Abstract
Coronavirus disease 2019 (COVID-19) causes a wide range of symptoms, including several unexpected symptoms such as loss of taste, skin changes, and eye problems. We recently observed patients with documented COVID-19 develop de novo severe genitourinary symptoms, most notably urinary frequency of ≥ 13 episodes/24 h and nocturia ≥ 4 episodes/night. We call these associated urinary symptoms COVID-19 associate cystitis (CAC). COVID-19 severity is associated with inflammation. We collected urine samples from COVID-19 patients, including patients with CAC, and found elevation of proinflammatory cytokines also in the urine. It has been previously shown that patients with urinary incontinence and ulcerative interstitial cystitis/bladder pain syndrome have elevated urinary inflammatory cytokines compared to normal controls. We therefore hypothesize that CAC, with presentation of de novo severe urinary symptoms, can occur in COVID-19 and is caused by increased inflammatory cytokines that are released into the urine and/or expressed in the bladder.
The most important implications of our hypothesis are: 1) Physician caring for COVID-19 patients should be aware of COVID-19 associate cystitis (CAC); 2) De novo urinary symptoms should be included in the symptom complex associated with COVID-19; and 3) COVID-19 inflammation may result in bladder dysfunction.
Keywords: COVID-19, Clinical symptoms, Bladder, Inflammation, Nocturia, Cytokine, SARS-CoV-2
Introduction/Background
Coronavirus disease 2019 (COVID-19), caused by infection with Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) virus, can cause a wide range of symptoms with the most common being fever, dry cough, and tiredness. Other symptoms may include headache, sore throat, shortness of breath or difficulty breathing, muscle aches, or chills. However, COVID-19 can also result in unexpected symptoms including loss of taste or small, itchy lesions on hands and feet, swollen eyelids and increase eye discharge, and gastrointestinal symptoms, including a loss of appetite, nausea, vomiting and diarrhea. Recently, Mumm and colleagues reported increased urinary frequency in COVID-19 patients [1]. We also observed confirmed COVID-19 patients develop de novo severe genitourinary symptoms, most notably urinary frequency of > 13 episodes/24 h and nocturia > 4 episodes/night [2]. Some patients also noted pain or discomfort associated with urination. We have called these associated urinary symptoms COVID-19 associate cystitis (CAC). Since COVID-19 severity is associated with inflammation and increased inflammatory cytokines, we then examined expression of inflammatory cytokines in the urine of these COVID-19 patients. We observed that the urine of confirmed COVID-19 patients had elevated levels of pro-inflammatory cytokines.
The hypothesis/theory
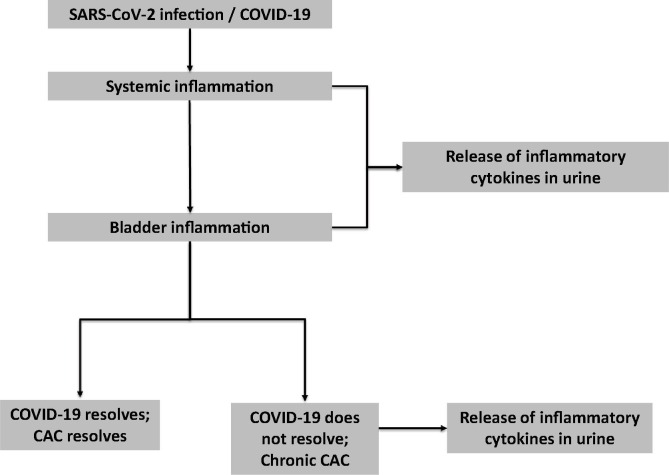
We hypothesize that CAC, with presentation of de novo severe urinary symptoms, can occur in COVID-19 patients and is caused by increased inflammatory cytokines that are released into the urine and/or expressed in the bladder (Fig. 1 ). We further hypothesize that chronic COVID-19 Associated Cystitis may occur in COVID-19 patients who do not fully recover and have a chronic inflammatory condition ongoing.
Fig. 1.
COVID-19 Associated Cystitis (CAC) Hypothesis.
Evaluation of the hypothesis/idea
Our hypothesis is supported by several lines of evidence. First, Mumm and colleagues as well as our group have observed alterations in bladder function in COVID-19 patients [1]. Mumm et al first observed increased urinary frequency in 7 males out of 57 patients admitted to their COVID-19 wards [1]. All patients tested positive for SARS-CoV-2 in nasopharyngeal swabs and developed pulmonary symptoms detectable on imaging. These patients did not have urinary infections, acute renal injury, nor prostatitis and were negative for urine cultures. These patients reported an average of 13.7 urinary voids per day on the day of admission and 11.6 on day 5; in comparison most people urinate between six and eight times a day. In our observations, all the COVID-19 positive patients (7 females and 32 males; all African American) developed de novo urinary symptoms without urinary tract infection by urine culture [2].
Second, other viral infections are known to result in lower urinary tract symptoms (LUTS), which can include storage symptoms (urgency, urgency incontinence, frequency, and nocturia), voiding symptoms (slow or interrupted stream, hesitancy, or straining to urinate), or post-micturition symptoms (dribble or sensation of incomplete emptying of bladder). For example, Human immunodeficiency virus (HIV) status is an independent risk factor for bothersome LUTS and the odds of severe LUTS are greater in HIV infected men with a history of AIDS [3]. Human T cell lymphotropic virus‐1 (HTLV‐1) infection can also result in bothersome LUTS including nocturia, urgency, frequency, and dysuria [4]. BK polyomavirus (BKPyV) after allogeneic hematopoietic cell transplantation can cause macroscopic hematuria and cystitis, including urinary frequency, dysuria, gross hematuria, and urinary blood clots [5]. Lastly, UTIs can be caused by viruses including adenovirus and cytomegalovirus (CMV), although viral UTIs are relatively uncommon.
There have been reports that inflammatory cytokines are elevated in the urine in urinary incontinent and interstitial cystitis/bladder pain syndrome patients compared to normal controls [6], [7], [8], [9], [10], [11]. For example, in overactive bladder (OAB), urinary cytokines elevated in OAB patients compared to controls included monocyte chemotactic protein-1 (MCP-1), soluble fraction of the CD40 ligand (sCD40L), macrophage inflammatory protein (MIP-1β), IL-12p70/p40, IL-5, epidermal growth factor (EGF), growth-related oncogene GRO-α, sIL-2Rα, and IL-10 [8]. The bladder tissue of interstitial cystitis patients compared to controls have increased IL-16, IL-18, CTACK, ICAM-1, MCP-3, SCGFβ, TRAIL, and VCAM-1 [7]. Urinary cytokines are also elevated in interstitial cystitis with Hunner’s lesions, specifically IL-6, IL-8, and GRO [6].
Cystitis, or bladder inflammation, can cause bladder dysfunction [12], [13]. Inflammation can occur from pathogenic (bacterial or viral), non-pathogenic (i.e. radiation, medication, chemicals, etc.), or idiopathic (i.e. interstitial cystitis) causes. Pro-inflammation mediators, including cytokines, can damage and irritate the bladder mucosa, the bladder tissue that is in contact with urine [12], [13]. The bladder mucosa is a dynamic sensory structure that can sense bladder fullness and can modulate bladder nerve and muscle function. Inflammation damage to the mucosa can thus result in bothersome lower urinary tract symptoms as the damaged urothelium may activate afferent nerves as well as locally influence bladder muscle function [12], [13]. Infection with SARS-CoV-2 in some can result in systemic inflammation and abnormally high immunologic response is associated with more severe COVID-19 symptoms and outcomes, including death [14], [15], [16]. This has been referred to as the cytokine storm syndrome or cytokine release syndrome. Increased expression of cytokines, especially IL-6, IL-1β, C-reactive protein, and TNF-α, are detectable in serum of COVID-19 patients with IL-6 being the best single prognostic indicator of COVID-19 severity [17].
In our observations detailed below, we have observed that several cytokines are elevated and detectable in the urine of COVID-19 patients with CAC. The presence of these cytokines in the urine could impact bladder sensation and thus function, resulting in the observed increased urinary frequency and nocturia. It is also possible that the virus may directly damage the bladder tissue, impacting bladder function. Lastly, impaired kidney function may cause some of the symptoms observed with CAC.
If our hypothesis is correct, we predict that drugs that are useful in helping treat overactive bladder may also be considered in CAC. Given that rest and sleep are important during illness for recovery, improving nocturia and frequency could help improve the speed and quality of recovery of COVID-19 patients. Furthermore, monitoring urinary cytokines may serve as a prognostic indicator for COVID-19 progression and severity when serum collection is not feasible or practical.
Empirical data
Clean catch urine samples were collected from 8 adult patients with normal renal function, 4 with COVID-19 and de novo urinary tract symptoms and 4 age matched normal control without COVID-19 and no history of urinary tract diseases. COVID-19 was diagnosed by SARS-CoV-2 molecular testing and presence of COVID-19 symptoms. IRB approval and written participant consent obtained and urine cultures were negative. Urine samples were analyzed in duplicate for cytokine expression level using a Luminex multiplex assay. The cytokines analyzed included interleukin-6 (IL-6), interleukin-8 (IL-8) and IP-10. The 4 COVID-19 positive patients with CAC were 3 female and 1 male African American, mean age of 68 years (range 51–85). The control were 2 female and 2 male African American, mean age of 66 years (range 50–78). The 4 COVID-19 positive CAC patients had de novo urological symptoms of urinary urgency (4/4), urge incontinence (4/4) and urinary frequency/24 hrs of 11–12 episodes (n = 1), 13–14 episodes (n = 1) and 15 + episodes/24 hrs (n = 2). All COVID-19 patients report de novo nocturia of 3, (n = 1), 4 (n = 2) and 5 episodes/night (n = 1). All COVID-19 positive patients had elevated levels of inflammatory cytokines IL-6, IL-8, and IP-10 in their urine compared to COVID-19 negative patients, with IL-6 and IL-8 being statistically significant (Table 1 ). There was variance between individuals that may reflect the severity and duration of their disease, and/or the presence of comorbidities. This data supports that COVID-19 patients with CAC have increased inflammatory cytokines in the urine.
Table 1.
Cytokine expression in urine of asymptomatic controls (n = 4) and COVID-19 patients with de novo urinary symptoms (n = 4). SEM = Standard error mean. P-value calculated from a one-tailed, two-sample T test with unequal variances.
| Patient | COVID-19 | SEX | AGE | IL-6 (pg/mL) | IL-8 (pg/mL) | IP-10 (pg/mL) |
|---|---|---|---|---|---|---|
| N1 | Negative | F | 50 | 7.02 | 48.1 | 0 |
| N2 | Negative | M | 71 | 0 | 1.01 | 0 |
| N3 | Negative | M | 78 | 1.51 | 1.79 | 154.89 |
| N4 | Negative | F | 65 | 0 | 2.33 | 0 |
| AVERAGE ± SEM | 2.13 ± 1.67 | 13.31 ± 11.60 | 38.72 ± 38.72 | |||
| P1 | Positive | F | 57 | 5.42 | 329.31 | 2307.64 |
| P2 | Positive | F | 80 | 39.36 | 260.2 | 68.96 |
| P3 | Positive | M | 85 | 12.81 | 287.9 | 527.79 |
| P4 | Positive | F | 51 | 21.18 | 155.06 | 548.11 |
| AVERAGE ± SEM | 19.69 ± 7.30 | 258.12 ± 37.17 | 863.13 ± 494.05 | |||
| p-value | 0.046 | 0.002 | 0.097 | |||
Consequences of the hypothesis and discussion
We propose that COVID-19 Associate Cystitis (CAC), with presentation of de novo severe urinary symptoms, can occur in COVID-19 and is caused by increased inflammatory cytokines that are released into the urine and/or expressed in the bladder. The most important implications of our hypothesis are: 1) Physicians and other clinicians caring for COVID-19 patients should be aware of CAC; 2) De novo urinary symptoms should be included in the symptoms complex associated with COVID-19; and 3) COVID-19 inflammation may result in bladder dysfunction. We further hypothesize that chronic COVID-19 Associated Cystitis may occur in COVID-19 patients who do not fully recover and have a chronic inflammatory condition ongoing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Dr. Bernadette Zwaans, Sarah Bartolone, and Elijah Ward for their critical review of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110375.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mumm JN, Osterman A, Ruzicka M, Stihl C, Vilsmaier T, Munker D, et al. Urinary Frequency as a Possibly Overlooked Symptom in COVID-19 Patients: Does SARS-CoV-2 Cause Viral Cystitis? European urology. 2020 May 19. PubMed PMID: 32475747. Pubmed Central PMCID: 7236674. [DOI] [PMC free article] [PubMed]
- 2.Dhar N., Dhar S., Timar R., Lucas S., Lamb L.E., Chancellor M.B. De Novo Urinary Symptoms Associated with COVID-19: COVID-19 Associated Cystitis (CAC) J Clin Med Res. 2020 doi: 10.14740/jocmr4294. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breyer B.N., Van den Eeden S.K., Horberg M.A., Eisenberg M.L., Deng D.Y., Smith J.F. HIV status is an independent risk factor for reporting lower urinary tract symptoms. J Urol. 2011;185(5):1710–1715. doi: 10.1016/j.juro.2010.12.043. PubMed PMID: 21420120. Pubmed Central PMCID: PMC3565615. Epub 2011/03/2eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro N.M., Rodrigues W., Jr., Freitas D.M., Muniz A., Oliveira P., Carvalho E.M. Urinary symptoms associated with human T-cell lymphotropic virus type I infection: evidence of urinary manifestations in large group of HTLV-I carriers. Urology. 2007;69(5):813–818. doi: 10.1016/j.urology.2007.01.052. PubMed PMID: 17482910. Epub 2007/05/08. eng. [DOI] [PubMed] [Google Scholar]
- 5.Imlay H., Xie H., Leisenring W.M., Duke E.R., Kimball L.E., Huang M.-L. Presentation of BK polyomavirus–associated hemorrhagic cystitis after allogeneic hematopoietic cell transplantation. Blood Adv. 2020;4(4):617–628. doi: 10.1182/bloodadvances.2019000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb L.E., Janicki J.J., Bartolone S.N., Peters K.M., Chancellor M.B. Development of an interstitial cystitis risk score for bladder permeability. PLoS ONE. 2017;12(10):e0185686. doi: 10.1371/journal.pone.0185686. PubMed PMID: 29088231. Pubmed Central PMCID: PMC5663335 methods for diagnosing interstitial cystitis. All other authors have declared no conflicts of interest exist. This does not alter our adherence to PLOS ONE policies on sharing data and materials. Epub 2017/11/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corcoran A.T., Yoshimura N., Tyagi V., Jacobs B., Leng W., Tyagi P. Mapping the cytokine profile of painful bladder syndrome/interstitial cystitis in human bladder and urine specimens. World J Urol. 2013;31(1):241–246. doi: 10.1007/s00345-012-0852-y. PubMed PMID: 22441309. Pubmed Central PMCID: PMC367457Epub 2012/03/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyagi P., Barclay D., Zamora R., Yoshimura N., Peters K., Vodovotz Y. Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol. 2010;42(3):629–635. doi: 10.1007/s11255-009-9647-5. PubMed PMID: 19784793. Epub 2009/09/29. eng. [DOI] [PubMed] [Google Scholar]
- 9.Liu H.T., Jiang Y.H., Kuo H.C. Alteration of Urothelial Inflammation, Apoptosis, and Junction Protein in Patients with Various Bladder Conditions and Storage Bladder Symptoms Suggest Common Pathway Involved in Underlying Pathophysiology. Lower Urinary Tract Symptoms. 2015;7(2):102–107. doi: 10.1111/luts.12062. PubMed PMID: 26663690. Epub 2015/12/15. eng. [DOI] [PubMed] [Google Scholar]
- 10.Kuo H.C. Potential urine and serum biomarkers for patients with bladder pain syndrome/interstitial cystitis. Int J Urol. 2014;21(Suppl 1):34–41. doi: 10.1111/iju.12311. PubMed PMID: 24807491. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C.O., Li Z.L., Kong C.Z. APF, HB-EGF, and EGF biomarkers in patients with ulcerative vs. non-ulcerative interstitial cystitis. BMC Urol. 2005;29(5):7. doi: 10.1186/1471-2490-5-7. PubMed PMID: 15862132. Pubmed Central PMCID: PMC1131910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grover S., Srivastava A., Lee R., Tewari A.K., Te A.E. Role of inflammation in bladder function and interstitial cystitis. Therapeutic Adv Urol. 2011;3(1):19–33. doi: 10.1177/1756287211398255. PubMed PMID: 21789096. Pubmed Central PMCID: 3126088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fry CH, Vahabi B. The Role of the Mucosa in Normal and Abnormal Bladder Function. Basic & clinical pharmacology & toxicology. 2016 Oct;119 Suppl 3:57-62. PubMed PMID: 27228303. Pubmed Central PMCID: 5555362. [DOI] [PMC free article] [PubMed]
- 14.Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, et al. Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. JAMA. 2020 Jun 8. PubMed PMID: 32511676. Pubmed Central PMCID: 7281352. [DOI] [PMC free article] [PubMed]
- 15.Levy HR. COVID-19 and cytokine storm syndrome2020 07/24/2020]. Available from: https://www.mlo-online.com/continuing-education/article/21138224/covid19-and-cytokine-storm-syndrome.
- 16.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clinical immunology. 2020;214 doi: 10.1016/j.clim.2020.108393. PubMed PMID: 32222466. Pubmed Central PMCID: 7102614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu T, Zhang J, Yang Y, Ma H, Li Z, Zhang J, et al. The potential role of IL-6 in monitoring severe case of coronavirus disease 2019. medRxiv. 2020:2020.03.01.20029769.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.