ABSTRACT
Objectives
The aim of this systematic review was to test the hypothesis of no difference in complications and donor site morbidity following harvesting of autogenous bone graft from the ascending mandibular ramus compared with the chin region.
Material and Methods
MEDLINE (PubMed), Embase and Cochrane Library search in combination with a hand-search of relevant journals was conducted including human studies published in English through June 26, 2020. Randomized and controlled trials were included. Outcome measures included pain, infection, mucosal dehiscence, altered sensation or vitality of adjacent tooth/teeth, neurosensory disturbances and patient-reported outcome measures. Risk of bias was assessed by Cochrane risk of bias tool and Newcastle-Ottawa Scale.
Results
Ten controlled trials of high-quality fulfilled inclusion criteria. Risk of infection and mucosal dehiscence seems to be comparable with the two treatment modalities. However, harvesting from the chin seems to be associated with increased risk of pain, altered sensation or loss of tooth vitality, and neurosensory disturbances. Willingness to undergo the same treatment again was reported with both treatment modalities, but significant higher satisfaction, lower discomfort and acceptance of the surgical procedure was reported following harvesting from the ascending mandibular ramus.
Conclusions
The hypothesis was rejected due to higher prevalence and severity of complications and donor site morbidity following harvesting of autogenous bone graft from the chin region. Dissimilar evaluation methods and various methodological confounding factors posed serious restrictions for literature review in a quantitative systematic manner. Conclusions drawn from results of this systematic review should therefore be interpreted with caution.
Keywords: alveolar bone grafting, alveolar ridge augmentation, dental implants, oral surgical procedures, review
INTRODUCTION
Alveolar ridge augmentation prior to or in conjunction with implant placement is frequently necessary when dimensions of the alveolar process are inadequate for placement of implants in an optimal position for the prosthodontic restoration [1-3]. Autogenous bone graft is considered the preferred grafting material for alveolar ridge augmentation due to its osteoinductive, osteogenic, and osteoconductive characteristics [4]. However, harvesting of autogenous bone graft is associated with risk of donor site morbidity, prolonged treatment time, and possibility of injury to adjacent vital structures [5-10]. Furthermore, extraoral harvesting requires general anaesthesia and hospitalization [7-11].
Various anatomic donor sites are available for harvesting of autogenous bone graft including iliac crest, calvaria, ribs, tibia, fibula, coronoid process, zygomatic buttress, tuber maxillae, ascending mandibular ramus, and the chin region [5-11]. Intraoral donor sites offer several advantages compared with extraoral locations including proximity of donor and recipient sites, avoidance of cutaneous scarring, can be performed under local anaesthesia on an outpatient basis, and convenient surgical accessibility [7,12,13]. The ascending mandibular ramus and the chin region are the most commonly used donor site for harvesting of intraoral autogenous bone graft and usually provides enough grafting material for reconstruction of localized alveolar ridge defects [7,14]. Autogenous bone graft from the ascending mandibular ramus is characterized by dense cortical bone with less amount of cancellous bone, whereas chin bone graft is characterized by higher quantity of cancellous bone [15]. It has been reported that the amount of autogenous bone graft that can be harvested from intraoral donor sites is highest in the chin region [16]. However, selection a specific intraoral donor site for harvesting of autogenous bone graft is based on different aspects including surgeon’s preference, quantity and quality of bone required, access to the donor site, and potential surgical complications.
Pain, swelling, bleeding, infection, mucosal dehiscence, altered sensation or loss of tooth vitality, limited mouth opening, changes in the contour of the donor area, and transient or permanent neurosensory disturbances of the inferior alveolar nerve are the most commonly reported complications following harvesting of intraoral autogenous bone graft [5-7,12,13]. Previous systematic reviews have concluded that harvesting of autogenous bone graft from the chin is associated with a higher prevalence and severity of complications as well as donor site morbidity compared with the ascending mandibular ramus [7,17]. Moreover, a long-term study revealed few complications and negligible donor site morbidity following harvesting of autogenous bone graft from the ascending mandibular ramus [18]. From a patient perspective, awareness of the invasiveness of the harvesting procedure as well as risk of complications and donor site morbidity are important issues before definitive acceptance of a specified treatment modality. Thus, the appropriate procedure for harvesting of intraoral autogenous bone graft should therefore be less invasive with least risk of complications [19]. The objective of the present systematic review is therefore to test the hypothesis of no difference in complications and donor site morbidity following harvesting of autogenous bone graft from the ascending mandibular ramus compared with the chin region.
MATERIAL AND METHODS
Protocol and registration
Review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews [20]. Methods of the analysis and inclusion criteria were specified in advance and documented in a protocol and registered in PROSPERO, an international prospective register of systematic reviews.
Registration number: CRD42020196671.
The protocol can be accessed at:
https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020196671.
Focus question
Focus question was developed according to the Patient, Intervention, Comparison and Outcome (PICO) framework as described in Table 1.
Table 1.
PICOS guidelines
| Patient and population (P) | Healthy patients in need of implant treatment undergoing harvesting of intraoral autogenous bone graft. |
| Intervention (I) | Autogenous bone graft from the ascending mandibular ramus. |
| Comparator or control group (C) | Autogenous bone graft from the chin region. |
| Outcomes (O) | Pain, infection, mucosal dehiscence, altered sensation or vitality of adjacent tooth/teeth, neurosensory disturbances of the inferior alveolar nerve or vestibular area, and patient-reported outcome measures. |
| Study design (S) | Randomized controlled trials and controlled trials. |
| Focused question | Are there any differences in complications and donor site morbidity following harvesting of autogenous bone graft from the ascending mandibular ramus compared with the chin region? |
Eligibility criteria for considering studies for this review
Randomized controlled trials and controlled trials in humans assessing complications and donor site morbidity following harvesting of autogenous bone graft from the ascending mandibular ramus compared with the chin region.
Types of outcome measures
Pain.
Infection.
Mucosal dehiscence.
Altered sensation or vitality of adjacent tooth/teeth.
Neurosensory sensory disturbances of the inferior alveolar nerve or vestibular area.
Patient-reported outcome measures (PROM).
Information sources
The search strategy incorporated examinations of electronic databases, supplemented by a thorough hand-search page by page of relevant journals including “British Journal of Oral and Maxillofacial Surgery”, “Clinical Implant Dentistry and Related Research”, “Clinical Oral Implants Research”, “European Journal of Oral Implantology”, “Implant Dentistry”, “International Journal of Oral and Maxillofacial Implants”, “International Journal of Oral and Maxillofacial Surgery”, “International Journal of Periodontics and Restorative Dentistry”, “International Journal of Prosthodontics”, “Journal of Clinical Periodontology”, “Journal of Dental Research”, “Journal of Oral Implantology”, “Journal of Oral & Maxillofacial Research”, “Journal of Periodontology”, “Journal of Prosthetic Dentistry”, “Journal of Craniofacial Surgery”, “Journal of Cranio-Maxillo-Facial Surgery”, “Journal of Oral and Maxillofacial Surgery”, “Periodontology 2000”, “Oral and Maxillofacial Surgery” and “Oral Surgery Oral Medicine Oral Pathology Oral Radiology”. The manual search also included the bibliographies of all articles selected for full-text screening as well as previously published reviews relevant for the present systematic review. One reviewer (T.S-J.) performed the search.
Search strategy for identification of studies
MEDLINE (PubMed), Embase, and Cochrane Library search was conducted. Human studies published in English through June 26, 2020 were included. Grey literature, unpublished literature as well as other databases like Scopus, Google Scholar, or Research Gate were not included in the search strategy. The search strategy was performed in collaboration with a librarian and utilized a combination of Medical subject heading (MeSH) and free text terms. A detailed description of the search strategy is presented in Appendices 1, 2, 3 and 4.
Selection of studies
PRISMA flow diagram presents an overview of the selection process (Figure 1). Titles of identified reports were initially screened with duplicates removed. Abstracts were assessed when titles indicated that the study was relevant. Full-text analysis was obtained for those with apparent relevance or when the abstract was unavailable. References of papers identified and previously published systematic reviews assessing complications and donor site morbidity following harvesting of autogenous bone graft from the ascending mandibular ramus or chin region were cross-checked for unidentified articles. Study selection was performed by one reviewer (T.S-J.).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram demonstrating results of systematic literature search.
Electronic search resulted in 291 entries. No additional articles were identified through hand-searching. Of these 291 articles, 66 were excluded because they had been retrieved in more than one search. A total of 31 abstracts were reviewed and full-text analysis included 13 articles. Ten controlled clinical trials were finally included in the present systematic review.
Inclusion criteria
Studies assessing complications and donor site morbidity following harvesting of autogenous bone graft from the ascending mandibular ramus compared with the chin region were included by addressing the previously described outcome measures. The review exclusively focused on studies with more than five patients and an observation period of at least three months.
Exclusion criteria
Following exclusion criteria were applied: unspecified number of included patients, harvesting procedures and complications as well as studies involving medically compromised patients were excluded. Moreover, letters, editorials, PhD theses, letters to the editor, case reports, abstracts, technical reports, conference proceedings, cadaveric studies, animal or in vitro studies and literature review papers were excluded.
Data extraction
Data were extracted by one reviewer (T.S-J.) according to a data-collection form ensuring systematic recording of the outcome measures. In addition, relevant characteristics of the study were recorded. Corresponding authors were contacted by e-mail in the absence of important information or ambiguities.
Data items
Following items were collected and arranged in following fields: study, year of publication, study design, patient, donor site, observation period, pain, infection, mucosal dehiscence, altered sensation or vitality of adjacent tooth/teeth, neurosensory disturbances of the inferior alveolar nerve or vestibular area and PROM.
Quality and risk-of-bias assessment
Quality assessment was undertaken by one review author (T.S-J.) as part of the data extraction process. Cochrane Collaboration’s tool for assessing risk of bias suggested in the Cochrane Handbook for Systematic Reviews of Interventions was used for included randomized controlled trials (version 5.1.0) [21]. Following items were evaluated:
Random sequence generation;
Allocation concealment;
Patient blinding;
Outcome blinding;
Incomplete outcome data addressed;
Selective reporting.
Publications were grouped into the following categories [22]:
Low risk of bias (possible bias not seriously affecting results) if all criteria were met.
High risk of bias (possible bias seriously weakening reliability of results) if one or more criteria were not met.
Unclear risk of bias when too few details were available for classification as high or low risk.
Newcastle-Ottawa scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) was applied for non-randomized studies [23]. Following items were evaluated:
Selection of studies;
Comparability of cohorts;
Ascertainment of either the exposure or outcome of interest.
Stars were awarded with highest quality studies awarded up to nine stars. Included non-randomized studies were categorized as:
Low-quality (0 - 3 stars);
Moderate quality (4 - 6 stars);
High quality (7 - 9 stars).
Statistical analysis
Meta-analysis (with random effect) where conducted using Sidik-Jonkman estimation method for pain, infection, mucosal dehiscence, temporary and permanent neurosensory disturbances of the inferior alveolar nerve or vestibular area. Risk difference with 95% confidence interval (CI) was calculated between mandibular ramus and chin region. Statistical significance level was defined at P = 0.05.
Assessment of heterogeneity
The significance of any discrepancies in the estimates of the treatment effects of the different studies was assessed by means of Cochran’s test for heterogeneity and the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance. Heterogeneity by Cochran’s test was considered statistically significant if P < 0.1. A rough guide to the interpretation of I2 given in the Cochrane Handbook for Systematic Reviews of Interventions is as follows:
0 - 40% the heterogeneity might not be important;
30 - 60% may represent moderate heterogeneity;
50 - 90% may represent substantial heterogeneity;
75 - 100% may represent considerable heterogeneity.
RESULTS
Study selection
Search results are outlined in Figure 1. Electronic search resulted in 291 entries. No additional articles were identified through hand-searching. Of these 291 articles, 66 were excluded due to being retrieved in more than one search. A total of 31 abstracts were reviewed and full-text analysis included 13 articles. Finally, ten comparative clinical trials were included [24-33].
Exclusion of studies
Reasons for excluding three studies after full-text assessment were: complications or donor site morbidity were not specified (n = 1) [34], unspecified number of harvesting procedures (n = 1) [35], and less than five patients in each group [36].
Characteristics of the studies included
No randomized controlled trials were identified. Ten controlled clinical trials of high quality were included in the present systematic review [24-33]. Power calculation of the sample size was performed in one study [33]. Age and gender distribution were clearly specified in seven studies [26,28-33]. Defined inclusion and exclusion criteria were described in three studies [31-33]. The choice of donor site was determined by the defect morphology and recipient site location [24], initially harvested from the chin region and subsequently from the mandibular ramus [26], by chance [28], consecutively from the chin region or mandibular ramus [31], patient-specific anatomical handicaps (root length, mouth opening, shallow vestibular sulcus depth, presence of third molar) [32], or no information was provide about the reason for the choice of donor site [25,27,29,30,33]. The surgical procedure was performed by one surgeon [26,28,30,31], two surgeons [33], or by an unknown number of surgeons [24,25,27,29,32]. Harvesting of autogenous bone graft was performed under local anaesthetics [25,28,29,32,33], local anaesthetics including intravenous or oral conscious sedation [24,26,29,30], general anaesthesia [29], or type of anaesthetics was not reported [27,31]. Patient perception of pain was assessed by verbal response [25,28], self-administrated questionnaire [26], review of medical records [27], interview [29], visual analogue scale [28-31], or no information’s was provided about the assessment method [33]. Infection and/or mucosal dehiscence were assessed by clinical examination [24,25,28,32], or review of medical records [27]. Altered sensation or vitality of tooth/teeth was assessed by verbal response [24,25], interview [29], cold vitality with carbon dioxide snow [30], response to cold [28], electric pulp test [31], radiographic examination including periapical radiolucency [28,30] or no information was provided about the assessment method [32,33]. Neurosensory disturbances were assessed by verbal response [24,25,32,33], self-administrated questionnaire [26,28,33], review of medical records [27], interview [29], point-blunt test [30,31] or two-point discrimination test including threshold values of lower than 7 mm (no alteration), between 7 mm and 11 mm (slight alteration), and larger than 11 mm (impairment of the skin sensitivity) [30,31]. PROM were reported by self-administrated questionnaire [26,28,30], method using a number between 0 and 10 [28], interview [29], or no information was provided about the assessment method [33]. Information about drops-out was reported in one study [30]. Methods for examiner training or calibration was reported in one study [30].
Methodological quality
Quality of the included studies are summarized in Table 2.
Table 2.
Newcastle-Ottawa scale for assessing quality of non-randomized studies categorized as low-quality (0 - 3 stars), moderate quality (4 - 6 stars), and high quality (7 - 9 stars)
| Study |
Year of publication |
Selection (maximum 4 stars) |
Comparability (maximum 2 stars) |
Outcome (maximum 3 stars) |
Total score/ quality |
|---|---|---|---|---|---|
| Misch et al [24] | 1997 | ★★★★ | ★★ | ☆★☆ | 7 stars/high quality |
| Cordaro et al. [25] | 2002 | ★★★★ | ★★ | ☆★☆ | 7 stars/high quality |
| Clavero et al. [26] | 2003 | ★★★★ | ★★ | ☆★☆ | 7 stars/high quality |
| Silva et al. [27] | 2006 | ★★★★ | ★★ | ☆★☆ | 7 stars/high quality |
| Raghoebar et al. [28] | 2007 | ★★★★ | ★★ | ☆★☆ | 7 stars/high quality |
| Andersson et al. [29] | 2008 | ★★★★ | ★★ | ☆★☆ | 7 stars/high quality |
| Cordaro et al. [30] | 2011 | ★★★★ | ★★ | ★★★ | 9 stars/high quality |
| Altiparmak et al. [31] | 2015 | ★★★★ | ★★ | ☆★☆ | 7 stars/high quality |
| Ersanli et al. [32] | 2016 | ★★★★ | ★★ | ☆★☆ | 7 stars/high quality |
| Pereira et al. [33] | 2019 | ★★★★ | ★★ | ☆★☆ | 7 stars/high quality |
Outcome measures
Results of complications and donor site morbidity following harvesting of autogenous bone graft from the ascending mandibular ramus compared with the chin region are presented below and outlined in Table 3. All reported numerical values are presented as mean values. For each outcome measure, a summary is provided.
Table 3.
Complications and donor site morbidity following harvesting of autogenous bone graft from the ascending mandibular ramus compared with the chin region
| Study | Material and methods | Outcome measures | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year of publication | Study design | Number of patients | Donor site | Observation period | Pain | Infection | Mucosal dehiscence |
Altered sensation or vitality of tooth/teeth |
Neurosensory disturbances of IAN or vestibular area |
Patient-reported outcome measures | ||||
| Temporary | Permanent | |||||||||||||
| Misch et al [24] | 1997 | CT | 31 | Chin | 4 - 6 months | NR | 6% | 11% | 29% | Verbal response | NR | |||
| 9.6% | NR | |||||||||||||
| 19 | Mandibular ramus | 0% | 0% | 0% | 0% | |||||||||
| Cordaro et al. [25] | 2002 | CT | 13 | Chin | 4 - 38 months | No difference | 0% | 0% |
Temporary: 7%; permanent: 0% |
Verbal response | NR | |||
| 5 | Mandibular ramus | 0% | 0% | 0% | ||||||||||
| Clavero et al. [26] | 2003 | CT | 29 | Chin | 18 months | Higher pain | 0% | NR | NR | Self-administrated questionnaire |
Met pretreatment expectations: 91% Undergo same treatment again: 94% |
|||
| 76% | 52% | |||||||||||||
| 24 | Mandibular ramus | Less pain | 21% | 4% | ||||||||||
| Silva et al. [27] | 2006 | CT | 50 | Chin | 120 days | NR | 0% | 0% | NR | Review of medical records | NR | |||
| 16% | ||||||||||||||
| 36 | Mandibular ramus | 8% | ||||||||||||
| Raghoebar et al. [28] | 2007 | CT | 15 | Chin | 12 months | 33% | 0% | NR |
Temporary: 13%; permanent: 0% |
Self-administrated questionnaire | Acceptance of the surgical procedure was significantly higher after harvesting of mandibular ramus bone and third molar removala | |||
| 40% | 20% | |||||||||||||
|
Temporary: 0%; permanent: 0% |
0% | |||||||||||||
| 15 | Mandibular ramus | 20% | 7% | |||||||||||
| 15 | Mandibular ramus and third molar | 20% | 7% | |||||||||||
| Andersson et al. [29] | 2008 | CT | 16 | Chin | 3 - 5 years | Higher pain | NR | NR | Sensitivity to cold: 12.5% | Interview | Significant lower discomfortc and higher satisfaction after harvesting of mandibular ramusd | |||
| NR | 33% | |||||||||||||
| 12 | Mandibular ramus | Less painb | Sensitivity to cold: 0% | 0% | ||||||||||
| Cordaro et al. [30] | 2011 | CT | 37 | Chin | 18 - 42 months | Less pain | NR | NR |
Negative pulp sensitivity: 13%; root canal treatment: 0.7% |
PBT | TPDT | Verbal response | Patient´s perception of morbidity did not differ between chin and mandibular ramusj | |
|
Mucosa: 16.2%; skin: 16.2% |
43.2% | 40% | 13.5% | |||||||||||
| 43 | Mandibular ramus | Higher paine |
Negative pulp sensitivity: 3%f; root canal treatment: 0%g |
Mucosa: 0%; skin: 11.6% |
41.9% | 16%h | 2.3%i | |||||||
| Altiparmak et al. [31] | 2015 | CT | 44 | Chin | 6 months | VAS: | NR | NR |
Negative pulp sensitivity: 13.8%; root canal treatment: 1.4% |
PBT | TPDT | PBT | TPDT | NR |
| 1.5 (0 - 5.8) |
Mucosa: 43.2%; skin: 13.6% |
Mucosa: 34.1%; skin: 13.6% |
Mucosa: 0%; skin: 0% |
|||||||||||
| 31 | Mandibular ramus | 1.3 (0 - 4)k |
Negative pulp sensitivity: 13.3%l; root canal treatment: 0%m |
Mucosa: 9.7%n; skin: 12.9% |
Mucosa: 16.1%; skin: 0% |
|||||||||
| Ersanli et al. [32] | 2016 | CT | 18 | Chin | 12 months | NR | 13% | 13% | 13% | NR | NR | NR | ||
| 14 | Mandibular ramus | 9% | 18% | 0% | ||||||||||
| Pereira et al. [33] | 2019 | CT | 29 | Chin | 12 months | 5.6% | NR | NR | 1.9% | Self-administered questionnaire |
Satisfied with treatment: 91%; recommend the procedure: 91% |
|||
| 62.1% | 13.8%o | |||||||||||||
| 28 | Mandibular ramus | 35.7% | 3.5%p | |||||||||||
aStatistically significant at level P < 0.05 (Student t-test); bstatistically significant at level P = 0.002 (Mann-Whitney test); cstatistically significant at level P = 0.006 (Mann-Whitney test); dstatistically significant at level P = 0.027 (Mann-Whitney test); estatistically significant at level P = 0.003 (Mann-Whitney test); f,gstatistically significant at level P < 0.001 (Mann-Whitney test); hstatistically significant at level P = 0.03 (Chi-squared test); istatistically non-significant at level P > 0.05 (Mann-Whitney Test); jstatistically significant at level P = 0.004 (Chi-squared test); kstatistically non-significant at level P = 0.862 (Mann-Whitney test); lstatistically non-significant at level P = 1 (Fisher´s exact test); mstatistically non-significant at level P = 1 (continuity corrected Chi-squared test); nstatistically significant at P = 0.004 (Mann-Whitney test); ostatistically significant at level P < 0.05 (Mann-Whitney test); pstatistically significant at level P < 0.05 (Mann-Whitney test).
CT = controlled trial; IAN = inferior alveolar nerve; NR = not reported; PBT = pointed-blunt test; RS = retrospective study; SD = standard deviation; TPDT = two-point discrimination test (threshold values of 7 mm and 11 mm); VAS = visual analogue scale.
Pain
No difference in pain following harvesting of autogenous bone graft from the chin region or mandibular ramus was reported in one study [25]. No statistical analysis was conducted [25].
Intensity and duration of pain as well as need for analgesics were more pronounced following harvesting of autogenous bone graft from the chin region compared with the mandibular ramus as evaluated by questionnaire [26]. No statistical analysis was conducted [26].
Prolonged pain was experienced by 33%, 20%, and 20% following harvesting of autogenous bone graft from the chin region, mandibular ramus or mandibular ramus including removal of third molar [28]. No statistical analysis was conducted [28].
Visual analogue scale score of pain was 3.8 and 1.8 following harvesting of autogenous bone graft from the chin region compared with the mandibular ramus after one week, respectively [29]. The difference was statistically significant (P = 0.002) [29].
Increased pain during chewing was reported after harvesting of autogenous bone graft from the mandibular ramus compared with the chin region [30]. The difference was statistically significant (P = 0.003) [30].
Visual analogue scale score of pain was 1.5 (range 0 to 5.8) and 1.3 (range 0 to 4) following harvesting of autogenous bone graft from the chin region and mandibular ramus, respectively [31]. The difference was not statistically significant (P = 0.862) [31].
Pain was reported in 5.6% of the patients, without differentiating between harvesting of autogenous bone graft from the chin region or mandibular ramus [33].
Pain was not reported in three studies [24,27,32].
Summary
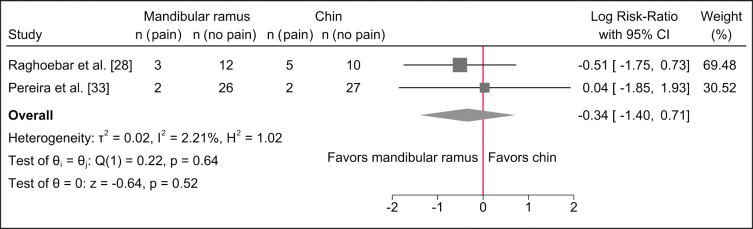
There seems to be a tendency for higher pain score, prolonged period of pain as well as increased need for analgesics following harvesting of autogenous bone graft from the chin region compared with the ascending mandibular ramus as evaluated by questionnaire and VAS, although pain during chewing was significantly higher following harvesting from the mandibular ramus.
However, meta-analysis revealed no statistically significant differences between the two treatment modalities (Figure 2).
Figure 2.
Random-effects meta-analyses using Sidik-Jonkman estimation method demonstrated no statistically significant differences in pain with the two treatment modalities.
Infection
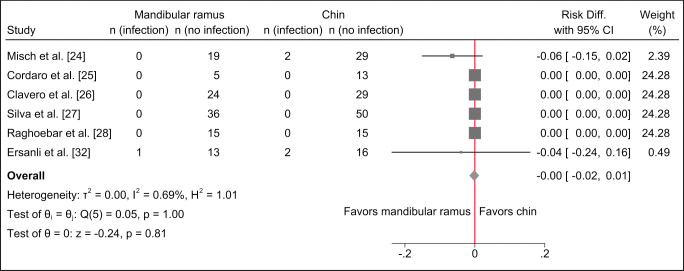
No difference in infection following harvesting of autogenous bone graft from the chin region or mandibular ramus was reported in four studies [25-28].
Infection following harvesting of autogenous bone graft from the chin region compared with the mandibular ramus was 6% and 0%, respectively [24]. No statistical analysis was conducted [24].
Infection following harvesting of autogenous bone graft from the chin region compared with the mandibular ramus was 13% and 9%, respectively [32]. No statistical analysis was conducted [32].
Infection was not reported in four studies [29-31,33].
Summary
Risk of infection seems to be comparable with the two treatment modalities and meta-analysis revealed no statistically significant differences (Figure 3).
Figure 3.
Random-effects meta-analyses using Sidik-Jonkman estimation method demonstrated no statistically significant differences in infection with the two treatment modalities.
Mucosal dehiscence
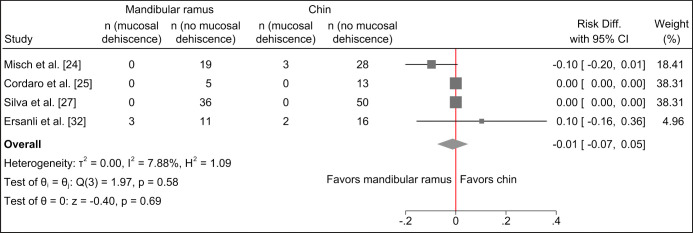
No difference in mucosal dehiscence following harvesting of autogenous bone graft from the chin region or mandibular ramus was reported in two studies [25,27].
Mucosal dehiscence following harvesting of autogenous bone graft from the chin region compared with the mandibular ramus was 11% and 0%, respectively [24]. No statistical analysis was conducted [24].
Mucosal dehiscence following harvesting of autogenous bone graft from the chin region compared with the mandibular ramus was 13% and 18%, respectively [32]. No statistical analysis was conducted [32].
Mucosal dehiscence was not reported in six studies [26,28-31,33].
Summary
Risk of mucosal dehiscence seems to be comparable with the two treatment modalities and meta-analysis revealed no statistically significant differences (Figure 4).
Figure 4.
Random-effects meta-analyses using Sidik-Jonkman estimation method demonstrated no statistically significant differences in mucosal dehiscence with the two treatment modalities.
Altered sensation or vitality of adjacent tooth/teeth
Altered sensation of the lower incisors was reported by 29% of the patients following harvesting of autogenous bone graft from the chin region, whereas no altered sensation of the adjacent teeth was reported after harvesting from the mandibular ramus [24]. No statistical analysis was conducted [24].
Numbness of the lower incisors during chewing was reported by 7% of the patients following harvesting of autogenous bone graft from the chin region, whereas no altered sensation of the adjacent teeth was reported after harvesting from the mandibular ramus [25]. No statistical analysis was conducted [25].
Altered sensation of the lower incisors with vital pulp response to cold was reported by 13% of the patients following harvesting of autogenous bone graft from the chin region, whereas no altered sensation of the adjacent teeth was reported after harvesting from the mandibular ramus with or without removal of third molar [28]. No statistical analysis was conducted [28].
A heightened sensitivity to cold temperatures was reported by 12.5% of the patients following harvesting of autogenous bone graft from the chin region, whereas no altered sensation of the adjacent teeth was reported after harvesting from the mandibular ramus [29]. No statistical analysis was conducted [29].
Negative pulp sensitivity test was measured in 13% and 3% of the adjacent teeth following harvesting of autogenous bone graft from the chin region or mandibular ramus [30]. Radiographic examination revealed that 0.7% of the lower incisors were treated endodontically after harvesting of autogenous bone from the chin region, whereas no teeth required root canal treatment after harvesting from the mandibular ramus. The difference between chin region and mandibular ramus was statistically significant (P < 0.001) [30].
Negative pulp sensitivity test was measured in 13.8% and 13.3% of the adjacent teeth following harvesting of autogenous bone graft from the chin region or mandibular ramus [31]. The difference was not statistically significant (P = 1). Root canal treatment was needed in 1.4% of the lower incisors after harvesting of autogenous bone from the chin region, whereas no teeth required root canal treatment after harvesting from the mandibular ramus. The difference was not statistically significant (P = 1) [31].
Numbness of the adjacent teeth was reported by 13% and 0% of the patients following harvesting of autogenous bone graft from the chin region compared with the mandibular ramus, respectively [32]. No statistical analysis was conducted [32].
Dental hypersensitivity was reported in 1.9% of the patients, without differentiating between harvesting of autogenous bone from the chin region or mandibular ramus [33].
Altered sensation or vitality of adjacent tooth/teeth was not reported in two studies [26,27].
Summary
Harvesting of autogenous bone graft from the chin region seems to be associated with significantly higher risk of altered sensation, numbness, heightened sensitivity or loss of tooth vitality of adjacent teeth compared with harvesting from the mandibular ramus as evaluated by verbal response, electric pulp test, cold vitality with carbon dioxide snow, response to cold and radiographic examination.
Neurosensory disturbances of the inferior alveolar nerve or vestibular area
No transient or permanent neurosensory disturbances of the inferior alveolar nerve were reported in one study following harvesting of autogenous bone graft from the chin region or mandibular ramus [25].
Transient neurosensory disturbances of the inferior alveolar nerve following harvesting of autogenous bone graft from the chin region compared with the mandibular ramus were 9.6% and 0% after 4 to 6 months, respectively [24]. No statistical analysis was conducted [24].
Transient neurosensory disturbances in the mental and lower lip area were reported by 76% of the patients using questionnaire following harvesting of autogenous bone graft from the chin region, whereas altered sensation in the posterior vestibular area was expressed by 21% after harvesting from the mandibular ramus, respectively [26]. Permanent neurosensory disturbances were reported by 52% and 4% following harvesting of autogenous bone graft from the chin region or mandibular ramus after 18 months, respectively. No statistical analysis was conducted [26].
Neurosensory disturbances in the mental and lower lip area were 16% and 8.3% as evaluated by medical records following harvesting of autogenous bone graft from the chin region or mandibular ramus after 120 days, respectively [27]. No statistical analysis was conducted [24].
Transient neurosensory disturbance of the inferior alveolar nerve was 40% and 7% following harvesting of autogenous bone graft from the chin region or the mandibular ramus with or without third molar removal, respectively [28]. Permanent neurosensory disturbances of the inferior alveolar nerve were 20% and 0% following harvesting from the chin region or the mandibular ramus with or without third molar removal, respectively. No statistical analysis was conducted [28].
Neurosensory disturbance involving numbness or changed sensation at the donor site were 33% and 0% as evaluated by dichotomous questions following harvesting of autogenous bone graft from the chin region or mandibular ramus after 3 to 5 years, respectively [29]. No statistical analysis was conducted [29].
Pointed-blunt test revealed that 16.2% and 16.2% suffered from hyperesthesia, hypoesthesia, paraesthesia, and anaesthesia of the skin and oral mucosa following harvesting of bone graft from the chin region, respectively [30]. Corresponding measurements were 11.6% and 0% for mandibular ramus. The difference was statistically significant (P = 0.001). Two-points discrimination test was 56.8% (< 7 mm), 43.2% (between 7 and 11 mm), and 0% (> 11 mm) following harvesting of bone graft from the chin region. Corresponding measurements were 58.1%, 41.9%, and 0% for mandibular ramus. The difference was not significant (P = 0.9). Transient and permanent neurosensory disturbances were reported by 40% and 13.5% of the patients following harvesting of bone graft from the chin region. Corresponding measurements were 16% and 2.3% for mandibular ramus. The difference was statistically significant (P = 0.03) [30].
Pointed-blunt test revealed that 13.6% and 43.2% of the patients suffered from transient paraesthesia of the skin and oral mucosa following harvesting of bone graft from the chin region [31]. Corresponding measurements were 12.9% and 9.7% for mandibular ramus. The difference was statistically significant (P = 0.004). Two-point discrimination test of the skin was 86.4% (< 7 mm), 13.6% (between 7 and 11 mm), and 0% (>11 mm) following harvesting of bone graft from the chin region. Corresponding measurements were 100%, 0%, and 0% for mandibular ramus. The difference was not significant (P = 0.039). Two-point discrimination test of the mucosa was 65.9% (< 7 mm), 34.1% (between 7 and 11 mm), and 0% (> 11 mm) following harvesting of bone graft from the chin region. Corresponding measurements were 83.9%, 16.1%, and 0% for mandibular ramus. The difference was not significant (P = 0.142).
Pointed blunt test and two-point discrimination test revealed no permanent neurosensory disturbances with the two treatment modalities [31].
Percentage of transient and permanent neurosensory disturbances following harvesting of autogenous bone graft from the chin region were 62.1% and 13.8% [33]. Corresponding measurements for the mandibular ramus were 35.7% and 3.5%. The difference was statistically significant (P < 0.05) [33].
Neurosensory disturbances of the inferior alveolar nerve or vestibular area was not reported in one study [32].
Summary
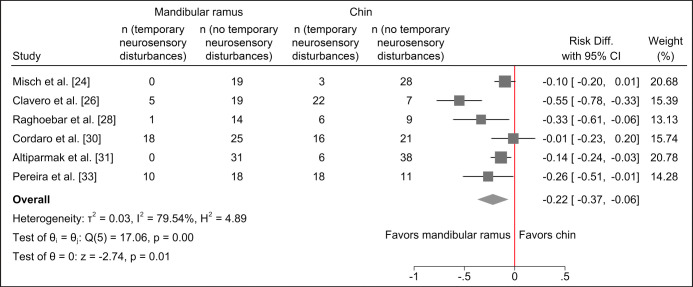
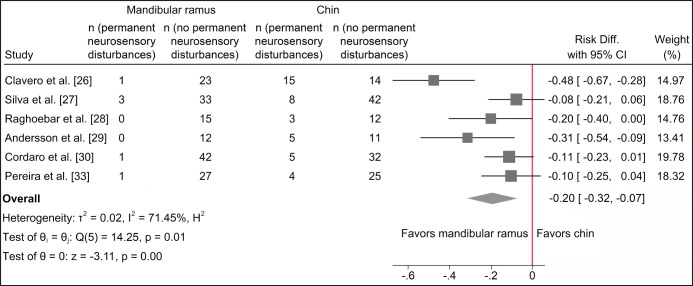
Harvesting of autogenous bone graft from the chin region seems to be associated with a statistically significantly higher risk of transient and permanent neurosensory disturbances of the inferior alveolar nerve compared with harvesting from the mandibular ramus as evaluated by questionnaire, medical records, point-blunt test and two-point discrimination test (Figure 5 and 6).
Figure 5.
Random-effects meta-analyses using Sidik-Jonkman estimation method demonstrated a statistically significant higher risk of temporary neurosensory disturbances following harvesting of autogenous bone graft from the chin region.
Figure 6.
Random-effects meta-analyses using Sidik-Jonkman estimation method demonstrated a statistically significant higher risk of permanent neurosensory disturbances following harvesting of autogenous bone graft from the chin region.
Patient-reported outcome measures
PROM as evaluated by questionnaire revealed that 91% of the patients felt that the treatment involving harvesting of autogenous bone graft from the chin region and mandibular ramus had met their pre-treatment expectations, and 94% stated that they would consider undergoing the same treatment again, if necessary [26].
PROM as evaluated by questionnaire revealed that all patients would be willing to repeat the procedure when necessary [28]. However, the acceptance of the surgical procedure was statistically significantly higher after harvesting of mandibular ramus bone graft including third molar removal compared with mandibular ramus bone without third molar removal or the chin region (P < 0.05) [28].
Significant lower discomfort (P = 0.006) and higher satisfaction (P = 0.027) were reported after harvesting of autogenous bone graft from the mandibular ramus compared with the chin region as evaluated by interview after 3 to 5 years [29].
Patient´s perception of morbidity following harvesting of autogenous bone from the chin region or mandibular ramus did not differ significantly (P > 0.05) [30].
PROM was not reported in five studies [24,25,27,31,32].
Summary
Willingness to undergo the same treatment again was reported with both treatment modalities as evaluated by questionnaire. However, harvesting of autogenous bone graft from the ascending mandibular ramus was associated with significant higher satisfaction, lower discomfort and acceptance of the surgical procedure compared with harvesting from the chin region.
DISCUSSION
The objective of the present systematic review was to test the hypothesis of no difference in complications and donor site morbidity following harvesting of autogenous bone graft from the ascending mandibular ramus compared with the chin region. No randomized controlled trials were identified, but ten controlled trials of high-quality fulfilled the inclusion criteria. Risk of infection and mucosal dehiscence seems to be comparable with the two treatment modalities. However, harvesting of autogenous bone graft from the chin region seems to be associated with increased risk of postoperative pain, altered sensation or loss of tooth vitality, as well as transient and permanent neurosensory disturbances of the oral mucosa and skin. Willingness to undergo the same treatment again was reported with both treatment modalities, but significant higher satisfaction, lower discomfort and acceptance of the surgical procedure was reported following harvesting of autogenous bone graft from the ascending mandibular ramus. The hypothesis was therefore rejected due to a significant higher prevalence and severity of complications and donor site morbidity following harvesting of autogenous bone graft from the chin region compared with the ascending mandibular ramus. However, dissimilar assessment methods and methodological confounding factors posed serious restrictions for literature review in a quantitative systematic manner. Conclusions drawn from results of this systematic review should therefore be interpreted with caution.
Postoperative complications are unanticipated or unfavourable outcome following a surgical intervention and the severity of a complication is usually associated with the type of surgery. Pain, swelling, bruising, bleeding, limited mouth opening, infection, and mucosal dehiscence are common sequelae following harvesting of intraoral autogenous bone graft [7,17], which is normally treated sufficiently with pharmacological therapies, cryotherapy, compression or drain of the infected area. The incidence of pain, infection, and mucosal dehiscence following harvesting of autogenous bone graft from the ascending mandibular ramus was compared with the chin region in the present systematic review revealing comparable outcome [24-33]. Smoking, increasing age, poor oral hygiene, and history of periodontitis are well-known risk factors for development of postoperative complications following harvesting of intraoral autogenous bone graft [37]. However, correlation between smoking habits, age, oral hygiene or reason for tooth loss and postoperative complications were not conducted in any of the included studies of the present systematic review [24-33].
Altered sensation, numbness or endodontic therapy of teeth adjacent to the donor site as well as permanent neurosensory disturbances of the skin and oral mucosa area are irreversible complications arising from harvesting of intraoral autogenous bone graft, which may cause dissatisfaction, severe discomfort, and impaired oral health-related quality of life [7,17,36]. Loss of pulp sensitivity and apical pathology of the anterior lower teeth following harvesting of autogenous bone graft from the chin region is a well-known complication [6,7,17]. Previous studies have revealed transient and permanent negative pulp sensitivity of 80% and 3 - 12% following harvesting from the chin region [38-42], whereas temporary and permanent changes in pulp sensitivity following harvesting of autogenous bone graft from the ascending mandibular ramus are seldom reported [5,18,43]. These results are in accordance with the results of the present systematic review disclosing a significant higher incidence of negative pulp sensitivity and loss of tooth vitality following harvesting of autogenous bone graft from the chin region compared with the ascending mandibular ramus. Consequently, a safety margin of at least 8 mm below the tooth apices with a maximum harvest depth of 4 mm have been suggested to diminish the risk of altered tooth sensitivity/vitality following harvesting of autogenous bone graft from the chin region [44].
Transient and permanent neurosensory disturbances of the skin and oral mucosa following harvesting of autogenous bone graft from the ascending mandibular ramus and chin region have previous been compared in systematic reviews [7,17]. A significant higher incidence of transient and permanent neurosensory disturbances of the skin and oral mucosa are related to harvesting of autogenous bone graft from the chin, which is in accordance with the results of the present systematic review [7,17]. Self-administrated questionnaires as well as quantitative and semiquantitative sensory test are the most commonly used subjective tests for assessment of neurosensory disturbances of the skin and oral mucosa. In the present systematic review, transient and permanent neurosensory disturbances of the skin and oral mucosa were assessed by verbal response, questionnaire, medical records, interview, pointed-blunt test, and two-point discrimination test at dissimilar time points [24-31,33]. The absence of a standardized terminology, uniform subjective tests, and length of observation period influence the results and conclusions of the present systematic review [24-31,33]. Moreover, risk of transient and permanent neurosensory disturbances of the skin and oral mucosa following harvesting of intraoral autogenous bone graft are strongly related to age, gender and intraoperative injury of the nerve, but none of the included studies reported percentage of intraoperative injury or visibility of the inferior alveolar nerve or correlated transient and permanent neurosensory disturbances of the skin or oral mucosa with age and gender [24-31,33]. Further randomized controlled trials assessing complications and donor site morbidity following harvesting of autogenous bone graft from the ascending mandibular ramus and chin region should therefore include validated subjective tests as well as correlation of complications with age and gender.
Numbness or altered sensation in the lower lip, chin and oral mucosa can compromise normal oral function and influence oral health-related quality of life due to the distorted perception of touch and thermal stimulus, which can change the ability to eat, drink, speak, kiss, and smile. However, a previous study assessing patients’ perceptions of alterations in facial aesthetics, eating, speaking, and lower lip movement following harvesting of autogenous bone graft from the chin region revealed that the preoperative VAS score was unaffected after one year [45]. Harvesting of intraoral autogenous bone graft for alveolar ridge augmentation of localized defects are generally accepted by patients, if it is necessary to allow placing implants [7,35]. In the present systematic review, willingness to undergo the same treatment again was reported with both treatment modalities, but significant higher satisfaction, lower discomfort and acceptance of the surgical procedure was reported following harvesting of autogenous bone graft from the ascending mandibular ramus compared with the chin region [26,28,29]. A previous study assessing PROM using questionnaire and interviews revealed that harvesting of autogenous bone graft from the ascending mandibular ramus was preferred by patients compared with harvesting from the chin region [35]. Patients concerns of complications and donor site morbidity are important criteria for selection of a specific donor site in elective preprosthetic surgery and minimally invasive treatment alternatives are generally preferred [7,35]. Thus, evidence-based information about risk of potential complications and donor site morbidity following harvesting of intraoral autogenous bone graft must be provided to guide patients in the choice of the most appropriate treatment option.
CONCLUSIONS
The hypothesis of no difference in complications or donor site morbidity following harvesting of autogenous bone graft from the ascending mandibular ramus compared with the chin region is rejected since a higher prevalence and severity of complications and donor site morbidity were observed when autogenous bone graft was harvested from the chin region. However, dissimilar evaluation methods and various methodological confounding factors posed serious restrictions for literature review in a quantitative systematic manner. Hence, conclusions drawn from results of this systematic review should be interpreted with caution.
Acknowledgments
ACKNOWLEDGMENTS AND DISCLOSURE STATEMENTS
The authors declare that there are no financial or other conflicts of interest related to this publication. The authors would like to give a special thanks to Jette Frost Jepsen (Medical Library, Aalborg University Hospital, Aalborg, Denmark) for her assistance with the search strategy. There were no sources of funding for this systematic review.
APPENDIX 1
Adobe PDF File
APPENDIX 2
Adobe PDF File
APPENDIX 3
Adobe PDF File
APPENDIX 4
Adobe PDF File
REFERENCES
- 1.Aludden HC, Mordenfeld A, Hallman M, Dahlin C, Jensen T. Lateral ridge augmentation with Bio-Oss alone or Bio-Oss mixed with particulate autogenous bone graft: a systematic review. Int J Oral Maxillofac Surg. 2017 Aug;46(8): 1030-1038. [DOI] [PubMed]
- 2.Troeltzsch M, Troeltzsch M, Kauffmann P, Gruber R, Brockmeyer P, Moser N, Rau A, Schliephake H. Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J Craniomaxillofac Surg. 2016 Oct;44(10): 1618-1629. [DOI] [PubMed]
- 3.Sanz-Sánchez I, Ortiz-Vigón A, Sanz-Martín I, Figuero E, Sanz M. Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review and Meta-analysis. J Dent Res. 2015 Sep;94(9 Suppl):128S-42S. [DOI] [PubMed]
- 4.Sakkas A, Wilde F, Heufelder M, Winter K, Schramm A. Autogenous bone grafts in oral implantology-is it still a "gold standard"? A consecutive review of 279 patients with 456 clinical procedures. Int J Implant Dent. 2017 Dec;3(1):23. [DOI] [PMC free article] [PubMed]
- 5.Carlsen A, Gorst-Rasmussen A, Jensen T. Donor site morbidity associated with autogenous bone harvesting from the ascending mandibular ramus. Implant Dent. 2013 Oct;22(5):503-6. [DOI] [PubMed]
- 6.Weibull L, Widmark G, Ivanoff CJ, Borg E, Rasmusson L. Morbidity after chin bone harvesting--a retrospective long-term follow-up study. Clin Implant Dent Relat Res. 2009 Jun;11(2):149-57. [DOI] [PubMed]
- 7.Nkenke E, Neukam FW. Autogenous bone harvesting and grafting in advanced jaw resorption: morbidity, resorption and implant survival. Eur J Oral Implantol. 2014 Summer;7 Suppl 2:S203-17. [PubMed]
- 8.Cricchio G, Lundgren S. Donor site morbidity in two different approaches to anterior iliac crest bone harvesting. Clin Implant Dent Relat Res. 2003;5(3):161-9. [DOI] [PubMed]
- 9.Scheerlinck LM, Muradin MS, van der Bilt A, Meijer GJ, Koole R, Van Cann EM. Donor site complications in bone grafting: comparison of iliac crest, calvarial, and mandibular ramus bone. Int J Oral Maxillofac Implants. 2013 Jan-Feb;28(1):222-7. [DOI] [PubMed]
- 10.Falkensammer N, Kirmeier R, Arnetzl C, Wildburger A, Eskici A, Jakse N. Modified iliac bone harvesting--morbidity and patients' experience. J Oral Maxillofac Surg. 2009 Aug;67(8):1700-5. [DOI] [PubMed]
- 11.Jakoi AM, Iorio JA, Cahill PJ. Autologous bone graft harvesting: a review of grafts and surgical techniques. Musculoskelet Surg. 2015 Dec;99(3):171-8. [DOI] [PubMed]
- 12.Yates DM, Brockhoff HC 2nd, Finn R, Phillips C. Comparison of intraoral harvest sites for Yates DM, Brockhoff HC 2nd, Finn R, Phillips C. Comparison of intraoral harvest sites for corticocancellous bone grafts. J Oral Maxillofac Surg. 2013 Mar;71(3):497-504. [DOI] [PubMed]
- 13.Sittitavornwong S, Gutta R. Bone graft harvesting from regional sites. Oral Maxillofac Surg Clin North Am. 2010 Aug;22(3):317-30, v-vi. [DOI] [PubMed]
- 14.Aloy-Prósper A, Peñarrocha-Oltra D, Peñarrocha-Diago M, Peñarrocha-Diago M. The outcome of intraoral onlay block bone grafts on alveolar ridge augmentations: a systematic review. Med Oral Patol Oral Cir Bucal. 2015 Mar 1;20(2): e251-8. [DOI] [PMC free article] [PubMed]
- 15.Kamal M, Gremse F, Rosenhain S, Bartella AK, Hölzle F, Kessler P, Lethaus B. Comparison of Bone Grafts From Various Donor Sites in Human Bone Specimens. J Craniofac Surg. 2018 Sep;29(6):1661-1665. [DOI] [PubMed]
- 16.Zeltner M, Flückiger LB, Hämmerle CH, Hüsler J, Benic GI. Volumetric analysis of chin and mandibular retromolar region as donor sites for cortico-cancellous bone blocks. Clin Oral Implants Res. 2016 Aug;27(8):999-1004. [DOI] [PubMed]
- 17.Reininger D, Cobo-Vázquez C, Monteserín-Matesanz M, López-Quiles J. Complications in the use of the mandibular body, ramus and symphysis as donor sites in bone graft surgery. A systematic review. Med Oral Patol Oral Cir Bucal. 2016 Mar 1;21(2):e241-9. [DOI] [PMC free article] [PubMed]
- 18.Khoury F, Hanser T. Mandibular bone block harvesting from the retromolar region: a 10-year prospective clinical study. Int J Oral Maxillofac Implants. 2015 May-Jun;30(3):688-97. [DOI] [PubMed]
- 19.Chiapasco M, Casentini P, Zaniboni M. Bone augmentation procedures in implant dentistry. Int J Oral Maxillofac Implants. 2009;24 Suppl:237-59. [PubMed]
- 20.Welch V, Petticrew M, Tugwell P, Moher D, O'Neill J, Waters E, White H. PRISMA-Equity Bellagio group. PRISMA-Equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity. PLoS Med. 2012;9(10):e1001333. [DOI] [PMC free article] [PubMed]
- 21.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration. 2011. URL: http://handbook.cochrane.org/
- 22.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011 Oct 18;343:d5928. [DOI] [PMC free article] [PubMed]
- 23.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 24.Misch CM. Comparison of intraoral donor sites for onlay grafting prior to implant placement. Int J Oral Maxillofac Implants. 1997 Nov-Dec;12(6):767-76. [PubMed]
- 25.Cordaro L, Amadé DS, Cordaro M. Clinical results of alveolar ridge augmentation with mandibular block bone grafts in partially edentulous patients prior to implant placement. Clin Oral Implants Res. 2002 Feb;13(1):103-11. [DOI] [PubMed]
- 26.Clavero J, Lundgren S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin Implant Dent Relat Res. 2003;5(3):154-60. [DOI] [PubMed]
- 27.Silva FM, Cortez AL, Moreira RW, Mazzonetto R. Complications of intraoral donor site for bone grafting prior to implant placement. Implant Dent. 2006 Dec;15(4):420-6. [DOI] [PubMed]
- 28.Raghoebar GM, Meijndert L, Kalk WW, Vissink A. Morbidity of mandibular bone harvesting: a comparative study. Int J Oral Maxillofac Implants. 2007 May-Jun;22(3):359-65. [PubMed]
- 29.Andersson L. Patient self-evaluation of intra-oral bone grafting treatment to the maxillary frontal region. Dent Traumatol. 2008 Apr;24(2):164-9. [DOI] [PubMed]
- 30.Cordaro L, Torsello F, Miuccio MT, di Torresanto VM, Eliopoulos D. Mandibular bone harvesting for alveolar reconstruction and implant placement: subjective and objective cross-sectional evaluation of donor and recipient site up to 4 years. Clin Oral Implants Res. 2011 Nov;22(11):1320-6. [DOI] [PubMed]
- 31.Altiparmak N, Soydan SS, Uckan S. The effect of conventional surgery and piezoelectric surgery bone harvesting techniques on the donor site morbidity of the mandibular ramus and symphysis. Int J Oral Maxillofac Surg. 2015 Sep;44(9):1131-7. [DOI] [PubMed]
- 32.Ersanli S, Arısan V, Bedeloğlu E. Evaluation of the autogenous bone block transfer for dental implant placement: Symphysal or ramus harvesting? BMC Oral Health. 2016 Jan 26;16:4. [DOI] [PMC free article] [PubMed]
- 33.Pereira RS, Pavelski MD, Griza GL, Boos FBJD, Hochuli-Vieira E. Prospective evaluation of morbidity in patients who underwent autogenous bone-graft harvesting from the mandibular symphysis and retromolar regions. Clin Implant Dent Relat Res. 2019 Aug;21(4):753-757. [DOI] [PubMed]
- 34.Stübinger S, Nuss K, Landes C, von Rechenberg B, Sader R. Harvesting of intraoral autogenous block grafts from the chin and ramus region: preliminary results with a variable square pulse Er:YAG laser. Lasers Surg Med. 2008 Jul;40(5):312-8. [DOI] [PubMed]
- 35.Hof M, Tepper G, Semo B, Arnhart C, Watzek G, Pommer B. Patients' perspectives on dental implant and bone graft surgery: questionnaire-based interview survey. Clin Oral Implants Res. 2014 Jan;25(1):42-5. [DOI] [PubMed]
- 36.Reissmann DR, Dietze B, Vogeler M, Schmelzeisen R, Heydecke G. Impact of donor site for bone graft harvesting for dental implants on health-related and oral health-related quality of life. Clin Oral Implants Res. 2013 Jun;24(6):698-705. [DOI] [PubMed]
- 37.Sakkas A, Schramm A, Winter K, Wilde F. Risk factors for post-operative complications after procedures for autologous bone augmentation from different donor sites. J Craniomaxillofac Surg. 2018 Feb;46(2):312-322. [DOI] [PubMed]
- 38.Chiapasco M, Abati S, Romeo E, Vogel G. Clinical outcome of autogenous bone blocks or guided bone regeneration with e-PTFE membranes for the reconstruction of narrow edentulous ridges. Clin Oral Implants Res. 1999 Aug;10(4):278-88. [DOI] [PubMed]
- 39.Nkenke E, Schultze-Mosgau S, Radespiel-Tröger M, Kloss F, Neukam FW. Morbidity of harvesting of chin grafts: a prospective study. Clin Oral Implants Res. 2001 Oct;12(5):495-502. [DOI] [PubMed]
- 40.Joshi A. An investigation of post-operative morbidity following chin graft surgery. Br Dent J. 2004 Feb 28;196(4):215-8; discussion 211. [DOI] [PubMed]
- 41.von Arx T, Häfliger J, Chappuis V. Neurosensory disturbances following bone harvesting in the symphysis: a prospective clinical study. Clin Oral Implants Res. 2005 Aug;16(4):432-9. [DOI] [PubMed]
- 42.Nóia CF, Ortega-Lopes R, Fernandes Moreira RW, Mazzonetto R. Prospective clinical assessment of pulp sensitivity after chin bone harvesting. Implant Dent. 2013 Apr;22(2):199-202. [DOI] [PubMed]
- 43.Nkenke E, Radespiel-Tröger M, Wiltfang J, Schultze-Mosgau S, Winkler G, Neukam FW. Morbidity of harvesting of retromolar bone grafts: a prospective study. Clin Oral Implants Res. 2002 Oct;13(5):514-21. [DOI] [PubMed]
- 44.Pommer B, Tepper G, Gahleitner A, Zechner W, Watzek G. New safety margins for chin bone harvesting based on the course of the mandibular incisive canal in CT. Clin Oral Implants Res. 2008 Dec;19(12):1312-6. [DOI] [PubMed]
- 45.Nóia CF, Ortega-Lopes R, Ricardo de Albergaria Barbosa J, Barbeiro RH, Mazzonetto R. Evaluation of patients' perceptions of alterations after chin bone graft harvesting. Implant Dent. 2012 Oct;21(5):411-4. [DOI] [PubMed]