ABSTRACT
We recently performed two studies exploring the presence of Epstein–Barr virus (EBV) and high-risk human papillomaviruses (HPVs) types 16, 18, 31, 33 and 35 in human colorectal cancers from the Syrian population. Herein, we report that EBV and high-risk HPVs are co-present in colorectal cancers from Syria. We reveal that 17 (~17%) of 102 cancer samples are positive for both EBV and high-risk HPVs and their co-presence is associated with high/intermediate grade invasive carcinomas. These data suggest that EBV and high-risk HPVs are co-present in human colorectal cancers where they might cooperate on the initiation and/or progression of these cancers. Thus, we believe that future studies are necessary to confirm the co-presence of these oncoviruses and their cooperative role in human colorectal carcinogenesis.
KEYWORDS: EBV, high-risk HPV, colorectal cancer, Syrian population, cancer phenotype
Introduction
Colorectal cancers are the most frequent cause of cancer-related deaths worldwide with approximately 2 million new cases diagnosed annually; approximately 55% of these cancer cases occur in more developed regions (WHO).1 On the other hand, it has been indicated that environmental conditions and lifestyle in addition to sequential genetic changes and possibly viral components are major risk factors for colorectal cancers. Meanwhile, it is well known that the majority of cancer deaths are the result of metastasis, either directly due to tumor involvement of critical organs or indirectly due to therapeutic resistance and the inability of available therapy to control tumor progression.
Approximately 20% of human cancers are linked to virus infections including Epstein–Barr virus (EBV) and high-risk human papillomaviruses (HPV) especially types 16, 18 and 33, which cumulatively infect 80–90% of the population worldwide.2,3 Accordingly, we have recently explored the presence of EBV in a cohort of 102 colorectal cancer samples from a Syrian population. Our study showed that 37 (36.27%) of the 102 samples are EBV positive. Additionally, we reported that LMP1 oncoprotein expression of EBV is correlated with invasive colorectal cancer phenotype in the majority of examined cases.4 Several recent studies showed clearly that EBV is present in ~20–52% of human colorectal cancers,5-12 while some investigations could not detect EBV in human colorectal cancer samples.6,7,13 Thus, studies confirming the presence of EBV in CRCs are important, especially given the well-known biological actions of EBV-derived oncoproteins. Moreover, it is possible that EBV infection alone and/or in cooperation with other oncoviruses can play an important role in the initiation and/or progression of this malignancy. This is based on our own studies as well as other investigations about the possible role of EBV oncoproteins in the progression in several types of human carcinomas including oral, colorectal and cervical.14-16
On the other hand, the presence of high-risk HPVs in human colorectal cancer was proven by several recent meta-analysis studies, which showed that the incidence of HPVs varies between 40 to 50%.17-20 Moreover, studies report a high incidence of HPV-associated colorectal cancer especially in South American/Latin regions of Brazil and Cuba. According to studies, high-risk HPV types 16,21,22 33,22 and 5821 are the most prevalent. In the Middle East, the largest number of studies are reported from Iran, and they confirm the presence of HPV in colorectal cancer. Most of these studies report that high-risk HPV types 16,23,24 18,23,24 51,25 5625 and 5821 are the most frequently observed. More specifically, in one of our earlier studies26 on the presence of HPV in colorectal cancer samples in Syria, we observed that 46%, 38%, 8%, 46% and 19% of the samples were positive for high-risk HPV types 16, 18, 31, 33 and 35 respectively. While, it is important to highlight that there are only few studies in the Middle East covering four countries, Syria, Iran, Turkey and Israel, on the presence of HPVs in colorectal cancer;24,26-29 nevertheless, there are no studies regarding the co-presence of EBV and high-risk HPVs in colorectal cancer cases in the ME; thus, we herein explore, for the first time, the co-presence of these oncoviruses in Syrian colorectal cancer samples.
Materials and methods
Colorectal cancer samples
In this investigation, formalin-fixed, paraffin-embedded (FFPE) tumor samples were obtained from the Department of Pathology of the University of Aleppo and its hospitals. A cohort of 102 FFPE blocks from colorectal cancer patients, (53 females and 49 males) with a median age of 49 years, were used for viral detection of EBV and HPV by PCR, tissue microarrays and immunohistochemistry as mentioned below. The use of these specimens and data in research was approved by the Ethics Committee of the Faculty of Medicine of Aleppo University.4,26
EBV and HPV detection by PCR
Twenty-five nanograms of purified genomic DNA from each sample was analyzed for EBV and HPV by polymerase chain reaction (PCR) as previously described30 using specific primers for LMP1 (Forward Primer: 5ʹ-TTGGAGATTCTCTGGCGACT-3ʹ and Reverse Primer: 5ʹ-AGTCATCGTGGTGGTGTTCA-3ʹ) as well as E6/E7 of HPV types: 16, 18, 31, 33, 35, 45, 51, 52 and 58. MY09/MY11 and GP5+/GP6+ primers were also used to amplify the L1 region of the viral genome which is commonly used for HPV detection in clinical and histological studies.30,31 GAPDH primers (Forward Primer: 5ʹ-GAAGGC-CATGCCAGTGAGCT-3ʹ and Reverse Primer: 5ʹ-CCGGGAAACTGTGGCGTGAT-3ʹ) were used as an internal control. Primers and analysis were performed as previously described by our group.30,33
Briefly, LMP1 and E6/E7 genes were amplified for an initial denaturation at 95°C for 10 minutes followed by 40 cycles of 95°C for 30 s, 61°C for 1 min, and 72°C for 1 minute. In parallel, HPV gene was amplified for an initial denaturation at 95°C for 10 minutes followed by 40 cycles of 95°C for 30 s, annealing at temperatures ranging from 50 to 62°C for 1 min depending on each primer’s melting temperature as previously described,30 and 72°C for 1 min. Samples were finally incubated for 10 minutes at 72°C for a final extension. The PCR product from each exon was resolved by using 1% agarose gel electrophoresis.
Tissue microarray (TMA)
Tissue microarray construction was performed as described previously by our group.33-34 Briefly, colorectal samples were embedded into a virgin paraffin TMA block using a manual tissue arrayer (Beecher Instruments, Silver Spring, MD, USA). All FFPE samples were de-identified and assembled without any previous knowledge of linked clinical or pathological staging information. Next, slides of the completed blocks were used for immunohistochemistry (IHC) assays (against LMP1 and E6 of EBV and high-risk HPV, respectively).
Immunohistochemistry (IHC)
Immunohistochemical analysis investigating the expression of LMP1 and E6 of EBV and high-risk HPVs, respectively, were performed using procedures as previously described.30 Briefly, TMA slides were further incubated for 35 minutes at 37°C with primary monoclonal antibodies for LMP1 of EBV and E6 of HPV (clone 1–4; Dako and clone C1P5; Calbiochem, Canada) using a fully automated immunostainer (Ventana Medical System, Tuscon, AZ). The fully automated Ventana Medical System uses an indirect biotin–avidin system with a universal biotinylated immunoglobulin secondary antibody. The slides were counterstained with hematoxylin prior to mounting. The staining procedures were completed according to the manufacturer’s recommendations. Negative controls were obtained by omitting specific primary antibody for LMP1 and E6 as well as specific blocking solution. Normal colorectal mucosa served also as a negative control while HPV-positive cervical cancer and lymphatic tissue adjacent to cancer served as positive controls for HPV and EBV respectively. The tumors were considered positive for LMP1 and E6 if cancer cells exhibited any positivity ≥1% of the cells.34 The intensity of expression was graded on the scale from 0 (no expression) to 3+ (intense positivity) and was compared with the intensity in the controls (HPV+ cervical cancer and lymphatic tissues and tumor infiltrating lymphocytes).
Statistical analysis
Statistical analysis was performed using IBM Statistical Package for the Social Sciences (version 25) and R. Data were calculated as non-parametric files. Spearman Correlation Rank test was used to assess the significance of EBV and HPV association. We utilized χ2 test with Yates correction to assess the significance of the association between clinic-pathological data (patient’s age, cancer grade and tumor stage) and the co-presence of EBV and HPVs. Statistical significance was achieved at p < .05.
Results
In this study, we investigated the co-presence of EBV and high-risk HPVs in colorectal cancer samples from the Syrian population. We report that 17 (~17%) of the 102 samples from colorectal cancer cases in Syria are positive for both EBV and high-risk HPVs (Table 1); the co-presence of EBV and high-risk HPVs was confirmed for all17 samples by PCR and IHC analysis using specific primers for LMP1 and E6/E7 as well as monoclonal antibodies for LMP1 and E6 of EBV and high-risk HPVs, respectively.
Table 1.
EBV and high-risk HPVs detection in human colorectal cancer cases and their association with tumor grade
| EBV/HPVs Statusa | EBV+/HPVs+ | EBV+/HPVs− | EBV−/HPVs+ | EBV−/HPVs− |
|---|---|---|---|---|
| Number of Samplesb | 17 (17) | 20 (20) | 38 (37) | 27 (26) |
| Tumor Grade | ||||
| High | 10 (59) | 3 (15) | 8 (21) | 2 (7) |
| Intermediate | 7 (41) | 9 (45) | 20 (53) | 11 (41) |
| Low | 0 (0) | 8 (40) | 10 (26) | 14 (52) |
| High & Intermediatec | 17 (100) | 12 (60) | 28 (74) | 13 (48) |
| p-value | .0031** | .0155* | .0002** | |
| Intermediate & Lowd | 7 (41) | 17 (85) | 30 (79) | 25 (93) |
| p-value | .0056** | .0020** | .0001** |
aThese two methodologies, PCR and IHC, were used to detect the presence of EBV and high-risk HPVs.
bThe total number of samples examined in this study is 102.
() percentage
cHigh & Intermediate indicate the number of the samples of high and intermediate grade and their respective EBV/HPVs statuses and P value as follows: .0031 for EBV+/HPVs−, .0155 for EBV−/HPVs+ and .0002 for EBV−/HPVs−.
dIntermediate & Low show the number of the samples of intermediate and low grade with their respective EBV/HPVs ranks as well as P value: .0056 for EBV+/HPVs−, .0020 for EBV−/HPVs+ and .0001 for EBV−/HPVs−.
More significantly, we found that the co-expression of LMP1 and E6 oncoproteins of EBV and high-risk HPVs in all 17 positive cases is associated with high-/intermediate-grade invasive carcinoma phenotypes in comparison with 60% of EBV+/HPV-, 74% of EBV-/HPV+, or 48% of EBV-/HPV- samples, with P values of 0.0031, 0.0155 and 0.0002 for the co-expressed group of 17 samples vs. each of the latter three groups. respectively. Correspondingly, 85%, 79% and 93% of EBV+/HPV-, EBV-/HPV+ and EBV-/HPV- samples are revealed to be low-/intermediate-grade with P-values vs. the co-expressed group of 0.0056, 0.0020 and 0.0001, respectively (Table 1).
More specifically, we noted that in moderately-differentiated and high-grade (Grades 2 and 3) intestinal adenocarcinomas, E6 protein expression by IHC (Figure 1a) is predominantly diffused (90–100% of cancer cells positive) and strong (2–3+ intensity on the scale 0–3+), while there is lack of E6 positivity in adjacent cancer stroma (Figure 1b). Similarly, moderately-differentiated/high-grade carcinomas overexpress LMP1 oncoprotein in predominantly diffused nuclear pattern (>75% of the cells positive at 1–2+ intensity on the scale 0–3+) with occasional positivity in stromal cells (tumor infiltrating lymphocytes), which served as positive control (Figure 1c,d). In contrast, normal colorectal mucosa and epithelial cells, which served as controls, were negative for both EBV and high-risk HPVs.
Figure 1.
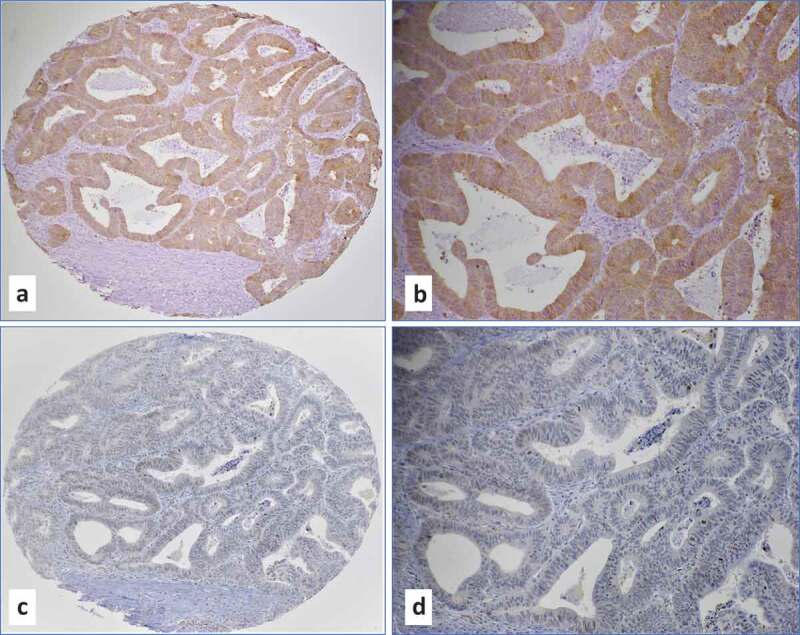
(a-d) Representative IHC images of moderately differentiated (Grade 2) intestinal adenocarcinoma with co-expression of E6 of high-risk HPVs (a-b, magnifications 10x and 20x) and LMP1 of EBV (c-d, magnifications 10x and 20x). Note a diffused (100% of cancer cells positive) and strong (3+ intensity on the scale 0-3+) E6 protein expression in cancer cells and lack of E6 positivity in adjacent cancer stroma (a and b). In case of LMP1 (c and d), protein expression was predominantly observed in cancer cells (nuclear expression in 75% of the cells at 1-2+ intensity on the scale 0-3+) with occasional positivity in tumor infiltrating lymphocytes. Co-presence of both viruses was confirmed by PCR using specific primers for LMP1, EBNA1 and E6/E7 genes.4,26
Discussion
This is, to the best of our knowledge, the first report on the co-presence of EBV and high-risk HPVs in human colorectal cancer and the relation of this co-incidence with cancer phenotypes in the Middle East area. However, based on several recent investigations including ours, it has been revealed that EBV and high-risk HPVs can be co-present in several types of human cancers.30,31 These data raise the possibility that oncoproteins of EBV and HPVs might be able to cooperate in the initiation and/or progression of human carcinomas via the crosstalk of oncoproteins and their pathways since they share β-catenin, JAK/STAT/SRC, PI3k/Akt/mTOR, and/or RAS/MEK/ERK signaling pathways.32,33
In our present study, we demonstrated that the co-presence of EBV and high-risk HPVs is associated with high/intermediate grade invasive carcinomas in all positive cases in comparison with EBV or HPVs positive samples alone in addition to EBV and HPVs negative, which are low/intermediate grade carcinomas. Accordingly, we presume that oncoproteins of EBV (LMP1 and/or EBNA1) might interact with those of high-risk HPVs (E5 and E6/E7) in human carcinoma development and consequently in their metastasis via the acceleration of the epithelial-mesenchymal transition event, as suggested earlier by our group.32 Thus, we consider that further studies are necessary to elucidate the cooperative role of EBV and high-risk HPVs in the development of human colorectal malignancy; especially since EBV and HPVs nonavalent vaccines are presently under clinical trials and available, respectively.35,36 In addition, further reports on the intricate mechanisms involved in the crosstalk between the oncoproteins expressed during the co-infection of different oncoviruses will be beneficial in providing an in-depth understanding of malignant transformation and its progression to metastasis. Such information is necessary for future drug development that might selectively target the signaling pathways and other molecular mechanisms that are modulated through co-infecting oncoviruses.
Finally, it is important to highlight that our investigation in the Syrian population, is limited to a small number of cases that are confined to a single region in Syria; therefore, it is essential to perform larger scale studies that would involve samples from different regions of the country and ultimately several studies from the Middle East in general in order to confirm our findings. This may identify as one of the limitations of this study.
In conclusion, our data pointed out for the first time the possibility of co-presence of EBV and high-risk HPVs in colorectal cancers, and that their co-presence is associated with high/intermediate grade invasive carcinoma phenotypes. This understanding has the potential to contribute to the eventual development of drugs that target signaling of viral oncoproteins, paving the way for a long-term goal of altering the course of carcinomas development; thereby preventing their progression to a metastatic form and decreasing cancer-related deaths. Ultimately, these data suggest that, for at least some populations, colorectal cancer incidence and biology could be eventually altered using public health programs that would include viral infection associated preventive measures.
Acknowledgments
We would like to thank Mrs. A. Kassab for her critical reading of the manuscript.
Funding Statement
This work was supported by grants from Qatar University: QUHI-CMED-19/20-1 and GCC # 2017-002 QU/KU.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1. doi: 10.1002/ijc.31937. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer. 2019;144:1941–53. [DOI] [PubMed] [Google Scholar]
- 2.Al Moustafa A-E. E5 and E6/E7 of high-risk HPVs cooperate to enhance cancer progression through EMT initiation. Cell Adh Migr. 2015;9(5):392–93. doi: 10.1080/19336918.2015.1042197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akram N, Imran M, Noreen M, Ahmed F, Atif M, Fatima Z, Bilal Waqar A.. Oncogenic role of Tumor viruses in humans. Viral Immunol. 2017;30(1):20–27. doi: 10.1089/vim.2016.0109. [DOI] [PubMed] [Google Scholar]
- 4.Al-Antary N, Farghaly H, Aboulkassim T, Yasmeen A, Akil N, Al Moustafa A-E. Epstein-Barr virus and its association with Fascin expression in colorectal cancers in the Syrian population: a tissue microarray study. Hum Vaccin Immunother. 2017;13(7):1573–78. doi: 10.1080/21645515.2017.1302046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song LB, Zhang X, Zhang CQ, Zhang Y, Pan -Z-Z, Liao W-T, Li M-Z, Zeng M-S. Infection of Epstein-Barr virus in colorectal cancer in Chinese. Ai Zheng. 2006;25:1356–60. [PubMed] [Google Scholar]
- 6.Mehrabani-Khasraghi S, Ameli M, Khalily F. Demonstration of herpes simplex virus, cytomegalovirus, and Epstein-Barr virus in colorectal cancer. Iran Biomed J. 2016;20(5):302–06. doi: 10.22045/ibj.2016.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salyakina D, Tsinoremas NF. Viral expression associated with gastrointestinal adenocarcinomas in TCGA high-throughput sequencing data. Hum Genomics. 2013;7(1):23. doi: 10.1186/1479-7364-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Militello V, Trevisan M, Squarzon L, Biasolo MA, Rugge M, Militello C, Palù G, Barzon L. Investigation on the presence of polyomavirus, herpesvirus, and papillomavirus sequences in colorectal neoplasms and their association with cancer. Int J Cancer. 2009;124(10):2501–03. doi: 10.1002/ijc.24224. [DOI] [PubMed] [Google Scholar]
- 9.Tafvizi F, Fard ZT, Assareh R. Epstein-Barr virus DNA in colorectal carcinoma in Iranian patients. Pol J Pathol. 2015;66(2):154–60. doi: 10.5114/pjp.2015.53012. [DOI] [PubMed] [Google Scholar]
- 10.Park JM, Choi M-G, Kim SW, Chung I-S, Yang CW, Kim YS, Jung CK, Lee KY, Kang J-H. Increased incidence of colorectal malignancies in renal transplant recipients: a case control study. Am J Transplant. 2010;10(9):2043–50. doi: 10.1111/j.1600-6143.2010.03231.x. [DOI] [PubMed] [Google Scholar]
- 11.Fiorina L, Ricotti M, Vanoli A, Luinetti O, Dallera E, Riboni R, Paolucci S, Brugnatelli S, Paulli M, Pedrazzoli P, et al. Systematic analysis of human oncogenic viruses in colon cancer revealed EBV latency in lymphoid infiltrates. Infect Agent Cancer. 2014;9:18. doi: 10.1186/1750-9378-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karpinski P, Myszka A, Ramsey D, Kielan W, Sasiadek MM. Detection of viral DNA sequences in sporadic colorectal cancers in relation to CpG island methylation and methylator phenotype. Tumour Biol. 2011;32(4):653–59. doi: 10.1007/s13277-011-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedri S, Sultan AA, Alkhalaf M, Al Moustafa AE, Vranic S. Epstein-Barr virus (EBV) status in colorectal cancer: a mini review. Hum Vaccin Immunother. 2019;15(3):603–10. doi: 10.1080/21645515.2018.1543525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Moustafa A-E, Chen D, Ghabreau L, Akil N. Association between human papillomavirus and Epstein-Barr virus infections in human oral carcinogenesis. Med Hypotheses. 2009;73(2):184–86. doi: 10.1016/j.mehy.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Guidry JT, Birdwell CE, Scott RS. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018;24(4):497–508. doi: 10.1111/odi.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vranic S, Cyprian FS, Akhtar S, Al Moustafa A-E. The role of Epstein-Barr virus in cervical cancer: a brief update. Front Oncol. 2018;8:113. doi: 10.3389/fonc.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damin DC, Ziegelmann PK, Damin AP. Human papillomavirus infection and colorectal cancer risk: a meta-analysis. Colorectal Dis. 2013;15(8):e420–e8. doi: 10.1111/codi.12257. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzon L, Ferri M, Pilozzi E, Torrisi MR, Ziparo V, French D. Human papillomavirus and colorectal cancer: evidences and pitfalls of published literature. Int J Colorectal Dis. 2011;26(2):135–42. doi: 10.1007/s00384-010-1049-8. [DOI] [PubMed] [Google Scholar]
- 19.Pelizzer T, Dias CP, Poeta J, Torriani T, Roncada C. Colorectal cancer prevalence linked to human papillomavirus: a systematic review with meta-analysis. Rev Bras Epidemiol. 2016;19(4):791–802. doi: 10.1590/1980-5497201600040009. [DOI] [PubMed] [Google Scholar]
- 20.Ibragimova MK, Tsyganov MM, Litviakov NV. Human papillomavirus and colorectal cancer. Med Oncol. 2018;35(11):140. doi: 10.1007/s12032-018-1201-9. [DOI] [PubMed] [Google Scholar]
- 21.Tanzi E, Bianchi S, Frati ER, Amicizia D, Martinelli M, Bragazzi NL, Brisigotti MP, Colzani D, Fasoli E, Zehender G, et al. Human papillomavirus detection in paraffin-embedded colorectal cancer tissues. J Gen Virol. 2015;96(Pt 1):206–09. doi: 10.1099/vir.0.070672-0. [DOI] [PubMed] [Google Scholar]
- 22.Soto Y, Limia CM, Gonzalez L, Grá B, Hano OM, Martínez PA, Kourí V. Molecular evidence of high-risk human papillomavirus infection in colorectal tumours from Cuban patients. Mem Inst Oswaldo Cruz. 2016;111(12):731–36. doi: 10.1590/0074-02760160217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motlagh A, Azadeh P, Hashemi M, Molaei M, MajidSheibani K, Alidoosti A, et al. Human papillomavirus infection, p53 overexpression and histopathologic characteristics in colorectal cancer. 2012. [Google Scholar]
- 24.Mahmoudvand S, Safaei A, Erfani N, Sarvari J. Presence of human papillomavirus DNA in colorectal cancer tissues in Shiraz, Southwest Iran. Asian Pac J Cancer Prev. 2015;16(17):7883–87. doi: 10.7314/APJCP.2015.16.17.7883. [DOI] [PubMed] [Google Scholar]
- 25.Malekpour Afshar R, Deldar Z, Mollaei HR, Arabzadeh SA, Iranpour M. Evaluation of HPV DNA positivity in colorectal cancer patients in Kerman, Southeast Iran. Asian Pac J Cancer Prev. 2018;19(1):193–98. doi: 10.22034/APJCP.2018.19.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghabreau L, Segal E, Yasmeen A, Kassab A, Akil N, Al Moustafa A-E. High-risk human papillomavirus infections in colorectal cancer in the Syrian population and their association with Fascin, Id-1 and P-cadherin expressions: a tissue microarray study. Clin Cancer Investig J. 2012;1(1):26–30. doi: 10.4103/2278-0513.95016. [DOI] [Google Scholar]
- 27.Al Moustafa A-E, Al-Awadhi R, Missaoui N, Adam I, Durusoy R, Ghabreau L, Akil N, Ahmed HG, Yasmeen A, Alsbeih G, et al. Human papillomaviruses-related cancers. Hum Vaccin Immunother. 2014;10(7):1812–21. doi: 10.4161/hv.28742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meshkat M, Tayyebi Meibodi N, Sepahi S, Fadaee N, Salehpour M, Meshkat Z. The frequency of human papillomaviruses in colorectal cancer samples in Mashhad, northeastern Iran. Turk J Med Sci. 2014;44(3):501–03. doi: 10.3906/sag-1303-81. [DOI] [PubMed] [Google Scholar]
- 29.Karbalaie Niya MH, Keyvani H, Safarnezhad Tameshkel F, Salehi-Vaziri M, Teaghinezhad-S S, Bokharaei Salim F, Monavari SHR, Javanmard D. Human papillomavirus type 16 integration analysis by real-time PCR assay in associated Cancers. Transl Oncol. 2018;11(3):593–98. doi: 10.1016/j.tranon.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Thawadi H, Ghabreau L, Aboulkassim T, Yasmeen A, Vranic S, Batist G, Al Moustafa A-E. Co-incidence of Epstein-Barr virus and high-risk human papillomaviruses in cervical cancer of Syrian women. Front Oncol. 2018;8:250. doi: 10.3389/fonc.2018.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guidry JT, Scott RS. The interaction between human papillomavirus and other viruses. Virus Res. 2017;231:139–47. doi: 10.1016/j.virusres.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cyprian FS, Al-Farsi HF, Vranic S, Akhtar S, Al Moustafa A-E. Epstein-Barr Virus and human papillomaviruses interactions and their roles in the initiation of epithelial-mesenchymal transition and cancer progression. Front Oncol. 2018;8:111. doi: 10.3389/fonc.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al Moustafa A-E, Al-Antary N, Aboulkassim T, Akil N, Batist G, Yasmeen A. Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum Vaccin Immunother. 2016;12(7):1936–39. doi: 10.1080/21645515.2016.1139255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aboulkassim T, Yasmeen A, Akil N, Batist G, Al Moustafa A-E. Incidence of Epstein-Barr virus in Syrian women with breast cancer: a tissue microarray study. Hum Vaccin Immunother. 2015;11(4):951–55. doi: 10.1080/21645515.2015.1009342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capra G, Giovannelli L, Matranga D, Bellavia C, Guarneri MF, Fasciana T, Scaduto G, Firenze A, Vassiliadis A, Perino A, et al. Potential impact of a nonavalent HPV vaccine on HPV related low-and high-grade cervical intraepithelial lesions: a referral hospital-based study in Sicily. Hum Vaccin Immunother. 2017;13(8):1839–43. doi: 10.1080/21645515.2017.1319026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riethmuller D, Jacquard A-C, Lacau St Guily J, Aubin F, Carcopino X, Pradat P, Dahlab A, Prétet J-L. Potential impact of a nonavalent HPV vaccine on the occurrence of HPV-related diseases in France. BMC Public Health. 2015;15:453. doi: 10.1186/s12889-015-1779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


