the eradication of AML growth in xenograft models.46–49
City of Hope has developed a CD123CAR T cell product (MB-102, Mustang Bio) that utilizes a CD123-targeting construct with a CD28 co-stimulatory domain, which is currently undergoing a phase 1 study in patients with r/r AML or BPDCN (NCT02159495). Of 24 patients who have been enrolled at the time of data presentation in 2018, CD123CAR T cells were successfully manufactured for 88% (21 of 24) and administered to 50% (12 of 24). The study reported 1 PR and 1 MLFS in patients who received dose level 0 (n = 2), and 1 CRi, 2 CR and 2 stable disease for patients received dose level 1 (n = 5).50 A multicenter phase 1/2 study testing the same product (MB-102) is open and recruiting patients with r/r AML, high-grade MDS and BPDCN (NCT04109482).
UCART123 (Cellectis) is an off-the-shelf allogeneic CD123CAR T cell product. A phase 1 study to test the efficacy of UCART123 for the treatment of patients with AML or BPDCN was initiated in 2017 (NCT03190278). This study was placed temporary on clinical hold on September 2017 after a patient's death, but the hold was lifted after the study was amended to infuse a lower dosage of UCART123. The study is currently recruiting.
Other studies using CD123-redirected CAR T cells are actively recruiting, with results currently unavailable. Investigators at the University of Pennsylvania are currently investigating their CD123CAR T cells generated with a CAR construct that includes a 4–1BB co-stimulatory domain to treat r/r AML (NCT03766126). There are also several studies from China employing CAR T cells that target CD123 alone in r/r CD123+ acute leukemias (NCT03114670, NCT03556982, NCT03796390, NCT03585517) CAR T cells that target CD123 in combination with other targets such as CLL1 or other antigens that are frequently expressed on acute leukemias (NCT03473457, NCT03631576).
Interestingly, a case report describes a patient with chemo-refractory AML with FUS-ERG-fusion who relapsed after alloHCT and subsequently received donor-derived CD123CAR T cells as part of conditioning regimen for second haploidentical transplant, which resulted in full donor chimerism and leukemia remission. However, the patient died on d 56 with severe graft versus host disease (GVHD) and multi-organ failure.51
As mentioned previously, CD123 is often upregulated in cases of CD19-negative relapsed B-cell ALL after failing CD19-targeted immunotherapies. In a mouse model, dual delivery of both CD123 and CD19 CAR T cells prevents CD19 antigen loss in ALL.14 Tu et al. are conducting a clinical study (NCT0312557) in which pooled donor-derived CD123 and CD19 CAR T cells are administered to patients with relapsed B-cell ALL after alloHCT. In a preliminary report of three patients treated on the study, dual CD123xCD19 donor-derived CAR T cell therapy was safe with only grade 1 and 2 CRS, no neurotoxicity and one patient developed oral GVHD. All three patients achieved complete remission, with two achieving minimum residual disease-negative responses, and treated patients remained free of disease for 7 to 11 months.52
Overall, CD123-targeting CAR T cell therapy is a promising modality for the treatment of acute leukemia.
Toxicities of CD123-targeted therapies
As the result of the expression of CD123 on normal hematopoietic cells, although at decreased intensity compared to leukemic cells, concerns have emerged regarding the impact of CD123-directed therapies on normal human myelopoiesis and the risk of bone marrow failure.46 The incidence and severity of myelosuppression induced by CD123-targeted therapies vary based on the specific modality, and appears to be a more prominent with CAR T cell-based approaches compared to other modalities based on preclinical studies.46 This observation led the FDA to mandate a rescue plan that requires the identification of an available hematopoietic stem cell donor in case a transplant is urgently needed in the case of bone marrow failure for studies employing CD123-directed CAR T cells. Although clinical studies utilizing CD123CAR T cell therapy remain in early phases, Budde and colleagues at COH reported no treatment-related cytopenias in six patients treated in an ongoing phase 1 trial.50 In contrast, preclinical studies show that flotetuzumab therapy has no impact on normal HSC or progenitor cells at therapeutic doses, and no myelosuppression was observed in monkeys treated with flotetuzumab.33 In the phase 1/expansion study of flotetuzumab in r/r AML, treatment-emergent grade 3 thrombocytopenia was encountered in 12% of treated patients. However, patients who achieved remission usually recover their blood counts, suggesting that this toxicity is short term and reversible.35 In the phase 1 study of XmAb14045, no myelosuppression requiring dose modifications was reported.40 In contrast, prolonged neutropenia was reported as a DLT in the phase 1 study of the IMGN32 in r/r AML when given at the every 3-week dosing schedule (cohort A).31 Thus, the concern of myelosuppression as a toxicity associated with CD123-targeted therapies will require additional clinical data to validate the degree and durability, especially when CAR T cell therapy is used.
Like myelosuppression, other toxicities associated with CD123-targeted therapies depend on the specific modality. For CD123-targeted immunotherapies, such as bispecific antibody- and CAR T cell-based therapies, CRS is the most commonly encountered toxicity and incidence of CRS has been reported frequently in the current ongoing studies.35,40,50 In contrast, the toxicity of antibody-drug conjugate-based therapy may depend more on the specific cytotoxic-conjugate. For example, VOD is an uncommon but unique toxicity during IMGN632 administration,31 while CLS is observed during tagraxofusp therapy.25 In addition to VOD and CLS, other hepatotoxicity manifestations including transaminitis, hypoalbuminemia and hyperbilirubinemia were observed across various CD123-based targeting modalities25,31,35 and could be related to the underrecognized expression of CD123 in the liver.
Future directions
While early studies have shown the promise of targeting CD123 with different modalities of single agents, studies are currently being developed to combine these agents with other novel therapies to improve their anti-leukemic efficacy. Such combinations include checkpoint inhibitors (e.g. flotetuzumab with MGA012) and combinations that include other known active anti-leukemic agents such as Bcl-2 inhibitors (NCT03113643, NCT04086264). Simultaneously, other studies are evaluating the concept of dual and multi-targeted immunotherapies in CD123 CAR T cell therapy (NCT03125577, NCT03473457, NCT03631576). Additionally, efforts are being developed to extend the application of individual CD123-targeting therapies to new settings and indications (NCT03739606, NCT03113643).
Safety is a major concern when therapeutically targeting CD123. While we are still learning about toxicities associated with anti-CD123 therapy, antibody-drug conjugate-based targeting has shown increased risk of VOD and CLS. Safer use of these agents is warranted and should include a better patient selection process and optimization of patient pre-treatment status to reduce the risk of developing these toxicities. CRS appears to be the main the DLT for CD123-based immunotherapies, including those that utilize bispecific antibodies or CAR T cells. The introduction of multi-step dose escalation and early utilization of anti-IL-6 therapy seem to limit the severity of CRS. Other efforts to reduce the severity of CD123-immunotherapy-induced CRS include concurrent treatment with JAK2 inhibitors (NCT02152956). For CD123-targeted CAR T cell therapy, the safety plan to mitigate the potential toxicity of bone marrow failure includes the identification of an available donor for rescue allo-HCT. Thus, as we learn more about the etiology of toxicities associated with therapies targeting CD123, we can generate prevention and treatment protocols to ensure the safety of patients.
While CD123-targeted therapies are active in a subset of patients with advanced acute leukemia, studies are warranted to understand mechanisms of resistance in non-responders and methods of treatment failure and relapse. Such studies can lead to stratify patients who could benefit the most from these therapies.
Disclosure of potential conflicts of interest
I.A. received research support from MacroGenics. M.C., J.Y.S. and V.P. reported no relevant conflict of interest.
References
- 1.Silvennoinen O, Witthuhn BA, Quelle FW, Cleveland JL, Yi T, Ihle JN.. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci U S A. 1993;90(18):8429–33. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagley CJ, Woodcock JM, Stomski FC, Lopez AF. The structural and functional basis of cytokine receptor activation: lessons from the common beta subunit of the granulocyte-macrophage colony-stimulating factor, interleukin-3 (IL-3), and IL-5 receptors. Blood. 1997;89(5):1471–82. doi: 10.1182/blood.V89.5.1471. [DOI] [PubMed] [Google Scholar]
- 3.Munoz L, Nomdedeu JF, Lopez O, Carnicer MJ, Bellido M, Aventín A, Brunet S, Sierra J. Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 2001;86:1261–69. [PubMed] [Google Scholar]
- 4.Angelova E, Audette C, Kovtun Y, Daver N, Wang SA, Pierce S, Konoplev SN, Khogeer H, Jorgensen JL, Konopleva M. CD123 expression patterns and selective targeting with a CD123-targeted antibody-drug conjugate (IMGN632) in acute lymphoblastic leukemia. Haematologica. 2019;104(4):749–55. doi: 10.3324/haematol.2018.205252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djokic M, Bjorklund E, Blennow E, Mazur J, Soderhall S, Porwit A. Overexpression of CD123 correlates with the hyperdiploid genotype in acute lymphoblastic leukemia. Haematologica. 2009;94(7):1016–19. doi: 10.3324/haematol.2008.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lhermitte L, de Labarthe A, Dupret C, Lapillonne H, Millien C, Landman-Parker J, Hermine O, Baruchel A, Sigaux F, Macintyre E. Most immature T-ALLs express Ra-IL3 (CD123): possible target for DT-IL3 therapy. Leukemia. 2006;20(10):1908–10. doi: 10.1038/sj.leu.2404349. [DOI] [PubMed] [Google Scholar]
- 7.Du W, Li J, Liu W, He Y, Yao J, Liu Y, Lin J, Zheng J. Interleukin-3 receptor alpha chain (CD123) is preferentially expressed in immature T-ALL and may not associate with outcomes of chemotherapy. Tumour Biol. 2016;37(3):3817–21. doi: 10.1007/s13277-015-3272-y. [DOI] [PubMed] [Google Scholar]
- 8.Del Giudice I, Matutes E, Morilla R, Morilla A, Owusu-Ankomah K, Rafiq F, A’Hern R, Delgado J, Bazerbashi MB, Catovsky D. The diagnostic value of CD123 in B-cell disorders with hairy or villous lymphocytes. Haematologica. 2004;89:303–08. [PubMed] [Google Scholar]
- 9.Garnache-Ottou F, Feuillard J, Ferrand C, Biichle S, Trimoreau F, Seilles E, Salaun V, Garand R, Lepelley P, Maynadié M. Extended diagnostic criteria for plasmacytoid dendritic cell leukaemia. Br J Haematol. 2009;145(5):624–36. doi: 10.1111/j.1365-2141.2009.07679.x. [DOI] [PubMed] [Google Scholar]
- 10.Pardanani A, Reichard KK, Zblewski D, Abdelrahman RA, Wassie EA, Morice II WG, Brooks C, Grogg KL, Hanson CA, Tefferi A. CD123 immunostaining patterns in systemic mastocytosis: differential expression in disease subgroups and potential prognostic value. Leukemia. 2016;30(4):914–18. doi: 10.1038/leu.2015.348. [DOI] [PubMed] [Google Scholar]
- 11.Fromm JR. Flow cytometric analysis of CD123 is useful for immunophenotyping classical Hodgkin lymphoma. Cytometry B Clin Cytom. 2011;80(2):91–99. doi: 10.1002/cyto.b.20561. [DOI] [PubMed] [Google Scholar]
- 12.Testa U, Riccioni R, Diverio D, Rossini A, Lo Coco F, Peschle C. Interleukin-3 receptor in acute leukemia. Leukemia. 2004;18(2):219–26. doi: 10.1038/sj.leu.2403224. [DOI] [PubMed] [Google Scholar]
- 13.Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14(10):1777–84. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 14.Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, Klichinsky M, Aikawa V, Nazimuddin F, Kozlowski M. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126(10):3814–26. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, Guthridge MA, Thomas D, Barry EF, Boyd A. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5(1):31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Testa U, Riccioni R, Militi S. Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood. 2002;100:2980–88. [DOI] [PubMed] [Google Scholar]
- 17.Vergez F, Green AS, Tamburini J, Sarry J-E, Gaillard B, Cornillet-Lefebvre P, Pannetier M, Neyret A, Chapuis N, Ifrah N. High levels of CD34+CD38low/-CD123+ blasts are predictive of an adverse outcome in acute myeloid leukemia: a Groupe Ouest-Est des Leucemies Aigues et Maladies du Sang (GOELAMS) study. Haematologica. 2011;96(12):1792–98. doi: 10.3324/haematol.2011.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldoss I, Song J, Stiller T, Nguyen T, Palmer J, O’Donnell M, Stein AS, Marcucci G, Forman S, Pullarkat V. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2017;92(9):858–65. doi: 10.1002/ajh.24783. [DOI] [PubMed] [Google Scholar]
- 19.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Busfield SJ, Biondo M, Wong M, Ramshaw HS, Lee EM, Ghosh S, Braley H, Panousis C, Roberts AW, He SZ. Targeting of acute myeloid leukemia in vitro and in vivo with an anti-CD123 mAb engineered for optimal ADCC. Leukemia. 2014;28(11):2213–21. doi: 10.1038/leu.2014.128. [DOI] [PubMed] [Google Scholar]
- 21.Cuesta-Mateos C, Alcaraz-Serna A, Somovilla-Crespo B, Munoz-Calleja C. Monoclonal antibody therapies for hematological malignancies: not just lineage-specific targets. Front Immunol. 2017;8:1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubasch AS, Schulze F, Giagounidis A. Single agent talacotuzumab demonstrates limited efficacy but considerable toxicity in elderly high-risk MDS or AML patients failing hypomethylating agents. Leukemia. 2019;34:1182–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montesinos P, Roboz GJ, Bulabois C-E. 2020. Safety and efficacy of talacotuzumab plus decitabine or decitabine alone in patients with acute myeloid leukemia not eligible for chemotherapy: results from a multicenter, randomized, phase 2/3 study. Leukemia. doi: 10.1038/s41375-020-0773-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frankel AE, Ramage J, Kiser M, Alexander R, Kucera G, Miller MS. Characterization of diphtheria fusion proteins targeted to the human interleukin-3 receptor. Protein Eng. 2000;13(8):575–81. doi: 10.1093/protein/13.8.575. [DOI] [PubMed] [Google Scholar]
- 25.Pemmaraju N, Lane AA, Sweet KL, Stein AS, Vasu S, Blum W, Rizzieri DA, Wang ES, Duvic M, Sloan JM. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2019;380(17):1628–37. doi: 10.1056/NEJMoa1815105. [DOI] [PubMed] [Google Scholar]
- 26.Jen EY, Gao X, Li L, Zhuang L, Simpson NE, Aryal B, Wang R, Przepiorka D, Shen YL, Leong R. FDA approval summary: tagraxofusp-erzs for treatment of blastic plasmacytoid dendritic cell neoplasm. Clin Cancer Res. 2020;26(3):532–36. doi: 10.1158/1078-0432.CCR-19-2329. [DOI] [PubMed] [Google Scholar]
- 27.Togami K, Pastika T, Stephansky J, Ghandi M, Christie AL, Jones KL, Johnson CA, Lindsay RW, Brooks CL, Letai A. DNA methyltransferase inhibition overcomes diphthamide pathway deficiencies underlying CD123-targeted treatment resistance. J Clin Invest. 2019;129(11):5005–19. doi: 10.1172/JCI128571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frankel A, Liu JS, Rizzieri D, Hogge D. Phase I clinical study of diphtheria toxin-interleukin 3 fusion protein in patients with acute myeloid leukemia and myelodysplasia. Leuk Lymphoma. 2008;49(3):543–53. doi: 10.1080/10428190701799035. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Sutherland MK, Yu C, Walter RB, Westendorf L, Valliere-Douglass J, Pan L, Cronkite A, Sussman D, Klussman K. Characterization of SGN-CD123A, A potent CD123-directed antibody-drug conjugate for acute myeloid leukemia. Mol Cancer Ther. 2018;17(2):554–64. doi: 10.1158/1535-7163.MCT-17-0742. [DOI] [PubMed] [Google Scholar]
- 30.Kovtun Y, Jones GE, Adams S, Harvey L, Audette CA, Wilhelm A, Bai C, Rui L, Laleau R, Liu F. A CD123-targeting antibody-drug conjugate, IMGN632, designed to eradicate AML while sparing normal bone marrow cells. Blood Adv. 2018;2(8):848–58. doi: 10.1182/bloodadvances.2018017517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daver NG, Montesinos P, DeAngelo DJ, Wang ES, Papadantonakis N, Deconinck E, Erba HP, Pemmaraju N, Lane AA, Rizzieri DA. Clinical profile of IMGN632, a novel CD123-targeting Antibody-Drug Conjugate (ADC), in Patients with Relapsed/Refractory (R/R) Acute Myeloid Leukemia (AML) or Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN). Blood. 2019;134(Supplement_1):734–734. doi: 10.1182/blood-2019-128648. [DOI] [Google Scholar]
- 32.Daver NG, Erba HP, Papadantonakis N, DeAngelo DJ, Wang ES, Konopleva MY, Sloss CM, Wang J, Malcolm KE, Zweidler-McKay PA. A phase 1b/2 study of the CD123-targeting antibody-drug conjugate IMGN632 as monotherapy or in combination with venetoclax and/or azacitidine for patients with CD123-positive acute myeloid leukemia. Blood. 2019;134(Supplement_1):2601–2601. doi: 10.1182/blood-2019-128501. [DOI] [Google Scholar]
- 33.Chichili GR, Huang L, Li H. A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: preclinical activity and safety in nonhuman primates. Sci Transl Med. 2015;7(289):289ra282. doi: 10.1126/scitranslmed.aaa5693. [DOI] [PubMed] [Google Scholar]
- 34.Al-Hussaini M, Rettig MP, Ritchey JK, Karpova D, Uy GL, Eissenberg LG, Gao F, Eades WC, Bonvini E, Chichili GR. Targeting CD123 in acute myeloid leukemia using a T-cell-directed dual-affinity retargeting platform. Blood. 2016;127(1):122–31. doi: 10.1182/blood-2014-05-575704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uy GL, Aldoss I, Foster MC, Sallman DA, Sweet KL, Rizzieri DA, Sayre PH, Advani AS, Emadi A, Wieduwilt MJ. Flotetuzumab, an Investigational CD123 x CD3 bispecific dart® protein, in salvage therapy for primary refractory and early relapsed Acute Myeloid Leukemia (AML) patients. Blood. 2019;134(Supplement_1):733–733. doi: 10.1182/blood-2019-122073. [DOI] [Google Scholar]
- 36.Bakkacha O, Uy GL, Aldoss I, Foster MC, Sallman DA, Sweet KL, Rizzieri DA, Sayre PH, Advani AS, Emadi A. Improvement in cytokine release syndrome management for the treatment of AML patients with flotetuzumab, a CD123 x CD3 bispecific dart® molecule for t-cell redirected therapy. Blood. 2019;134(Supplement_1):5144–5144. doi: 10.1182/blood-2019-127138. [DOI] [Google Scholar]
- 37.Kenderian SS, Ruella M, Shestova O, Kim M, Klichinsky M, Chen F, Kengle N, Lacey S, Melenhorst J, June CH. 2 - ruxolitinib prevents cytokine release syndrome after car T-cell therapy without impairing the anti-tumor effect in a xenograft model. Biol Blood Marrow Transplant. 2017;23(3,Supplement):S19–S20. doi: 10.1016/j.bbmt.2016.12.003. [DOI] [Google Scholar]
- 38.Rettig MP, Godwin J, Vey N. Preliminary translational results from an ongoing phase 1 study of flotetuzumab, a CD123 x CD3 Dart®, in AML/MDS: rationale for combining flotetuzumab and anti-PD-1/PD-L1 immunotherapies. Blood. 2017;130:1365–1365. [Google Scholar]
- 39.Vadakekolathu J, Minden MD, Hood T, Church SE, Reeder S, Altmann H, Sullivan AH, Viboch EJ, Patel T, Ibrahimova N. Immune landscapes predict chemotherapy resistance and anti-leukemic activity of flotetuzumab, an investigational CD123×CD3 bispecific dart® molecule, in patients with relapsed/refractory acute myeloid leukemia. Blood. 2019;134(Supplement_1):460–460. doi: 10.1182/blood-2019-121870. [DOI] [Google Scholar]
- 40.Ravandi F, Bashey A, Foran JM, Stock W, Mawad R, Blum W, Saville MW, Johnson CM, Vanasse KGJ, Ly T. Complete responses in relapsed/refractory acute myeloid leukemia (AML) patients on a weekly dosing schedule of XmAb14045, a CD123 x CD3 T cell-engaging bispecific antibody: initial results of a phase 1 study. Blood. 2018;132(Supplement 1):763–763. doi: 10.1182/blood-2018-99-119786. [DOI] [Google Scholar]
- 41.Gaudet F, Nemeth JF, McDaid R, Li Y, Harman B, Millar H, Teplyakov A, Wheeler J, Luo J, Tam S. Development of a CD123xCD3 bispecific antibody (JNJ-63709178) for the treatment of acute myeloid leukemia (AML). Blood. 2016;128(22):2824–2824. doi: 10.1182/blood.V128.22.2824.2824.27663672 [DOI] [Google Scholar]
- 42.Hernandez-Hoyos G, Sewell T, Bader R, Bannink J, Chenault RA, Daugherty M, Dasovich M, Fang H, Gottschalk R, Kumer J. MOR209/ES414, a novel bispecific antibody targeting PSMA for the treatment of metastatic castration-resistant prostate cancer. Mol Cancer Ther. 2016;15(9):2155–65. doi: 10.1158/1535-7163.MCT-15-0242. [DOI] [PubMed] [Google Scholar]
- 43.Comeau MR, Miller RE, Bader R. Abstract 1786: APVO436, a bispecific anti-CD123 x anti-CD3 ADAPTIR™ molecule for redirected T-cell cytotoxicity, induces potent T-cell activation, proliferation and cytotoxicity with limited cytokine release. Cancer Res. 2018;78:1786–1786. [Google Scholar]
- 44.Park JH, Riviere I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–59. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, Carroll M, Danet-Desnoyers G, Scholler J, Grupp SA. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123(15):2343–54. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thokala R, Olivares S, Mi T, Maiti S, Deniger D, Huls H, Torikai H, Singh H, Champlin RE, Laskowski T. Redirecting specificity of T cells using the sleeping beauty system to express chimeric antigen receptors by mix-and-matching of VL and VH domains targeting CD123+ tumors. PLoS One. 2016;11(8):e0159477. doi: 10.1371/journal.pone.0159477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tettamanti S, Marin V, Pizzitola I, Magnani CF, Giordano Attianese GMP, Cribioli E, Maltese F, Galimberti S, Lopez AF, Biondi A. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematol. 2013;161(3):389–401. doi: 10.1111/bjh.12282. [DOI] [PubMed] [Google Scholar]
- 49.Mardiros A, Dos Santos C, McDonald T, Brown CE, Wang X, Budde LE, Hoffman L, Aguilar B, Chang W-C, Bretzlaff W. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122(18):3138–48. doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Budde L, Song JY, Kim Y. Remissions of acute myeloid leukemia and blastic plasmacytoid dendritic cell neoplasm following treatment with CD123-specific CAR T cells: A first-in-human clinical trial. Blood. 2017;130:811–811. [Google Scholar]
- 51.Yao S, Jianlin C, Yarong L, Botao L, Qinghan W, Hongliang F, Lu Z, Hongmei N, Pin W, Hu C. Donor-derived CD123-targeted CAR T Cell serves as a RIC regimen for haploidentical transplantation in a patient with FUS-ERG+ AML. Front Oncol. 2019;9:1358. doi: 10.3389/fonc.2019.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tu S, Deng L, Huang R, Zhou X, Yang J, Zhou W, Li M, Yue C, Wu S, Guo Z. A novel chimeric antigen receptor T cells therapy strategy that dual targeting CD19 and CD123 to treat relapsed acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation. Blood. 2018;132(Supplement 1):4015–4015. doi: 10.1182/blood-2018-99-118526. [DOI] [Google Scholar]


