Abstract
Objective:
To determine whether a modifiable risk factor, endotracheal tube size, is associated with the diagnosis of post-extubation aspiration in survivors of acute respiratory failure (ARF).
Design:
Prospective cohort study
Setting:
Intensive care units (ICUs) at four academic tertiary care medical centers.
Patients:
Two hundred ten patients who were at least 18 years old, admitted to an intensive care unit (ICU), and mechanically ventilated with an endotracheal tube for longer than 48 hours were enrolled.
Interventions:
Within 72 hours of extubation, all patients received a flexible endoscopic evaluation of swallowing (FEES) exam that entailed administration of ice, thin liquid, thick liquid, puree, and cracker boluses. Patient demographics, treatment variables, and hospital outcomes were abstracted from the patient’s medical records. Endotracheal tube size was independently selected by the patient’s treating physicians.
Measurements and Main Results:
For each FEES exam, laryngeal pathology was evaluated, and for each bolus, a Penetration Aspiration Scale (PAS) score was assigned. Aspiration (PAS score ≥6) was further categorized into non-silent aspiration (PAS=6 or 7) and silent aspiration (PAS=8). One third (n=68) of patients aspirated (PAS≥6) on at least one bolus, 13.6% (n=29) exhibited silent aspiration, and 23.8% (n=50) exhibited non-silent aspiration. In a multivariable analysis, endotrachael tube (ETT) size (≤7.5 vs. ≥8.0) was significantly associated with patients exhibiting any aspiration (PAS≥6) (p=0.016, OR=2.17, 95% CI=1.14-4.13) and with risk of developing laryngeal granulation tissue (p=0.02).
Conclusions:
Larger ETT size was associated with increased risk of aspiration and laryngeal granulation tissue. Using smaller ETTs may reduce the risk of post-extubation aspiration.
Keywords: Deglutition Disorders; Respiratory Distress Syndrome, Adult; Intratracheal Intubation; Respiratory Aspiration; Airway Extubation
INTRODUCTION
Each year, approximately 790,000 patients in the United States develop acute respiratory failure (ARF) that requires intubation and mechanical ventilation. With a hospital mortality rate of approximately 35%, over 500,000 ARF patients will be extubated and discharged to home or to a rehabilitation facility.[1-4]
ARF survivors suffer from a range of long-term complications including neuromuscular, psychiatric, cognitive, and pulmonary disorders that are associated with worse functional status, decreased quality of life, and increased caregiver burden.[1, 5-10] Recently, there has been an increased focus on post-extubation dysphagia in these patients.[11-14] Identifying and managing post-extubation dysphagia is critical to ensure patient safety, proper medication administration, and adequate nutrition and hydration.[15] One of the more serious complications of post-extubation dysphagia is aspiration, which is associated with an increased risk of reintubation, pneumonia, prolonged hospital length of stay, and mortality.[13, 16-19]
Several epidemiological studies have identified patient age [20-22], duration of intubation[16, 18-20, 23, 24], and lower preadmission functional status[17, 25] as risk factors for post-extubation aspiration. However, most of these risk factors are not modifiable. Endotracheal tube (ETT) size is a potentially modifiable factor that has been associated with an increased risk of post-extubation laryngeal injury.[26] Shinn and colleagues reported that patients with 7.5 or larger ETTs experienced greater rates of laryngeal injury and significantly worse breathing and voicing 10 weeks post-extubation as compared to patients with 7.0 or smaller ETTs, but did not evaluate impact of ETT size on aspiration or dysphagia rates. In a previous pilot study, the authors also identified ETT size as a potential risk factor for aspiration,[27] but its effects on post-extubation aspiration remains relatively unexplored.[28, 29]
In this current multi-center prospective cohort study, we hypothesized that endotracheal tube size may be a modifiable risk factor associated with the development of post-extubation aspiration.
METHODS
Patient population:
As part of a multicenter National Institutes of Health (NIH) funded, prospective, multi-site cohort study, patients were enrolled from four university medical centers. Patients were eligible if they were at least 18 years old, admitted to an intensive care unit (ICU), had a diagnosis of acute respiratory failure, and were mechanically ventilated with an endotracheal tube for longer than 48 hours. A requirement of at least 48 hours of intubation was used because while aspiration can occur in extubated patients with shorter durations of mechanical ventilation, pathogenic changes in oral bacterial flora occur after 48 hours.[30, 31] Patients who aspirate after 48 hours of mechanical ventilation are therefore at greater risk of developing health-care associated pneumonia. Exclusion criteria included contraindications to enteral nutrition, pre-existing or acute primary, central, or peripheral neuromuscular disorders, pre-existing history of dysphagia or head and neck cancer or surgery, presence of a tracheostomy, coagulopathy resulting in uncontrolled nasal or pharyngeal bleeding, altered mentation, or extubation for greater than 72 hours. Enrollment within 72 hours of extubation represented a clinically realistic timeframe when considering initial dysphagia screening, medical team order placement for SLP services, and variable SLP staff availability on weekends. All patients (or their surrogates) were approached and consented for study participation if they met the inclusion/exclusion criteria. As such, the patient cohort was not limited just to patients with moderate-severe dysphagia who would preferentially receive orders for instrumental swallow studies. If an eligible patient (based on inclusion/exclusion criteria) had not been referred to the speech and swallow service, the speech and swallow service would request and receive a referral from the medical teams who fully supported this study. This study was approved by the Institutional Review Board at each of the four centers.
After enrollment, demographic and treatment related information was recorded including age, race, gender, APACHE II score, Charlson Comorbidity Index, Body Mass Index (BMI), length of hospital stay, duration of intubation, primary reason for admission, and endotracheal tube size. The size of the endotracheal tube was independently determined prior to study enrollment by the treating physician at the time of intubation and in the context of routine clinical care.
Study procedures:
Flexible endoscopic evaluations of swallowing (FEES) examinations were performed by study clinicians. Optional intranasal lidocaine (0.2ml 4%) spray, which has been shown to increase patient comfort but not to impact patient swallowing[32], could be administered before laryngoscope insertion. The study clinician then administered five standard consistencies. In order to enhance detection of aspiration, all bolus consistencies were dyed green and additional white food coloring was added to thin liquid bolus trials. Boluses were administered in the following order: ½ teaspoon and full teaspoon of ice chips; 5 ml, 15 ml and 2 oz nectar-thick liquid (Thick & Easy®, Hormel HealthLabs, Austin, MN); 5 ml and 10 ml puree (applesauce); 5 ml, 15 ml, and 2 oz thin liquid; ¼ piece of graham cracker; and 3 oz water swallow test. If aspiration was visualized on a small bolus (i.e. 5 ml), then the larger bolus within that consistency was skipped and the next consistency was presented. The 3 oz water swallow test was not performed if aspiration was visualized on any prior thin liquid bolus. Any of the bolus presentations were stopped if the clinician perceived significant risk to the patient. Following the bolus trials, the clinician assessed laryngeal sensation by advancing the endoscope tip to briefly contact each arytenoid. If the laryngeal adductor reflex was not readily apparent on one side, a second attempt was made.
All FEES examinations were recorded with sound. One of the study investigators (SEL) assigned a Penetration Aspiration Scale (PAS)[33] score to each of the per-protocol boluses. The PAS is scored in the following manner: 1=Material does not enter airway, 2=Material enters the airway, remains above the vocal folds, and is ejected from the airway, 3=Material enters the airway, remains above the vocal folds, and is not ejected from the airway, 4=Material enters the airway, contacts the vocal folds, and is ejected from the airway, 5=Material enters the airway, contacts the vocal folds, and is not ejected from the airway, 6=Material enters the airway, passes below the vocal folds, and is ejected into the larynx or out of the airway, 7=Material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort, and 8=Material enters the airway, passes below the vocal folds, and no effort is made to eject. Patients were classified as having aspirated if their PAS score was ≥6 on any bolus on any consistency. Patients who aspirated were then sub-classified into silent aspiration (if their PAS = 8) and non-silent aspiration (if their PAS = 6 or 7) on any consistency. Patients who experienced silent and non-silent aspiration on different boluses were classified into both groups. This study investigator (SEL) also confirmed the presence or absence of a laryngeal adductor reflex (LAR) during laryngeal sensation testing. Another investigator, who is board certified in otolaryngology (DF), independently reviewed and scored all of the FEES examinations for the presence of granulation tissue (none vs. abnormal), edema (none vs. abnormal), and vocal cord immobility (absent vs. present, and if present unilateral or bilateral). Neither of the experts were involved in patient enrollment nor in performing the clinical exams, and they were not given any demographic or clinical information about the patients. This allowed the raters to be blinded, and prevented any conscious or subconscious bias from influencing their ratings.
Statistical Analyses:
The sample size was based on a parallel study where 200 patients would yield an area under the curve of 0.75 for a decision tree algorithm. To be conservative, and to ensure adequate power to perform a logistic regression that would test the relationship between patient, treatment, and outcome variables, we enrolled 248 patients. A logistic regression with a sample size between 178 and 271 observations achieves 80% power at a 0.05 significance level to detect an odds ratio between 2.5 and 3.0.
Paired dichotomous data were analyzed using McNemar’s test. Fisher’s exact test was used for nominal dependent variables. Ordinal dependent variables were analyzed using the Wilcoxon Mann-Whitney exact test. When endotracheal tube size groups had less than 10 patients, we combined them with the closest endotracheal tube size group that had more than 10 patients. We conducted individual univariate analyses testing the association between patient / treatment variables and aspiration. Only variables that were associated (p < 0.1) with aspiration were entered into a multiple logistic regression analysis with endotracheal tube size as the primary independent variable and were tested for interaction. There were no significant interactions in our analyses. Odds ratios (ORs) and 95% CIs were determined for each independent variable in all multivariate logistic regression analyses. An alpha value of .05 was used for all statistical tests. Data was analyzed using JMP® Pro (Version 12.0.1, SAS Institute Inc., 2015).
RESULTS
Patient Demographics:
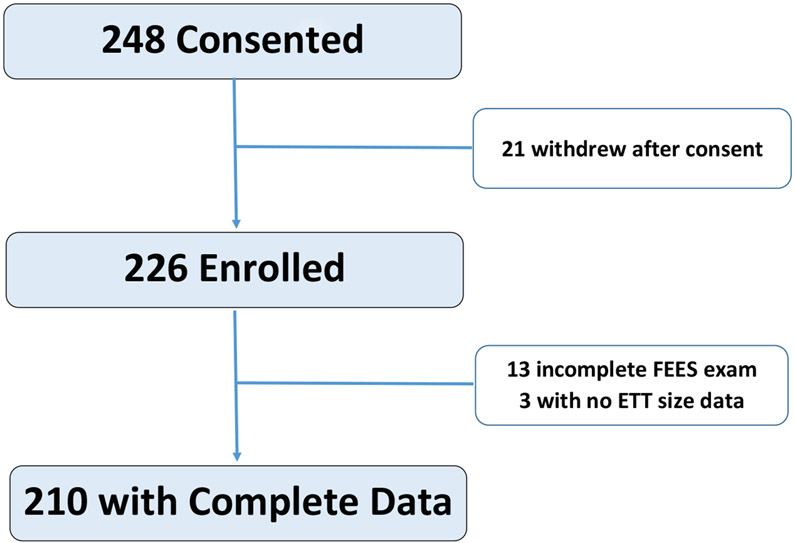
From August 2015 until July 2018, a total of 248 patients were consented. Twenty-one subjects withdrew from the study before the FEES exam was performed and an additional 13 patients did receive a FEES exam or had incomplete FEES examinations. Reasons for withdrawal, no FEES exam, or incomplete FEES exam are detailed in Supplemental Digital Content – Table 1. Three patients who did not have ETT size documented in their medical record were also excluded. A total of 210 patients were included in the final analysis (Figure 1).
Figure 1:
Consort Diagram
Patient demographics are included in Table 1. The median time from extubation until FEES examination was 26 hours [interquartile range: 22-47 hours]. Overall, 49% (n = 102) of patients had been intubated with an 8.0mm or greater diameter endotracheal tube, and 51% (n = 108) of patients with a 7.5mm or smaller diameter endotracheal tube.
Table 1:
Patient Demographics (N=210)
| Category | Distribution |
|---|---|
| Site | BUMC = 95 patients UCH = 75 patients Stanford* = 20 patients Yale* = 20 patients |
| Patient Age: median [IQR] | 57 [47-66] years |
| Gender (% male) | N=129 (61%) |
| Race | Caucasian = 115 (55%) African American = 54 (25%) Other = 41 (20%) |
| Primary service: N (%) | Medical ICU: 146 (70%) Cardiac ICU: 43 (20%) Surgical ICU: 20 (9%) Burn ICU: 1 (1%) |
| Co-morbidity | C0PD = 56 (27%) Diabetes = 52 (25%) Congestive heart failure = 45 (21%) Myocardial infarction = 35 (17%) Liver disease = 21 (10%) Peripheral vascular disease = 19 (9%) Cerebral vascular disease = 17 (8%) Dementia = 7 (3%) Rheumatological disorder = 5 (2%) Peptic ulcer disease = 5 (2%) |
| Time from admission to intubation: median hours [IQR] | 8 [0-48] hours |
| Length of intubation: median hours [IQR] | 133 [73-206] hours |
| Endotracheal tube size: N (%) | 8.5mm: 2 (1%) 8.0mm: 100 (48%) 7.5mm: 74 (35%) 7.0mm: 28 (13%) 6.5mm: 4 (2%) 6.0mm: 2 (1%) |
| Time from extubation to FEES exam: median hours [IQR] | 26 [22-47] hours |
| More than one intubation prior to enrollment: N (%) | 38 (18.1%) |
| Charlson Comorbidity Index (CCI): Median score [IQR] | 3 [2-5] |
| APACHE II score [IQR] | 19 [14-26] |
| Body Mass Index: median [IQR] | 27.8 [23.7-34.3] |
BUMC = Boston University Medical Campus; UCH = University of Colorado Health; IQR = interquartile range; ICU = Intensive Care Unit; FEES = Fiberoptic Endoscopic Evaluation of Swallowing;
Stanford and Yale were added in the final year of the study to boost total enrollment numbers so their enrollment numbers do not represent insufficient screening or enrollment efforts.
Association between patient/treatment variables and aspiration:
A total of 32.4% of patients (n = 68) had documented aspiration (PAS ≥ 6) on at least one bolus. The majority of aspiration events occurred with thin liquid boluses (n = 53). Overall, 13.8% of patients (n = 29) exhibited silent aspiration, and 23.8% (n=50) exhibited non-silent aspiration events (Table 2).
Table 2:
Aspiration events by bolus type where N represents the number of patients who aspirated on each bolus consistency and % is in proportion to the entire sample (N=210).
| Bolus Consistency | N(%) Patients who Aspirated | Silent Aspiration | Non-Silent Aspiration |
|---|---|---|---|
| Ice | N=17 (8%) | N=7 | N=10 |
| Puree | N=6 (3%) | N=4 | N=2 |
| Nectar | N=16 (8%) | N=6 | N=10 |
| Thin Liquid | N=53* (25%) | N=19* | N=37* |
| Ice | N=3 (1%) | N=0 | N=3 |
Since multiple boluses of a given consistency could have been given, a patient may have experienced both a silent and non-silent aspiration event on separate boluses. This phenomenon is thought to be due to the fact that dysphagic patients are more likely to sense larger boluses, which is the underlying rationale behind the water swallow screening exams.
Endotracheal tube size was significantly associated with overall aspiration (7.0 or less = 21% (7/34), 7.5 = 26% (19/74), and 8.0 or greater = 41% (42/102), p = 0.02. A graphical representation of the relationship between ETT size and frequency of aspiration can be found in Supplemental Digital Content – Figure 1. To determine how to best define ETT size as the independent variable, an initial multivariable analysis with ETT stratified into three groups (≤7.0, 7.5, and ≥ 8.0) was performed. Using ETT = 7.5 as the reference, there was no statistical difference between the <7.0 and 7.5 groups (p = 0.27), but there was a statistical difference between 7.5 and ≥ 8.0 groups (p = 0.02). A subgroup analysis of only the 174 patients with either a 7.5 ETT (n=74) or an 8.0 ETT (n=100) was performed. In a univariate analysis, overall aspiration occurred in 25.7% (19/74) of patients with a 7.5 ETT and 40.0% (40/100) of patients with an 8.0 ETT (p = 0.047).
In a backward elimination logistic multivariable regression analysis adjusting for patient age, duration of intubation, and Charlson Comorbidity Index scores, the effect of the endotracheal tube size was nearly associated with overall aspiration with a p value of 0.062. Accordingly, it was decided that the best way to analyze the data was to dichotomize ETT size by ≤7.5mm vs ≥8.0mm.
When endotracheal tube size was dichotomized into 7.5mm or smaller versus 8.0mm or greater, larger endotracheal tube size was significantly associated with overall aspiration (41% (42/102) vs. 24% (26/108), p = 0.008). Other variables found to be associated with aspiration included older patient age, male sex, longer duration of intubation, and higher Charlson Comorbidity Index scores (See Table 3). Importantly, Body Mass Index was not associated with aspiration (p = 0.66) or endotracheal tube size (p = 0.54)
Table 3:
Univariate analysis of risk factor association with aspiration.
| Demographic | Aspiration | No Aspiration | P value |
|---|---|---|---|
| Endotracheal tube size | 8.0 or greater: 41% 7.5 or less: 24% |
8.0 or greater: 59% 7.5: 76% |
0.008* |
| Age (in years) [IQR] | 61 [53-72] | 55 [44-65] | 0.0008 |
| Gender (female/male) | F = 24% M = 38% |
F = 76% M = 62% |
0.03 |
| Intubation length (hours) [IQR] | 176 [72-234] | 117 [72-184] | 0.02 |
| Charlson Comorbidity Index | 5.0 ± 3.2 | 3.3 ± 2.6 | < 0.001 |
| APACHE II scores | 21.3 ± 9.4 | 19.6 ± 8.2 | 0.18 |
| Body Mass Index (BMI) | 30.3 ± 11.3 | 31.1 ± 12.2 | 0.66 |
IQR = Interquartile range
The p-value for ETT size indicates that the proportion of people who aspirate is significantly greater if they have an 8.0mm or larger ETT as compared to the proportion of people who aspirate if they have a 7.5mm or smaller ETT.
In a multivariate analysis, the effect of endotracheal tube size (7.5 or less compared to 8.0 or greater) was independently associated with overall aspiration when adjusting for patient age, duration of intubation, and Charlson Comorbidity Index scores (p = 0.016, OR = 2.17 95% CI = 1.14-4.13). Two separate multivariable analyses were performed in which only silent aspiration or non-silent aspiration were included as the outcome variable. In these analyses, endotracheal tube size was not associated solely with non-silent aspiration or with silent aspiration; non-silent aspiration (p = 0.57, OR = 1.27, 95% CI 0.55- 3.05), and silent aspiration (p = 0.66, OR = 1.22, 95% CI = 0.44-3.53).
Association of endotracheal tube size and laryngeal pathology
Larger endotracheal tube size was associated with an increased frequency of severe granulation tissue, but was not associated with increased frequency of laryngeal edema, unilateral or bilateral vocal cord immobility, or with reduced laryngeal sensation. (See table 4)
Table 4:
Association between identified laryngeal trauma and ETT Size.
| Laryngeal Trauma | Smaller ETT size (≤ 7.5) (n=108) |
Large ETT size (≥ 8.0) (n=102) |
P-Value |
|---|---|---|---|
| Granulation Tissue Present | 14% (15/108) | 26% (27/102) | 0.02 |
| Airway Edema Present | 51% (55/108) | 60% (61/102) | 0.20 |
| Vocal Cord Immobility Present | 20% (22/108) | 24% (24/102) | 0.58 |
| Laryngeal sensation decreased | 43% (40/94) | 45% (38/84) | 0.71 |
DISCUSSION
In this study, 210 recently extubated ARF patients who did not have pre-existing dysphagia, who were not treated for an acute medical problem that could have caused a dysphagia, and who were cognitively intact to comply with a comprehensive FEES examination, were enrolled. Larger ETT size (≥8mm) was significantly associated with a higher incidence of any aspiration and laryngeal injury (presence of granulation tissue).
The FEES exam was chosen as the instrumental gold standard because it can be performed at bedside and has been shown to have superior sensitivity in identifying aspiration.[34] As compared to a bedside swallow exam assessment, direct visualization of aspiration also makes the FEES exam optimally relevant from a clinical standpoint because it minimizes the risk of false negative aspiration events. Some patients who do not aspirate on a FEES exam may still aspirate in a less controlled clinical setting, but the frequency or severity of such clinical aspiration would certainly be less than that experienced by someone who does aspirate on a FEES exam. As such, the FEES exam identifies with greatest existent precision which patients are at greatest risk of “clinically significant” aspiration that can result in deleterious clinical outcomes such as increased length of stay, mortality, and aspiration pneumonia.[18] Patients who aspirate on the FEES examination are therefore typically prescribed dietary modifications to decrease the risk of aspiration and any associated potential clinical consequences. However, it remains unknown how many patients, or what types of patients, who experience some degree of aspiration experience adverse clinical outcomes. An important future study would be to follow patients with some degree of aspiration on their FEES exam to determine the clinical significance of these findings, and to model which patients are at greatest risk of adverse clinical outcomes.
There is conflicting evidence in the literature regarding the correlation between non-modifiable patient or clinical variables and post-extubation dysphagia and aspiration.[13, 35] While length of intubation and endotracheal tube size have been associated with laryngeal trauma[26, 28, 29], the impact of ETT size on post-extubation aspiration remained relatively unexplored. To date, only one study has correlated ETT size with aspiration outcome in the ARF patient population. It was a study that enrolled 45 patients to assess the accuracy of the bedside swallowing evaluation among ARF survivors, and a statistically significant but unadjusted association between ETT size and aspiration was discovered.[27] This current larger study corroborated these findings; that larger endotracheal tube size (≥8.0) was significantly associated with overall post-extubation aspiration, even after controlling for a number of patient and treatment related variables. Given that aspiration in the critical care population has been associated with an increased risk of reintubation, pneumonia, prolonged hospital length of stay, and mortality, choosing a 7.5mm or smaller ET tube size (when possible) may be a modifiable factor that could at least partially attenuate these complications.[13, 16-19]
It is important, however, to balance the advantages that a smaller ETT size may have on laryngeal pathology, post-extubation aspiration, and the medical complications of aspiration, with the advantages that a larger ETT size may have on airway management of the critical care patient. Advantages of a larger ETT size include easier spontaneous breathing, easier aspiration of broncho-alveolar secretions, performing bronchoscopy without over occlusion of the ETT lumen, and prevention against the reduction in ETT luminal diameter caused by long-term buildup of biofilm. These are important considerations for patients who may need pulmonary toilets and who will be intubated for extended periods of time. Patients who may not need extensive use of bronchoscopy or relatively long term vent dependence may not benefit from larger ETT sizes which could place patients at greater risk of aspiration associated medical complications after extubation. Balancing the costs and benefits of smaller and larger ETTs will require better established algorithms to optimize both immediate clinical care and long-term patient outcomes.[36] A clinical trial designed to establish such algorithms is warranted.
Another interesting consideration regarding optimal ETT size may be the ratio between the diameter of the glottic space and external ETT diameter. Gender and height are often used to approximate proper ETT size in adults, but a person’s laryngeal anatomy and glottic space may vary considerably within gender and height. Trauma to the larynx be as much a function of laryngeal anatomy as absolute ETT size. A future study that assesses the relationship between laryngeal anatomy, ETT size, and laryngeal pathology/aspiration outcomes would be informative.
ETT size was not significantly associated with either of the defined subgroups of silent or non-silent aspiration, but it was significantly associated with aspiration when analyzed collectively (silent and non-silent). In other words, patients with larger ET tubes experienced greater rates of both silent and non-silent aspiration as compared to patients with smaller ET tubes. This lack of significance between ETT size and aspiration sub-group could be a true lack of relationship or could be due to a Type II error secondary to inadequate subgroup sample size. However, a proportional increase in silent aspiration suggests that ETT size may have some negative influence on laryngeal sensation. Since there was no association between ETT size and laryngeal sensation measured by laryngeal adductor reflex testing (table 4), reduced sensation to the supraglottic larynx may not be the cause. Instead, perhaps larger ETT size compromises subglottic sensation that is associated with the recurrent laryngeal nerve. If the recurrent laryngeal nerve was fully functioning, then a bolus passing below the vocal cords should elicit a cough[37-39], which would have resulted in an increase in non-silent aspiration but not silent aspiration. This theory is partially supported by Borders et. al. who showed no association between Laryngeal Adductor Reflex (LAR) testing (which tests supraglottic sensory response) and rate of silent aspiration.[40] Since non-silent aspiration also increased proportionally with larger ETT size, there are likely additional salient factors associated with this increase in aspiration risk. A recent study by Brodsky et. al. demonstrated slowed pharyngeal and laryngeal swallow timing in a cohort of recently extubated acute respiratory distress syndrome patients as compared to healthy controls.[41] Accordingly, the risk of aspiration in the ARF patient population may be a function of several factors including reduced sensation (perhaps subglottic), altered swallow timing, and general muscle weakness.
A study by Francois et. al[29] found a significant relationship between larger ETT size and presence of laryngeal edema. This current study did not corroborate this relationship but did find an association between larger ETT size and presence of granulation tissue which was recently also identified in a study by Shinn and colleagues.[26] How or if such laryngeal pathology could impact aspiration rates is still unknown. While laryngeal edema could in some cases cause aspiration secondary to anatomic changes that alter swallowing biomechanics, it is unclear if or how granulation tissue could. One possibility is that larger ETTs cause a combination of posterior glottic and subglottic subclinical hypoesthesia and granulation tissue that collectively increase the risk of reduced cricoarytenoid joint mobility and/or silent aspiration. Alternatively, granulation tissue may cause odynophagia which may promote a generally weak swallow, a contention that may be supported by the aforementioned study by Brodsky and colleagues.[41]
While reduced sensation and laryngeal trauma caused by the ETT can only be prevented by minimizing ETT sizes and potentially ETT cuff pressures as appropriate for an individual patient’s care, the risk of aspiration secondary to delayed or weak swallowing may be best attenuated by ensuring that ARF patients receive a FEES exam by a speech language pathologist. The SLP can then directly visualize and assess the reason for the impaired swallow, and can offer compensatory strategies such as diet modification or patient swallow maneuvers to reduce the risk of aspiration. They may also be able to work with the patient to stimulate a brisker swallow or to strengthen the swallow using FEES as a biofeedback tool.
Of the 210 patients that were enrolled in this study, 38 patients (18.1%) had multiple intubations prior to enrollment, of which 13 patients were re-intubated electively for an operative procedure and 25 patients were re-intubated urgently. If patients had different ETT sizes at different intubation events, they were classified by the size of the most recent ETT. While it makes sense that re-intubation has an effect on laryngeal trauma, the only reported outcome associated with re-intubation is increased hospital discharge times.[16] A 2018 systematic review on laryngeal injury and intubation by Brodsky et. al. did not report on any associations between multiple intubations and laryngeal injury nor aspiration.[28] The impact of multiple intubations on aspiration remains unexplored. This is likely because repeated intubation is confounded by the fact that re-intubation is correlated with a sicker patient population, longer intubation times, and hence also increased risk of trauma to the upper airway and post-extubation dysphagia and aspiration.[42] The associations between reintubation, laryngeal trauma, and post-extubation dysphagia need to be better studied. Unfortunately, the sample size of this current study was not adequate to determine the independent effect of multiple intubations on outcome.
This study has a few strengths and limitations. Strengths of this study include the fact that it was a prospective multi-center study with a relatively large and representative cohort that minimized bias and enhances the generalizability of the results. The study entailed comprehensive instrumental swallow evaluations that were reviewed by blinded experts. The use of FEES is a strength in this patient population since not all ICU patients are appropriate for transport to modified barium swallow exams in radiology, and can be performed at bedside in a natural position for the patient which reduces selection bias. FEES has also been shown to be more sensitive than MBS in a recent systematic review comparing the two exams.[34]
This study also has some limitations. The authors were not able to collect reasons for choosing ETT size which may have been associated with planned procedures such as bronchoscopy. This study also did not collect a few clinical variables, such as presence of nasogastric tubes, temperature probes, esophageal dopplers, or ultrasound cardiac probes that may have influenced the rate or severity of dysphagia in certain groups of patients. Another limitation is that the sample size was not large enough to determine whether timing of the post-extubation FEES exam could have influenced aspiration rates among certain patient subgroups. A study by Marvin et. al. demonstrated a reduction in aspiration rates in a heterogeneous group of 49 patients from 2 hours to 24 hours post-extubation,[43] but a larger clinical trial is needed to determine how timing impacts swallowing in the ARF patient population and in an increased window of time that extends to 72 hours post-extubation. While the institutions in this study have ETT cuff pressure monitoring protocols that prescribe upper and lower pressure limits, ETT cuff pressures were not monitored in this study and could be a confounding factor as previously identified by Nseir and colleagues.[44] Finally, only one blinded reviewer assessed PAS scores and presence of LAR, and only one blinded reviewer assessed laryngeal pathology. While this may be perceived as a limitation, the investigator who rated the endsocopically assessed PAS and LAR swallow outcomes developed the FEES exam and is the world’s foremost expert on FEES evaluations. Laryngeal pathology was simply rated as present or absent, so the expertise of a single board certified otolaryngologist seemed adequate to report on this dichotomous secondary outcome.
CONCLUSION
This study suggests that using a smaller ETT size may reduce post-extubation aspiration. However, a much larger and adequately powered multi-center clinical trial is needed to authoritatively establish causal links between modifiable and non-modifiable risk factors associated with aspiration and its associated morbidity and mortality in the ARF patient population.
Supplementary Material
Acknowledgments
Source of Funding
This study was funded by the following NIH grant: R21NR015886 (PI=Moss).
Footnotes
Conflicts of Interest
The authors declare that they have no conflict of interest.
Copyright form disclosure: Drs. Krisciunas, Landmore, Levitt, McKeehan, McNally, Scheel, Rubio, Vojnik, Warner, and Moss’s institutions received funding from National Institutes of Health (NIH). Drs. Krisciunas, Landmore, Gomez-Taborda, Levitt, McKeehan, McNally, Scheel, Rubio, Vojnik, Warner, and Moss received support for article research from the NIH. Dr. Siner’s institution received funding from the University of Colorado. The remaining authors have disclosed that they do not have any potential conflicts of interest.
ClinicalTrials.gov Identifier: NCT02363686
REFERENCES
- 1.Wunsch H, Linde-Zwirble WT, Angus DC, et al. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38(10):1947–1953. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Akca S, De Mendonca A, et al. The epidemiology of acute respiratory failure in critically ill patients(*). Chest. 2002;121(5):1602–1609. [DOI] [PubMed] [Google Scholar]
- 3.Behrendt CE. Acute respiratory failure in the United States: incidence and 31-day survival. Chest. 2000;118(4):1100–1105. [DOI] [PubMed] [Google Scholar]
- 4.Lewandowski K Contributions to the epidemiology of acute respiratory failure. Crit Care. 2003;7(4):288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adhikari NKJ, McAndrews MP, Tansey CM, et al. Self-reported symptoms of depression and memory dysfunction in survivors of ARDS. Chest. 2009;135(3):678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–2867. [DOI] [PubMed] [Google Scholar]
- 7.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39(2):371–379. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths RD, Jones C. Recovery from intensive care. BMJ. 1999;319(7207):427–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herridge MS, Tansey CM, Matté A, et al. Functional Disability 5 Years after Acute Respiratory Distress Syndrome. N Engl J Med. 2011;364(14):1293–1304. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(4):340–347. [DOI] [PubMed] [Google Scholar]
- 11.Heffner JE. Swallowing Complications After Endotracheal Extubation: Moving From Whether to How. Chest. 2010;137(3):509–510. [DOI] [PubMed] [Google Scholar]
- 12.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40(2):502–509. [DOI] [PubMed] [Google Scholar]
- 13.Skoretz SA, Flowers HL, Martino R. The incidence of dysphagia following endotracheal intubation: a systematic review. Chest. 2010;137(3):665–673. [DOI] [PubMed] [Google Scholar]
- 14.Macht M, Wimbish T, Bodine C, et al. ICU-acquired swallowing disorders. Crit Care Med. 2013;41(10):2396–2405. [DOI] [PubMed] [Google Scholar]
- 15.Brodsky MB, Mayfield EB, Gross RD. Clinical Decision Making in the ICU: Dysphagia Screening, Assessment, and Treatment. Semin Speech Lang. 2019;40(3):170–187. [DOI] [PubMed] [Google Scholar]
- 16.Barker J, Martino R, Reichardt B, et al. Incidence and impact of dysphagia in patients receiving prolonged endotracheal intubation after cardiac surgery. Can J Surg. 2009;52(2):119–124. [PMC free article] [PubMed] [Google Scholar]
- 17.El Solh A, Okada M, Bhat A, et al. Swallowing disorders post orotracheal intubation in the elderly. Intensive Care Med. 2003;29(9):1451–1455. [DOI] [PubMed] [Google Scholar]
- 18.Macht M, Wimbish T, Clark BJ, et al. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit Care. 2011;15(5):R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malandraki GA, Markaki V, Georgopoulos VC, et al. Postextubation Dysphagia in Critical Patients: A First Report From the Largest Step-Down Intensive Care Unit in Greece. Am J Speech Lang Pathol. 2016;25(2):150–156. [DOI] [PubMed] [Google Scholar]
- 20.Hogue CW, Lappas GD, Creswell LL, et al. Swallowing dysfunction after cardiac operations: Associated adverse outcomes and risk factors including intraoperative transesophageal echocardiography. J Thorac Cardiovasc Surg. 1995;110(2):517–522. [DOI] [PubMed] [Google Scholar]
- 21.Ferraris VA, Ferraris SP, Moritz DM, et al. Oropharyngeal dysphagia after cardiac operations. Ann Thorac Surg. 2001;71(6):1792–1796. [DOI] [PubMed] [Google Scholar]
- 22.Tsai MH, Ku SC, Wang TG, et al. Swallowing dysfunction following endotracheal intubation: Age matters. Medicine. 2016;95(24):e3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rousou JA, Tighe DA, Garb JL, et al. Risk of dysphagia after transesophageal echocardiography during cardiac operations. Ann Thorac Surg. 2000;69(2):486–489. [DOI] [PubMed] [Google Scholar]
- 24.Brodsky MB, Gellar JE, Dinglas VD, et al. Duration of oral endotracheal intubation is associated with dysphagia symptoms in acute lung injury patients. J Crit Care. 2014;29(4):574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leder SB, Cohn SM, Moller BA. Fiberoptic endoscopic documentation of the high incidence of aspiration following extubation in critically ill trauma patients. Dysphagia. 1998;13(4):208–212. [DOI] [PubMed] [Google Scholar]
- 26.Shinn JR, Kimura KS, Campbell BR, et al. Incidence and Outcomes of Acute Laryngeal Injury After Prolonged Mechanical Ventilation. Crit Care Med. 2019;47(12):1699–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch YT, Clark BJ, Macht M, et al. The accuracy of the bedside swallowing evaluation for detecting aspiration in survivors of acute respiratory failure. J Crit Care. 2017;39:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brodsky MB, Levy MJ, Jedlanek E, et al. Laryngeal Injury and Upper Airway Symptoms After Oral Endotracheal Intubation With Mechanical Ventilation During Critical Care: A Systematic Review. Crit Care Med. 2018;46(12):2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francois B, Bellissant E, Gissot V, et al. 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: a randomised double-blind trial. Lancet. 2007;369(9567):1083–1089. [DOI] [PubMed] [Google Scholar]
- 30.Bryant LR, Trinkle JK, Mobin-Uddin K, et al. Bacterial Colonization Profile With Tracheal Intubation and Mechanical Ventilation. Arch Surg. 1972:104(5):647–651. [DOI] [PubMed] [Google Scholar]
- 31.Johanson WG, Pierce AK, Sanford JP. Changing Pharyngeal Bacterial Flora of Hospitalized Patients. N Engl J Med. 1969;281(21):1137–1140. [DOI] [PubMed] [Google Scholar]
- 32.O'Dea MB, Langmore SE, Krisciunas GP, et al. Effect of Lidocaine on Swallowing During FEES in Patients With Dysphagia. Ann Otol Rhinol Laryngol. 2015;124(7):537–544. [DOI] [PubMed] [Google Scholar]
- 33.Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–98. [DOI] [PubMed] [Google Scholar]
- 34.Giraldo-Cadavid LF, Leal-Leano LR, Leon-Basantes GA, et al. Accuracy of endoscopic and videofluoroscopic evaluations of swallowing for oropharyngeal dysphagia. Laryngoscope. 2017;127(9):2002–2010. [DOI] [PubMed] [Google Scholar]
- 35.Zuercher P, Moret CS, Dziewas R, et al. Dysphagia in the intensive care unit: epidemiology, mechanisms, and clinical management. Crit Care. 2019;23(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrow S, Farrow C, Soni N. Size matters: choosing the right tracheal tube. Anaesthesia. 2012;67(8):815–819. [DOI] [PubMed] [Google Scholar]
- 37.Blitzer A Approaches to the patient with aspiration and swallowing disabilities. Dysphagia. 1990;5(3):129–137. [DOI] [PubMed] [Google Scholar]
- 38.Dua K, Surapaneni SN, Kuribayashi S, et al. Pharyngeal airway protective reflexes are triggered before the maximum volume of fluid that the hypopharynx can safely hold is exceeded. Am J Physiol Gastrointest Liver Physiol. 2011;301(2):G197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dua KS, Ren J, Bardan E, et al. Coordination of deglutitive glottal function and pharyngeal bolus transit during normal eating. Gastroenterology. 1997;112(1):73–83. [DOI] [PubMed] [Google Scholar]
- 40.Borders JC, Fink D, Levitt JE, et al. Relationship Between Laryngeal Sensation, Length of Intubation, and Aspiration in Patients with Acute Respiratory Failure. Dysphagia. 2019;34(4):521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brodsky MB, De I, Chilukuri K, et al. Coordination of Pharyngeal and Laryngeal Swallowing Events During Single Liquid Swallows After Oral Endotracheal Intubation for Patients with Acute Respiratory Distress Syndrome. Dysphagia. 2018;33(6):768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marquez-Lara A, Nandyala SV, Fineberg SJ, et al. Incidence, outcomes, and mortality of reintubation after anterior cervical fusion. Spine (Phila Pa 1976). 2014;39(2):134–139. [DOI] [PubMed] [Google Scholar]
- 43.Marvin S, Thibeault S, Ehlenbach WJ. Post-extubation Dysphagia: Does Timing of Evaluation Matter? Dysphagia. 2019;34(2):210–219. [DOI] [PubMed] [Google Scholar]
- 44.Nseir S, Brisson H, Marquette CH, et al. Variations in endotracheal cuff pressure in intubated critically ill patients: prevalence and risk factors. Eur J Anaesthesiol. 2009;26(3):229–234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.