Abstract
Lack of preformed long-chain polyunsaturated fatty acids (LCPUFA) in infant formula has been hypothesised as contributing to cognitive differences between breast-fed and formula-fed infants. Previous systematic reviews found no cognitive differences between infants fed formula with LCPUFA and those fed formula without, but focused on early developmental measures, such as Bayley Scales of Infant Development, which are poorly differentiating and not predictive of cognitive ability in childhood. This systematic review examined the effect of randomising infants to formula supplemented with LCUFA vs unsupplemented formula on cognitive function ≥ age 2.5 years. We searched Medline, Embase the Cochrane Central Register of Controlled Trials without date limit, following a pre-published protocol according to PRISMA guidelines. We conducted random effects meta-analyses in RevMan v5.4 and followed GRADE and Cochrane Guidelines to evaluate strength of evidence and potential for bias. We included 8 trial cohorts which randomised participants between 1993 and 2004 and analyse 6 previously unpublished outcomes provided by various trialists. Age at the last available cognitive test ranged from 3.3 to 16 years. The pooled mean difference in Wechsler Preschool and Primary Scale of Intelligence-Revised from four trials in term-born children showed no benefit of LCPUFA: -0.04 points (95% confidence interval -5.94 to 5.85, 95% prediction interval -14.17 to 14.25). The pooled mean difference in Wechsler Abbreviated Scale of Intelligence score from two trials in preterm-born children also showed no benefit of LCPUFA: -7.71 (95% CI -24.63 to 9.22, 95% PI -97.80 to 82.38). Overall quality of evidence was low, due to substantial heterogeneity, low rates of follow-up, and indications of selective publication. The long-term effect of LCPUFA supplementation in term and preterm-born infants on cognition is highly uncertain and includes potential for large benefit as well as large harm. Based on our findings, LCPUFA supplementation of infant formula is not recommended until further robust evidence excludes long-term harm.
Study registration
PROSPERO registration numbers CRD42018105196 and CRD42018088868.
Introduction
Long-chain polyunsaturated fatty acids (LCPUFA), such as docosahexaenoic acid (DHA) and arachidonic acid (AA), are important structural components of the human brain that mainly accumulate during the third trimester of pregnancy and early infancy [1–3]. Human breast milk contains DHA, AA, and their fatty acid precursors [4] but, historically, infant formula contained only the precursors alpha linoleic acid and linoleic acid, which infants, especially those born preterm, may not be able to effectively synthesise into DHA and AA [5].
Research suggests that breast-fed children have higher cognitive ability compared to formula-fed children [6–9]. Lack of preformed LCPUFA in infant formula has been hypothesised as contributing to these cognitive differences. Yet so far there is no clear evidence from published randomised controlled trials (RCTs) that LCPUFA-supplemented infant formula improves cognition compared with unsupplemented formula milk [5, 10]. Previous systematic reviews of RCTs may have failed to detect a difference in cognition because they mainly focused on early measures of cognition, such as Bayley Scales of Infant Development. Early measures of cognition are, however, not adequate to differentiate between cognitive skills potentially affected by nutrient supplementation and are poorly predictive of cognition during school age [11–13]. Follow-up later in childhood, using more reliable measures of cognitive function such as Intelligence Quotient (IQ) scores, might be more likely to detect an existing effect of LCPUFA-supplementation.
Clear evidence on the long-term effects of LCPUFA-supplementation is needed as the EU Commission (EC) recently mandated the addition of one type of LCPUFA, DHA, to all infant and follow-on formulae [14]. While the decision acknowledged the lack of evidence on cognitive benefits and was instead based around theoretical arguments, supplementation comes at a cost: a family can spend up to $400 extra per year on LCPUFA-supplemented- compared to unsupplemented infant formulas and mandatory supplementation may result in price rises across the market [15].
To our knowledge, no systematic review has previously focused on later childhood -when more accurate measures are available [11–13]- to study the cognitive effects of infant formula supplemented with LCPUFA. The present study combines published and previously unpublished trial data, acquired through contacting trial authors, to compare the long-term cognitive effects of LCPUFA-supplemented versus unsupplemented infant formula in children born at term and preterm.
Methods
Search strategy
This systematic review and meta-analysis follows two published protocols (one for terms and one for preterms) [16, 17], based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18]. We searched Medline, Embase, proceedings from major scientific meetings of child nutrition (S2 File) and the Cochrane Central Register of Controlled Trials in October 2019, without date or language restrictions. We reviewed the reference lists of the included studies and traced subsequent publications. We first identified RCT participant cohorts based on any infant formula supplementation with LCPUFA, independent of whether cognitive outcomes were reported. We then contacted a total of 18 trialists, ethics committees or industry representatives to identify potential unpublished data, clarify study details and to ask whether they knew of any other eligible trials that had measured cognitive outcomes ≥2.5 years of age (S4 and S5 Tables in S1 File). The cut-off of ≥2.5 years was based on the age where early development of the prefrontal cortex, the brain region associated with higher cognitive functions is completed [19]. Abstract review was done in duplicate by MV and SD; consensus was achieved by discussion. All extracted data is available within S1 Table in S1 File.
Inclusion and exclusion criteria
We based our selection on trial cohorts rather than publications. We included trial cohorts where infants were given either infant formula supplemented with LCPUFA (DHA alone or DHA together with AA, at any dose) compared with unsupplemented formula. Trial cohorts were eligible if commencement of the intervention began within 2 weeks of birth and they measured cognitive function age ≥ 2.5 years using validated measures including Wechsler and Stanford-Binet IQ scores. We excluded trial cohorts for which we could not find any cognitive outcomes ≥2.5 years of age (irrespective of whether this outcome was published).
Outcomes and data analysis
The primary outcome was the pooled difference of cognitive ability between supplemented and unsupplemented groups. We decided to use the cognitive test reported most frequently among the included studies rather than a combination of different tests, to increase the interpretability of the primary outcome and decrease heterogeneity. We aimed to use the mean difference (MD) when the cognitive measure was already standardised (e.g. IQ score) so as to increase interpretability, otherwise we would use the standardised mean difference (SMD). We performed separate analyses for term and preterm-born participants because healthy term infants are able to synthesise LCPUFA from fatty acid pre-cursors, whereas preterm babies are born with fewer LCPUFA reserves accumulated in utero and are less able to synthesise LCPUFA than term-born babies. We therefore hypothesised that term/preterm status could modify the effect of supplemented LCPUFA.
The secondary outcome was the pooled SMD of all available cognitive test scores. To not include the same participants multiple times, we only included one score per trial cohort. For this, we used the score at the oldest age available under the assumption that scores at a later age are a more accurate reflection of cognitive ability than scores at an earlier age.
All analyses were performed in RevMan v5.4 and included participants with the relevant outcome in the groups to which they were randomised. We defined statistically significant differences based on a p-value <0.05 and report all summary measures along with 95% confidence intervals and a measure of heterogeneity (I2). We also calculated the prediction interval (PI) to accurately reflect any uncertainty about clinical harms and benefits [20].
Strength of evidence and risk of bias assessment
We assessed the strength of evidence and risk of bias for each study using GRADE (S3 Table in S1 File) and the Cochrane Risk of Bias Tool II, which was also used as the template for data extraction (S2 Table in S1 File). Post-hoc, we explored potential for publication bias by plotting the SMDs for all available scores against their standard error (SE), to visualise the relative distribution of published and unpublished outcomes (S1 Fig in S1 File).
Results
We included eight unique trial cohorts [21–49] of which six were performed in infants born at term and two in infants born preterm (Fig 1). We obtained previously unpublished outcome data for two RCTs: Firstly, a two-centre trial of term babies in England [30, 31] provided unpublished follow-up data on IQ assessments using the Wechsler Preschool and Primary Scales of Intelligence (WPPSI) at age 4.5 years and the Wechsler Abbreviated Scales of Intelligence (WASI) at age 16 years. Secondly, a two-centre trial in England of babies born preterm provided unpublished data from their IQ assessments using the WASI at age 16 years [50]. We also received partly published outcome data from three trials, in a form that allowed them to be included in a pooled meta-analysis: IQ using the WASI at 9 years from a Dutch trial of term babies [35–38]; IQ using the WASI at age 16 years from the Kansas centre of the US based DIAMOND trial conducted in term infants [39, 41–46, 51]; and IQ assessments adjusted for maternal education at 10 years from a two-centre study in Scottish preterm children [48, 49].
Fig 1. Study selection process.
Table 1 shows the characteristics of all included trial cohorts. The total number of children randomised was not reported for one RCT [24–29]. Study randomisation was performed between 1992 and 2004 with the latest cognitive assessments conducted at mean ages 3.3–16 years. All studies randomised participants to infant formula supplemented with LCPUFA containing DHA and AA or to unsupplemented infant formula. DHA was sourced from egg, fish, fungi, algae or starflower oil and made up between 0.12 and 0.96% of total fat content. Ratios of DHA:AA ranged from 1:0.8 to 1:3.6. Duration of the intervention ranged from two to 12 months in term infants and three weeks to 9 months in infants born preterm. All trials, except for one, included a non-randomised breast-fed reference group but these were not analysed in this review. Randomised children in one preterm trial [48, 49] could receive some breastmilk during the first months, but the intake was balanced across groups.
Table 1. Characteristics of included RCT cohorts and associated publications.
| RCT Cohort (recruitment years) and [publication references] | Criteria | LCPUFA, % | Any breastmilk during study | Breastfed reference group | Start LCPUFA (age in days) | Duration LCPUFA (months) | Outcomes (age in years) | Unpublished outcome data? | %Follow-up: n followed-up1,2/ n randomised2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Term studies | wga | ||||||||||
| US: 3 centres (92–93) [21–23] | >36 | Egg DHA 0.12 + Egg AA 0.43 | No | Yes | <7 d | 12 | PPVT (3.3) SB IQ (3.3) | 79% | 72/91 | ||
| Europe: 6 centres* (92–93) [24–29] | 37–42 | Egg DHA 0.30§+ Egg AA 0.44§ | No | Yes | <3 d | 4 | WPPSI-R (6) | n/a | 147/ n/a** | ||
| ENG: 2 centres (93–95) [30, 31] | >36 | Egg DHA 0.32 + Egg AA 0.30 | No | Yes | <7 d | 6 | WPPSI-R (4.5) WASI (16) | Yes | 60% | 184/309 | |
| US: Dallas (93–95) [32–34] | 37–40 | Algae DHA 0.36 + Fungi AA 0.72 | No | Yes | <5 d | 3.9 | WPPSI-R (4) | 68% | 36/53 | ||
| NL: Groningen (97–99) [35–38] | 37–42 | Egg & Fish DHA 0.30 + Fungi AA 0.45 | No | Yes | <5 d | 2 | WASI (9) | partly‡ | 68% | 214/314 | |
| US: DIAMOND [39–46] | Dallas (02–04) | 37–42 | Algae DHA 0.32 + Fungi AA 0.64 | No | No | <10 d | 4 | PPVT (3.5), BBCS-R (2.5) | 46% | 42/92 | |
| Kansas (02–04) | No | WPPSI-R (6) | partly‡ | 38% | 30/80 | ||||||
| Preterm studies | bw, wga | ||||||||||
| ENG: 2 centres (93–96) [47] | <1750g, <37 | Algae DHA 0.32 + Fungi AA 0.64 | No | Yes | <11 d | 0.69 | WASI (16) | yes | 9% | 17/196 | |
| SCT: Glasgow (95–97) [48, 49] | ≤2000g, <35 | Egg DHA 0.17 + Egg AA 0.31 | Yes | No | 2–60 d | 9 | WASI (10) | partly‡ | 45% | 107/238 | |
bw: birthweight, wga: Weeks gestational age, PPVT: Peabody Picture Vocabulary Test, SB IQ Stanford-Binet IQ, BBCS-R: Bracken Basic Concept Scale-Revised, WASI: Wechsler Abbreviated Scale of Intelligence, WPPSI-R: Wechsler Preschool and Primary Scale of Intelligence-Revised;
*Two locations unknown, 1: When latest cognitive outcome was measured 2: Only dose/source of interest, some studies had more than one randomised dose/source group (only one per trial is included here);
§Two different concentrations were published: DHA = 0.30 [29] or 0.21 [54]–AA = 0.44 [29] or 0.35 [54])
‡data was published in graphical form or differently modelled; n/a: Not available;
** the 4 centres that were followed up randomised 237 infants between them but it is unknown how many were randomised in the remaining two centres and why they weren’t followed-up. It follows that the follow-up rate was < 62%.
Three RCTs had more than one randomised intervention group [21, 22, 32–34, 39–46, 52]. To ensure comparability with a previous Cochrane review [53], we included only the intervention group in our analysis that was most similar in DHA dose and source to the other included RCTs. One study [39–46] randomised babies in two centres and then conducted different cognitive assessments at different ages for children followed-up stratified by centre. We regarded these as independent and included both reported cognitive assessments in our analysis.
The most frequently reported measures of cognitive function were the WPPSI IQ at ages 4–6 years (in four term RCTs) and the WASI IQ at ages 9–16 years (two term and two preterm RCTs). Among term born participants, assessments at the oldest age comprised WASI at ages 9 and 16 years (2 RCTs), Stanford-Binet IQ at age 3.3 years (1 RCT), the Peabody Picture Vocabulary Test at age 3.5 years (1 RCT) and WPPSI 4–6 years (3 RCTs; see Table 1). Other reported measures in term were the Bracken Basic Concept Scale-Revised at age 2.5 years (one term RCT). Among preterm-born participants, the most frequently reported assessments were also those conducted at the oldest age.
Primary outcome
We pooled the data for infants born at term using random-effects because heterogeneity was judged to be high with an I2 of 72% (p = 0.01), despite homogeneity in terms of the assessment used. Fig 2 shows that, among term-born babies, the pooled MD from four trials suggests no difference at the 5% level in the WPPSI score between LCPUFA supplemented and control groups: MD -0.04 IQ points. Uncertainty around the effect estimate was extremely high: 95% CI -5.94 to 5.85 and 95% PI from -14.17 to 14.25.
Fig 2. Primary outcome term infants: WPPSI-R IQ at ages 4–6 years (mean difference).
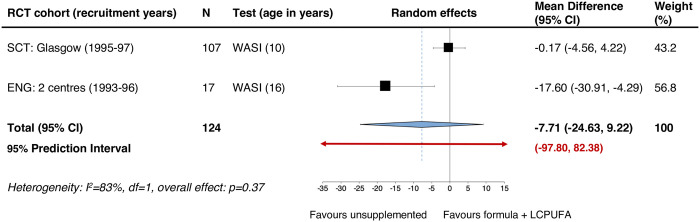
We pooled the data for infants born preterm also using random-effects because heterogeneity was judged to be high with an I2 of 83% (p = 0.01), despite homogeneity in terms of the assessment used. Fig 3 shows that among preterm-born babies, the pooled MD suggests no difference in the WASI Scale IQ at age 9–16 years: MD -7.71 IQ points. Again, uncertainty around the effect estimate was extremely high: 95% CI -24.63 to 9.22 and 95% PI from -97.80 to 82.38.
Fig 3. Primary outcome preterm infants: WASI IQ at ages 10–17 years (mean difference).
Secondary outcomes
Pooled cognitive tests scores performed at the oldest available age in each trial showed no evidence that children who received LCPUFA-supplemented infant formula differed from the control group: the SMD for term born children using random effects was -0.10, with a 95% CI of -0.32 to 0.12, with a 95% PI of -0.61 to 0.39 (Fig 4). The cognitive measures included were the Peabody Picture Vocabulary Test (PPVT) at age 3.5 years, Stanford-Binet IQ at 3.3 years, WASI at 9 and 16 years, and the WPPSI at age 4 and 6 years. There were no further available cognitive measures for infants born preterm.
Fig 4. Secondary outcome term infants: Cognitive function summary score (standardised mean difference).
Strength of evidence and risk of bias assessment
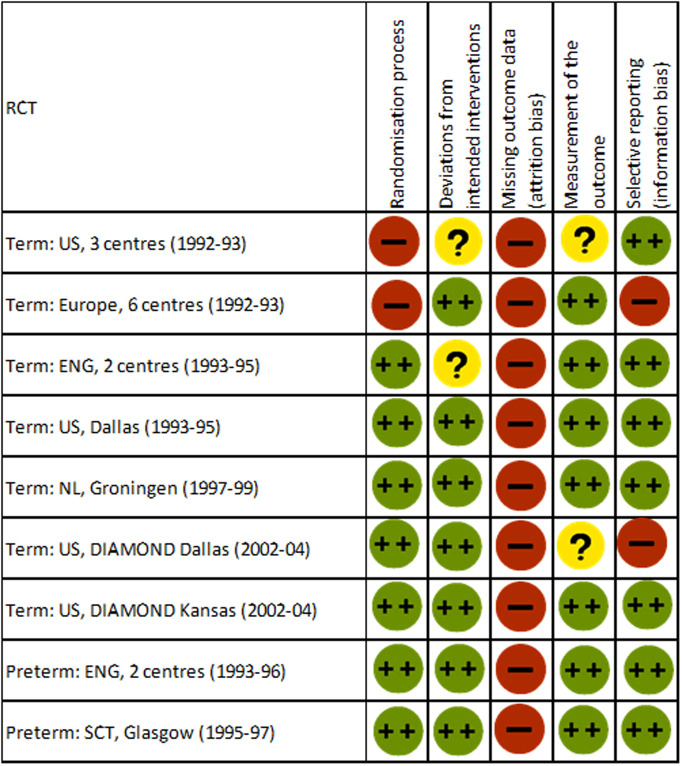
Fig 5 displays the results from the Cochrane Risk of Bias Tool. It highlights that potential for bias from attrition of study participants was a universal problem for all included trials. This is also reflected in the GRADE Summary of Findings (S3 Table in S1 File). Overall, the quality of available evidence was low, rated down for heterogeneity, attrition and potential for bias from selective publication. Potential for selective publication was based on correspondence from trialists about the (perceived) difficulty of publishing harmful results. S1 Fig in S1 File plots published and unpublished effect estimates against their standard error and indicates that unpublished effect estimates tend to be those that show harm. However, this should be interpreted with caution since visual analysis of potential for publication bias has limited reliability with <10 studies [55]. Completeness of follow-up for cognitive assessment was low, ranging from 9% to 79% of children initially randomised (median 52.6). Most studies reported balanced group characteristics at follow-up (S2 Table in S1 File).
Fig 5. Risk of bias summary: Based on latest available included cognitive outcome.
Discussion
We found no evidence that LCPUFA-supplemented infant formula improved long-term cognition among children born at term or preterm. Effect estimates were highly uncertain and included potential for large benefit and large harm.
This uncertainty should be taken seriously. While previous trials on LCPUFA-supplementation mostly reported either no effect or transiently favourable effects on developmental outcomes, negative effects are not without precedent. LCPUFA-supplementation has been associated with adverse effects on growth (including head growth) [56–58] and on development such as reduced vocabulary scores in term infants at age 14 months [52]. Furthermore, LCPUFA formula supplementation comprising DHA has been associated with potential harms in other domains, for example a higher risk of bronchopulmonary dysplasia in preterm infants [59].
Potential harms of LCPUFA might relate to the source of LCPUFA. LCPUFA in the included studies of this review were derived from egg, fish, algae and fungi and may not have the same functional effects as LCPUFA present in human milk. Furthermore, the DHA content of human milk is variable and heavily influenced by maternal diet. It therefore does not easily translate into an optimal dose [60]. This incertitude was reflected in the variety of doses administered in the included trials and likely drove the substantial heterogeneity that was observed in our meta-analyses. The plotted SMDs of all available outcomes also suggest that heterogeneity between studies is partly due to under-representation of outcomes showing a harmful effect of LCPUFA. We cannot determine the reason for non-publication for all trials, but a (perceived) difficulty in publishing negative results might play a role. Apart from that, trials were conducted in similar populations, using similar inclusion criteria, and the pooled primary outcomes were based on the same respective test.
We were not able to perform subgroup analyses to determine which factors accounted for the observed heterogeneity due to the small number of available trials. Yet even if findings are truly inconsistent, this would not change the conclusion of this study, namely that current evidence for supplementing infant formula with LCPUFA on the basis of cognitive benefits is weak and does not exclude potential for large harm.
Strengths of this review include a comprehensive search independent of reported outcomes, duplicate assessment of eligibility, and rigorous application of the GRADE and Cochrane Risk of Bias approach to rate quality of evidence and risk of bias. In contrast with previous systematic reviews, we included previously unpublished outcomes, which enabled a meta-analysis of measures of cognitive function beyond the first two years of life. As the child ages, cognitive assessments become more discriminatory and predictive of adult function than tests before two years of age and less dependent on the situation and outcome assessor [11–13].
The limitations of our review are related to the quality of the underlying evidence. We observed substantial statistical heterogeneity, potential for attrition bias in all outcomes, as well as potential for selective publication. Attrition of study participants is a universal problem in long-term nutrition studies [56–58]. Potentially negative effects of LCPUFA-supplementation could have resulted from selective follow-up. However, it seems unlikely that either 1) children with lower IQ who were previously assigned to the control group would be less likely to respond than children with lower IQ from the intervention group, or 2) children with higher IQ outcomes be more likely to respond if they were in the control group, especially as all trials were blinded.
While there was industry involvement in all of the original trials, only four of the ten follow-up studies reported in our analyses received industry funding. In six of the eight trials there were other potential conflicts of interest (S7 Table in S1 File). While industry involvement is very common in the area of infant nutrition studies it is not necessarily predictive of lower study quality. It is also necessary to emphasise that many of the outcomes included in our review were published at a time when reporting standards were lower than today. Importantly, the included studies represent the only available evidence on long-term cognitive outcomes.
More robust evidence of benefit, and certainty about absence of harm, is needed to justify mandatory LCPUFA-supplementation of infant formula. New trials would take time, are expensive and would suffer from the same problems of attrition and resulting biases as the studies included in this review. Recent methods, involving data linkage of extant trial data to administrative education and health data in adolescence and adulthood, offer a more rapid, less biased, and cost effective way of obtaining data on long-term outcomes. Linkage of historical trials to administrative education or health datasets is achievable where trial data and participant identifiers have been retained and governance arrangements allow secure linkage without re-consent [61, 62].
We found no evidence that LCPUFA-supplemented infant formula benefits cognitive function compared with unsupplemented formula in children born at term or preterm. Given the lack of benefit on other functional outcomes [5, 53] and the additional costs of supplemented formula [63], widespread addition of LCPUFA to infant and follow-on formula cannot be supported until further robust evidence excludes potential for future harm.
Supporting information
(DOCX)
(DOC)
Acknowledgments
The authors thank the investigators Nancy Auestad, Bridget Barrett-Reis, Eileen Birch, Susan Carlson, John Colombo, Mijna Hadders-Algra, Alan Lucas and Atul Singhal for contributing data or providing additional information.
Abbreviations
- AA
arachidonic acid
- BBCS-R
Basic Concept Scale-Revised
- CI
Confidence Interval
- DHA
docosahexaenoic acid
- EU
European Union
- IQ
intelligence quotient
- MD
mean difference
- PI
Prediction Interval
- PPVT
Peabody Picture Vocabulary Test
- RCT
randomised controlled trial
- SB IQ
Stanford-Binet IQ
- SE
standard error
- SMD
standardised mean difference
- WASI
Wechsler Abbreviated Scale of Intelligence
- WASI
Wechsler Adult Intelligence Scale
- WPPSI-R
Wechsler Preschool and Primary Scales of Intelligence-Revised
- LCPUFA
long-chain polyunsaturated fatty acid
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
This study was supported by funds from the Economic and Social Research Council, and the Great Ormond Street Hospital Charity. RG was supported by Health Data Research UK, an initiative funded by the UK Research and Innovation, Department of Health and Social Care (England) and the devolved administrations, and leading medical research charities. Research at UCL Great Ormond Street Institute of Child Health is supported by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Koletzko B., et al. , Pediatric nutrition in practice. 2015: Karger Medical and Scientific Publishers. [Google Scholar]
- 2.Neuringer M. and Connor W.E., n‐3 Fatty Acids in the Brain and Retina: Evidence for Their Essentiality. Nutrition Reviews, 1986. 44(9): p. 285–294. 10.1111/j.1753-4887.1986.tb07660.x [DOI] [PubMed] [Google Scholar]
- 3.Martinez M., Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr, 1992. 120(4 Pt 2): p. S129–38. 10.1016/s0022-3476(05)81247-8 [DOI] [PubMed] [Google Scholar]
- 4.Brenna J.T., et al. , Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. The American Journal of Clinical Nutrition, 2007. 85(6): p. 1457–1464. 10.1093/ajcn/85.6.1457 [DOI] [PubMed] [Google Scholar]
- 5.Moon K., et al. , Longchain polyunsaturted fatty acid supplementation in preterm infants: Updated cochrane review. Journal of Paediatrics and Child Health, 2017. 53 (Supplement 2): p. 69–70. [Google Scholar]
- 6.Lucas A., et al. , Breast milk and subsequent intelligence quotient in children born preterm. Lancet, 1992. 339(8788): p. 261–4. 10.1016/0140-6736(92)91329-7 [DOI] [PubMed] [Google Scholar]
- 7.Morrow-Tlucak M., Haude R.H., and Ernhart C.B., Breastfeeding and cognitive development in the first 2 years of life. Social Science & Medicine, 1988. 26(6): p. 635–639. 10.1016/0277-9536(88)90028-7 [DOI] [PubMed] [Google Scholar]
- 8.Anderson J.W., Johnstone B.M., and Remley D.T., Breast-feeding and cognitive development: a meta-analysis. Am J Clin Nutr, 1999. 70(4): p. 525–35. 10.1093/ajcn/70.4.525 [DOI] [PubMed] [Google Scholar]
- 9.Kramer M.S., et al. , Breastfeeding and Child Cognitive Development: New Evidence From a Large Randomized Trial. JAMA Psychiatry, 2008. 65(5): p. 578–584. 10.1001/archpsyc.65.5.578 [DOI] [PubMed] [Google Scholar]
- 10.Jasani B., et al. , Long chain polyunsaturated fatty acid supplementation in infants born at term. The Cochrane Library, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombo, J., Assessing Neurocognitive Development in Studies of Nutrition, in Recent Research in Nutrition and Growth. Nestlé Nutr Inst Workshop. 2018, Nestlé Nutrition Institute,: Switzerland. p. 143–154. [DOI] [PubMed]
- 12.Sun H., et al. , Infant formula and neurocognitive outcomes: impact of study end-point selection. Journal Of Perinatology, 2015. 35: p. 867 10.1038/jp.2015.87 [DOI] [PubMed] [Google Scholar]
- 13.Colombo J. and Carlson S.E., Is the measure the message: the BSID and nutritional interventions. Pediatrics, 2012. 129(6): p. 1166–1167. 10.1542/peds.2012-0934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EU Commission, Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow-on formula and as regards requirements on information relating to infant and young child feeding. OJEC, 2016. 59: p. 1–29. [Google Scholar]
- 15.Hughes H.K., Landa M.M., and Sharfstein J.M., Marketing claims for infant formula: The need for evidence. JAMA Pediatrics, 2017. 171(2): p. 105–106. 10.1001/jamapediatrics.2016.3837 [DOI] [PubMed] [Google Scholar]
- 16.Verfuerden, M. and S. Dib, Effect of long chain polyunsaturated fatty acid-supplemented infant formula in term infants on cognitive ability: a protocol for a systematic review of randomised controlled trials. CRD42018088868 PROSPERO 2018. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018088868.
- 17.Verfuerden, M. and S. Dib, Effect of long chain polyunsaturated fatty acid-supplemented infant formula in preterm infants on cognitive ability: a protocol for a systematic review of randomised controlled trials. CRD42018105196 PROSPERO, 2018. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018105196.
- 18.Moher D., et al. , Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews, 2015. 4(1): p. 1 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huttenlocher P.R. and Dabholkar A.S., Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol, 1997. 387(2): p. 167–78. [DOI] [PubMed] [Google Scholar]
- 20.IntHout J., et al. , Plea for routinely presenting prediction intervals in meta-analysis. BMJ open, 2016. 6(7): p. e010247–e010247. 10.1136/bmjopen-2015-010247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auestad N., et al. , Visual, cognitive, and language assessments at 39 months: a follow-up study of children fed formulas containing long-chain polyunsaturated fatty acids to 1 year of age. Pediatrics, 2003. 112(3 Pt 1): p. e177–83. 10.1542/peds.112.3.e177 [DOI] [PubMed] [Google Scholar]
- 22.Auestad N., et al. , Visual acuity, erythrocyte fatty acid composition, and growth in term infants fed formulas with long chain polyunsaturated fatty acids for one year. Ross Pediatric Lipid Study. Pediatr Res, 1997. 41(1): p. 1–10. 10.1203/00006450-199701000-00001 [DOI] [PubMed] [Google Scholar]
- 23.Scott D.T., et al. , Formula Supplementation With Long-chain Polyunsaturated Fatty Acids: Are There Developmental Benefits? Pediatrics, 1998. 102(5): p. e59–e59. 10.1542/peds.102.5.e59 [DOI] [PubMed] [Google Scholar]
- 24.Willatts P., et al. , Effects of long-chain PUFA supplementation in infant formula on cognitive function in later childhood. The American Journal of Clinical Nutrition, 2013. 98(2): p. 536S–542S. 10.3945/ajcn.112.038612 [DOI] [PubMed] [Google Scholar]
- 25.Willatts P., et al. , Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet, 1998. 352(9129): p. 688–91. 10.1016/s0140-6736(97)11374-5 [DOI] [PubMed] [Google Scholar]
- 26.Willatts P., et al. , Influence of long-chain polyunsaturated fatty acids on infant cognitive function. Lipids, 1998. 33(10): p. 973–80. 10.1007/s11745-998-0294-7 [DOI] [PubMed] [Google Scholar]
- 27.Forsyth J.S., et al. , Long chain polyunsaturated fatty acid supplementation in infant formula and blood pressure in later childhood: follow up of a randomised controlled trial. BMJ, 2003. 326(7396): p. 953 10.1136/bmj.326.7396.953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agostoni C., et al. , Neurodevelopmental Quotient of Healthy Term Infants at 4 Months and Feeding Practice: The Role of Long-Chain Polyunsaturated Fatty Acids. Pediatric Research, 1995. 38: p. 262 10.1203/00006450-199508000-00021 [DOI] [PubMed] [Google Scholar]
- 29.Agostoni C., et al. , Developmental quotient at 24 months and fatty acid composition of diet in early infancy: a follow up study. Archives of Disease in Childhood, 1997. 76(5): p. 421–424. 10.1136/adc.76.5.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas A., et al. , Efficacy and safety of long-chain polyunsaturated fatty acid supplementation of infant-formula milk: a randomised trial. Lancet, 1999. 354(9194): p. 1948–54. 10.1016/S0140-6736(99)02314-4 [DOI] [PubMed] [Google Scholar]
- 31.Lucas, A., et al., Long chain polyunsaturated fatty acids in infant formula and intelligence quotient at 4–5 years: a randomised trial. unpublished.
- 32.Birch E.E., et al. , Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatr Res, 1998. 44(2): p. 201–9. 10.1203/00006450-199808000-00011 [DOI] [PubMed] [Google Scholar]
- 33.Birch E.E., et al. , Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum Dev, 2007. 83(5): p. 279–84. 10.1016/j.earlhumdev.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 34.Birch E.E., et al. , A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol, 2000. 42(3): p. 174–81. 10.1017/s0012162200000311 [DOI] [PubMed] [Google Scholar]
- 35.de Jong C., et al. , Effects of long-chain polyunsaturated fatty acid supplementation of infant formula on cognition and behaviour at 9 years of age. Dev Med Child Neurol, 2012. 54(12): p. 1102–8. 10.1111/j.1469-8749.2012.04444.x [DOI] [PubMed] [Google Scholar]
- 36.de Jong C., et al. , The Groningen LCPUFA study: no effect of postnatal long-chain polyunsaturated fatty acids in healthy term infants on neurological condition at 9 years. British Journal of Nutrition, 2010. 104(4): p. 566–572. 10.1017/S0007114510000863 [DOI] [PubMed] [Google Scholar]
- 37.Bouwstra H., et al. , Long-chain polyunsaturated fatty acids and neurological developmental outcome at 18 months in healthy term infants. Acta Paediatr, 2005. 94(1): p. 26–32. 10.1111/j.1651-2227.2005.tb01783.x [DOI] [PubMed] [Google Scholar]
- 38.Bouwstra H., et al. , Long-chain polyunsaturated fatty acids have a positive effect on the quality of general movements of healthy term infants. Am J Clin Nutr, 2003. 78(2): p. 313–8. 10.1093/ajcn/78.2.313 [DOI] [PubMed] [Google Scholar]
- 39.Colombo J., et al. , Long-chain polyunsaturated fatty acid supplementation in infancy reduces heart rate and positively affects distribution of attention. Pediatr Res, 2011. 70(4): p. 406–10. 10.1203/PDR.0b013e31822a59f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colombo J., et al. , Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am J Clin Nutr, 2013. 98(2): p. 403–12. 10.3945/ajcn.112.040766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colombo J., et al. , Docosahexaenoic acid (DHA) and arachidonic acid (ARA) balance in developmental outcomes. Prostaglandins Leukot Essent Fatty Acids, 2017. 121: p. 52–56. 10.1016/j.plefa.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao K., et al. , Event-related potential differences in children supplemented with long-chain polyunsaturated fatty acids during infancy. Dev Sci, 2017. 20(5). 10.1111/desc.12455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birch E.E., et al. , The DIAMOND (DHA Intake And Measurement Of Neural Development) Study: a double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am J Clin Nutr, 2010. 91(4): p. 848–59. 10.3945/ajcn.2009.28557 [DOI] [PubMed] [Google Scholar]
- 44.Drover J.R., et al. , A randomized trial of DHA intake during infancy: school readiness and receptive vocabulary at 2–3.5 years of age. Early Hum Dev, 2012. 88(11): p. 885–91. 10.1016/j.earlhumdev.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 45.Drover J.R., et al. , Cognitive function in 18-month-old term infants of the DIAMOND study: a randomized, controlled clinical trial with multiple dietary levels of docosahexaenoic acid. Early Hum Dev, 2011. 87(3): p. 223–30. 10.1016/j.earlhumdev.2010.12.047 [DOI] [PubMed] [Google Scholar]
- 46.Drover J.R., et al. , Addendum to "Cognitive function in 18-month-old term infants of the DIAMOND study: a randomized, controlled clinical trial with multiple dietary levels of docosahexaenoic acid" [Early Hum. Dev. 87 (2011) 223–230]. Early Hum Dev, 2013. 89(3): p. 195 10.1016/j.earlhumdev.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 47.Fewtrell M.S., et al. , Double-blind, randomized trial of long-chain polyunsaturated fatty acid supplementation in formula fed to preterm infants. Pediatrics, 2002. 110(1 Pt 1): p. 73–82. 10.1542/peds.110.1.73 [DOI] [PubMed] [Google Scholar]
- 48.Fewtrell M.S., et al. , Randomized, double-blind trial of long-chain polyunsaturated fatty acid supplementation with fish oil and borage oil in preterm infants. J Pediatr, 2004. 144(4): p. 471–9. 10.1016/j.jpeds.2004.01.034 [DOI] [PubMed] [Google Scholar]
- 49.Isaacs E.B., et al. , 10-year cognition in preterms after random assignment to fatty acid supplementation in infancy. Pediatrics, 2011. 128(4): p. e890–8. 10.1542/peds.2010-3153 [DOI] [PubMed] [Google Scholar]
- 50.Fewtrell M.S., et al. , Double-Blind, Randomized Trial of Long-Chain Polyunsaturated Fatty Acid Supplementation in Formula Fed to Preterm Infants. Pediatrics, 2002. 110(1): p. 73–82. 10.1542/peds.110.1.73 [DOI] [PubMed] [Google Scholar]
- 51.Colombo J., et al. , Long-term effects of LCPUFA supplementation on childhood cognitive outcomes–. The American journal of clinical nutrition, 2013. 98(2): p. 403–412. 10.3945/ajcn.112.040766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott D.T., et al. , Formula supplementation with long-chain polyunsaturated fatty acids: are there developmental benefits? Pediatrics, 1998. 102(5): p. E59 10.1542/peds.102.5.e59 [DOI] [PubMed] [Google Scholar]
- 53.Jasani B., et al. , Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev, 2017. 3: p. CD000376 10.1002/14651858.CD000376.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willatts P., et al. , Effects of long-chain PUFA supplementation in infant formula on cognitive function in later childhood. Am J Clin Nutr, 2013. 98(2): p. 536S–42S. 10.3945/ajcn.112.038612 [DOI] [PubMed] [Google Scholar]
- 55.Higgins J.P., et al. , Cochrane handbook for systematic reviews of interventions. 2019: John Wiley & Sons. [Google Scholar]
- 56.Montalto M.B., et al. , Reduced Growth in Hospital Discharged Low Birth Weight (Lbw) Infants Fed Formulas with Added Marine Oil (Mo). † 1878. Pediatric Research, 1996. 39: p. 316–316. [Google Scholar]
- 57.Carlson S.E., et al. , First year growth of preterm infants fed standard compared to marine oil n-3 supplemented formula. Lipids, 1992. 27(11): p. 901–7. 10.1007/BF02535870 [DOI] [PubMed] [Google Scholar]
- 58.Carlson S.E., Werkman S.H., and Tolley E.A., Effect of long-chain n-3 fatty acid supplementation on visual acuity and growth of preterm infants with and without bronchopulmonary dysplasia. Am J Clin Nutr, 1996. 63(5): p. 687–97. 10.1093/ajcn/63.5.687 [DOI] [PubMed] [Google Scholar]
- 59.Collins C.T., et al. , Docosahexaenoic Acid and Bronchopulmonary Dysplasia in Preterm Infants. New England Journal of Medicine, 2017. 376(13): p. 1245–1255. 10.1056/NEJMoa1611942 [DOI] [PubMed] [Google Scholar]
- 60.Koletzko B., et al. , Physiological aspects of human milk lipids and implications for infant feeding: a workshop report. Acta Paediatr, 2011. 100(11): p. 1405–15. 10.1111/j.1651-2227.2011.02343.x [DOI] [PubMed] [Google Scholar]
- 61.Verfuerden M., et al. , Unconsented linkage between dormant trials and administrative data: practical and regulatory implications. International Journal of Population Data Science, 2018. 3(2). [Google Scholar]
- 62.Verfürden M., et al. , Infant formula composition and educational performance: a protocol to extend follow-up for a set of randomised controlled trials using linked administrative education records. BMJ Open, 2020. 10(7): p. e035968 10.1136/bmjopen-2019-035968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hughes H.K., Landa M.M., and Sharfstein J.M., Marketing Claims for Infant Formula: The Need for Evidence. JAMA Pediatr, 2017. 171(2): p. 105–106. 10.1001/jamapediatrics.2016.3837 [DOI] [PubMed] [Google Scholar]