Abstract
Schizophrenia is a mental illness characterized by symptoms such as hallucinations, delusions, disorganized speech, disorganized or catatonic behavior, and negative symptoms (emotional flatness, apathy, and lack of speech). It causes social and economic burdens to patients and their family. Although etiology of schizophrenia is still uncertain, dopamine dysregulation is traditionally considered as a main etiological factor of schizophrenia, which has been utilized to develop drugs for treating schizophrenia. Recently, inflammation has presented being a risk factor for schizophrenia in that neuroinflammation contributes to the pathophysiology of schizophrenia and the exacerbation of symptom severity. Various factors including diet can regulate inflammatory state. Specific foods or dietary patterns have anti- or pro-inflammatory potentials. Increased levels of pro-inflammatory cytokines and microglia activation have been reported in schizophrenia populations and were related to the pathogenesis of schizophrenia. Omega-3 fatty acids were often recommended to schizophrenia patients because of their anti-inflammatory activities. In this review, we investigate the inflammation-related pathogenesis of schizophrenia and summarize potential nutritional approaches to inhibit the manifestation of symptoms and to alleviate symptom severity using anti-inflammatory nutrients or functional components.
Keywords: Cytokines, Inflammation, Microglia, Omega-3 fatty acids
INTRODUCTION
Schizophrenia is a psychotic disorder with the prevalence of about 20 million people worldwide and has also affected about 450 thousand people in Republic of Korea [1,2]. The symptoms of schizophrenia are divided into positive symptoms (hallucinations, delusions, and racing thoughts, etc.) and negative symptoms (flat affect / inappropriate responses, apathy, and lack of speech). Although the causes of schizophrenia have not been evidently identified, interactions among genetic, environmental, and other psychological factors may involve with the development of schizophrenia [3,4].
The pathogenic mechanism of schizophrenia is not evidently elucidated, but the most leading hypothesis is the one that the altered dopamine levels in brain are involved with the development of schizophrenia [5]. Dopamine levels are high in patients with schizophrenia with the dysregulation of dopamine synthesis and secretion [5]. Also, the role of glutamate dysfunction due to N-methyl-D-aspartate receptor hypofunction has also been suggested to induce symptoms of schizophrenia [6,7]. With dopaminergic and glutamatergic dysfunction, complicated symptoms such as delusion, hallucination, cognitive deficits, and oscillation are followed [6,8]. In addition, patients with schizophrenia suffer from negative symptoms, for example, social isolation and lethargy, as a compensatory response of dopamine overstimulation. Antipsychotic drugs are prescribed as a main treatment that alleviates symptoms, prevents relapse of schizophrenia, and regulates the dopamine level to the normal. However, usage of antipsychotic drugs often has the side effects of increasing appetite and body weights by interfering ghrelin and leptin, so disturbance of dopaminergic neurotransmission may change food intake and increase body weight in schizophrenia population [9,10,11]. Also, schizophrenia patients had poor dietary patterns with more saturated fats, sugar and alcohol as well as less intakes of fish, vegetables, and fruits, which may be related to impaired cognitive function [12,13]. It is assumed that the combination of antipsychotic drugs and poor dietary patterns increases a risk for obesity and metabolic syndrome.
Immune dysregulation and infection have been discussed as risk factors of schizophrenia [14]. In addition, chronic inflammation due to dietary and environmental factors may affect the development of schizophrenia, and aggravate or alleviate symptoms of schizophrenia [15,16]. Therefore, the management of dietary factors is important for schizophrenia patients.
The aim of this review is to investigate the possible inflammatory mechanisms that evoke pathogenesis of schizophrenia and to link dietary inflammation with the risk of schizophrenia. Furthermore, existing evidence for nutritional interventions and strategies that alleviate the symptoms of schizophrenia is discussed.
INFLAMMATION AND SCHIZOPHRENIA
Schizophrenia occurs by interactions between genetic variations and environmental factors, which affect neurotransmission and subsequent behavioral and intellectual changes [3,4]. Dysregulation of neurotransmission systems (e.g., dopaminergic and glutamatergic neurotransmission) has been considered as a primary hypothesis of schizophrenia [5,6,8].
In the central nervous system (CNS), inflammation may be neuroprotective or neurotoxic depending on the interactions among genetic variations, environmental factors, and inflammatory responses [17]. Recent evidence regarding inflammation and schizophrenia suggests that inflammation is a possible risk factor to induce schizophrenia and to aggravate its symptoms [16,18,19,20]. The levels of pro-inflammatory cytokines including interleukin (IL)-6, tumor necrosis factor (TNF)-α, IL-1β, IL-12, and transforming growth factor (TGF)-β were elevated in subjects with schizophrenia [21,22]. Levels of pro-inflammatory cytokines in blood and brain and their relationship are not fully understood because pro-inflammatory cytokines are known to act in a paracrine way and blood brain barrier (BBB) protects brain from the influx of pro-inflammatory cytokines [15]. Nevertheless, excessive inflammatory responses could be a possible pathophysiological mechanism of schizophrenia. Increased microglia activation was observed in schizophrenia population, and activation levels of microglia increase the proportion to the severity of symptoms [23,24,25,26]. Microglia are neuronal support cells of CNS and play a crucial role in mediating neuroinflammation. Microglia induce inflammation in brain by low-level stimuli that let microglia produce pro-inflammatory cytokines [27]. Moreover, inflammation or immune imbalance affect the neurotransmission system. Excesses in pro-inflammatory cytokines inhibit striatal dopamine synthesis and release [28,29].
Increased pro-inflammatory cytokines
Immune responses are categorized as innate and adaptive immunity. Innate immune response is a rapid reaction to injury or damaged cells; however, has relatively low specificity and diversity compared with acquired immunity. On the other hand, acquired immunity recognizes and memorizes the antigen and stimulates memory cells to produce specific antibody. Cytokines are small signaling molecules released from various types of cells and produced as a component of acquired immunity playing a role in communication between immune and neuronal cells. For example, cytokines activate other cells located on distant parts of the body or cytokine-secreting cells themselves to induce differentiation or alteration of activity by autocrine, paracrine, or endocrine manners. Cytokines are categorized further into 5 groups; 1) pro-inflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α are produced by activated macrophages and exacerbate inflammatory responses, 2) T helper 1 cytokines IL-2, IL-12, and interferon (IFN)-γ are involved in cell-mediated immunity, and have a function in protecting cells from parasites inside the cells while T helper 1 cytokines produce pro-inflammatory responses and function in especially autoimmune diseases like multiple sclerosis and rheumatoid arthritis, 3) T helper 2 cytokines IL-4, IL-5, and IL-13 play a role in humoral immunity and defense against parasites and allergic reactions outside the cells, 4) T helper 17 cytokines such as IL-17 and IL-23 are chief mediators of pro-inflammatory processes, and 5) IL-10 and TGF-β are classified as T regulatory cytokines that play a role in suppressing immune responses [22].
There are several hypotheses for the etiology of schizophrenia relating to inflammation. “Inflammation and two-hit hypothesis” for schizophrenia considers genetic susceptibility and inflammatory stimuli (infection and inflammation responses) during prenatal period as a first hit and developmental insults (environmental factors, physical and mental stresses, and endocrine disruption) inducing pro-inflammatory state during adolescence and adulthood as a second hit [30]. Another hypothesis “inflammation and neural diathesis-stress hypothesis” is that inflammation mediates neural damages to the stress-susceptible regions [31]. It is in part overlapped with the inflammation and two-hit hypothesis in that both hypotheses can be explained by first and second hits.
Increases in IL-6 levels are relatively consistent in schizophrenia subjects [32,33,34], which is accompanied by the concomitant upregulation of soluble IL-6 receptor [35]. In addition to IL-6, plasma levels of IL-1β, IL-2, and IFN-γ were higher in schizophrenia subjects compared with controls [34]. In particular, IFN-γ and IL-12 levels were inversely correlated with whole-brain gray matter, and IL-1β levels were positively related to psychotic symptoms assessed by Scale for the Assessment of Positive Symptoms in schizophrenia subjects [34]. And, increases in serum levels of IL-6, IL-18, TNF-α, and soluble IL-2 receptor were reported in subjects with depression and schizophrenia without overt inflammation having high C-reactive protein (CRP) concentrations (CRP > 6 mg/L) [32]. These findings suggest that pro-inflammatory cytokines are significantly stimulated in schizophrenia subjects regardless of obvious inflammation and that anti-inflammatory strategies using diet and drugs may be utilized as a potential adjuvant therapy. In fact, some anti-psychotic drugs (e.g., clozapine, olanzapine, and risperidone) reduced the levels of pro-inflammatory cytokines including IL-8 and IL-12 in first episode and relapse state schizophrenia patients [36,37]. Although there has been inconsistency in alterations of cytokine levels and types of affected cytokines, several pro-inflammatory cytokines were relatively higher in schizophrenia subjects compared with controls, which was suppressed by the treatment of anti-psychotic drugs, resulting in alleviation of symptoms of schizophrenia.
Microglia activation
Microglia are immune cells in CNS, and play a role in mediating neuroinflammation. In normal condition, microglia regulate the homeostasis and resting state of neurons with the purpose of supporting neurons [38]. In a condition of brain damage, microglia rapidly shift into activated state by altering its phenotype, morphology, and even function. Along with these alterations, microglia release pro-inflammatory cytokines such as IL-1β and TNF-α, neurotoxic substances, reactive oxygen species and nitric oxide [39]. Inflammation brings about response of M1 type of microglia, whereas M2 phenotype microglia respond in a way of ameliorating inflammatory processes. It has been indicated that microglial dysfunction has either neuroprotective or neurotoxic capacities depending on which microglia phenotype reacts. In the situation of microglia reacted to M1 phenotype peripheral monocyte, this type of microglia would impair neurons by releasing pro-inflammatory cytokines and lead to neuroinflammation. Neuroinflammation is the mechanism that protects CNS from external damage and infectious agents. As shown by the structural changes of brain including white matter and grey matter loss, it has been received mounting attention that neuroinflammation is a possible mechanism for morphological changes of brain [39].
Microglia are sensitized or primed by low stimuli such as neurodegeneration, aging process and stress [15]. When excessive immune processes begin, additional low-level stimuli let microglia proliferate and produce pro-inflammatory cytokines. Microglia activation-mediated CNS inflammation is one of the key features of various neurodegenerative diseases including Parkinson's and Alzheimer's diseases [40,41]. In post-mortem studies, increased microglia activation and density have been presented in schizophrenia population [24,26,42]. Immunohistochemical analysis showed that microglial cell numbers in anterior cingulate cortex and mediodorsal thalamus were increased in schizophrenia subjects committed suicide compared with controls [43]. Furthermore, elevated microglia activation in grey matter of schizophrenia subjects has demonstrated that it has similar manifestation with neurodegenerative dementia [25,44]. The inflammation and neural diathesis-stress hypothesis considers microglia activation-mediated inflammation as a key component triggering the onset of schizophrenia and aggravation of its symptoms [31].
Anti-inflammatory potential of anti-psychotic drugs
From numerous trials, anti-inflammatory potentials of anti-psychotic drugs on schizophrenia have paid attention [45,45,47,48]. The effects of anti-psychotic drugs on inflammation have been inconsistent because anti-psychotic drugs act in 2 ways on inflammatory processes, direct anti-inflammatory and indirect pro-inflammatory ways on alterations of cytokine levels. For example, risperidone, which is a second generation atypical antipsychotics, decreased the IL-1β and IL-6 levels during the initial weeks of intervention; however, these levels rose again at six-month follow-up [48]. The result of this longitudinal study has indicated that anti-psychotics present both pro- and anti-inflammatory properties. Moreover, studies using anti-psychotic drugs and other medicines with anti-inflammatory properties (minocycline and non-steroidal anti-inflammatory drugs (NSAIDs)) have been conducted to investigate whether inflammation is a potential mechanism of pathophysiology of schizophrenia. Minocycline is a widely prescribed antibiotics that has anti-inflammatory potential on microglia [49,50]. Minocycline can cross BBB by itself so that minocycline is able to act directly on microglia in accordance with improving the symptoms of schizophrenia [51,52,53]. Taking NSAIDs as adjunct to anti-psychotics in a randomized controlled trial showed ameliorative effect of symptom severity of schizophrenia spectrum disorder [54]. With these accumulating evidence, alleviation of inflammation has been suggested as adjunctive approach for treating schizophrenia [55].
DIETARY INFLAMMATION AND SCHIZOPHRENIA
Dietary inflammation
Dietary inflammatory index (DII) used in recent analyses is the literature-derived scoring tool that calculates inflammatory potentials on overall diet [56]. Developing DII involved the processes of reviewing and grading the weight of research articles regarding the effect of food parameters on inflammatory markers. Research articles were weighted by type of study (human, animal, and cell culture studies) and study design (experimental, prospective cohort, case-control, and cross-sectional studies), and the effect was calculated for each food parameter on inflammatory markers including CRP, IL-1β, IL-4, IL-6, IL-10, and TNF-α. After grading articles, DII scores were standardized to world database for the 45 food parameters consisting of nutrients, whole foods and spices based on data from 11 countries [56]. Researches that examine the relationship between DII scores and inflammatory markers have been conducted to validate the DII [57,58]. DII has been proved its validity by linking several inflammatory markers including IL-1β, IL-4, IL-6, IL-10, CRP, and TNF-α. For Korean adult population, validation analysis of the DII scores was conducted by making a comparison between DII scores and high-sensitivity CRP (hs-CRP) concentrations indicating that higher DII scores are positively related to hs-CRP levels [58].
Several case-control studies analyzed the association between DII and risk for diseases such as colorectal cancer, obesity, and metabolic syndrome. It has been demonstrated that the patients' diets with pro-inflammatory potentials increased the risk for the development of above-mentioned diseases compared with controls [59,60,61].
Dietary inflammation and schizophrenia
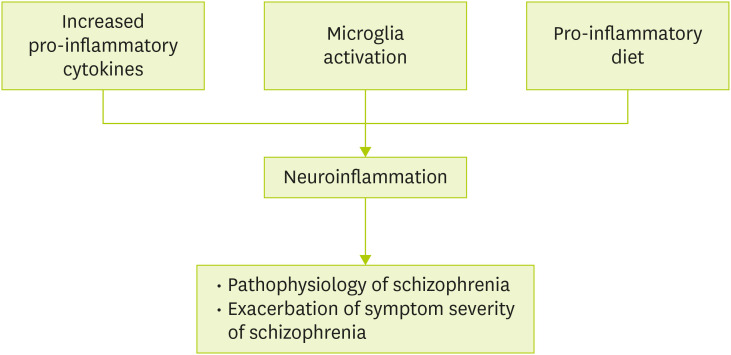
Evidence accumulated from previous studies indicates that inflammation has been noted for the etiology of schizophrenia. In addition to 2 major players in neuroinflammation, pro-inflammatory cytokines and microglia activation, pro-inflammatory diet aggravates neuroinflammation inducing the pathophysiological changes of schizophrenia and exacerbation of its symptom severity (Figure 1). Given the role that diets involve in the regulation of inflammatory markers, nutrients having anti-inflammatory properties such as omega-3 polyunsaturated fatty acids (PUFAs), vitamin D and vitamin B have been emphasized as adjunctive therapeutic means of improving either positive or negative symptoms of schizophrenia [41,62,63].
Figure 1. The suggested roles of inflammation in schizophrenia.
Gut-brain axis should be considered to explain the interaction between dietary inflammation and schizophrenia. Alterations in microbiota composition and microbial metabolites affect gut integrity and immune responses. Gut dysbiosis was observed in patients with schizophrenia, and the abundance of specific bacterial genera, Succinivibrio and Corynebacterium, were significantly associated with the symptom severity of schizophrenia [64]. The gut dysbiosis may affect the susceptibility to infection and inflammation, which can accelerate the development of schizophrenia and aggravate its symptom severity. It is assumed that dietary components considered in schizophrenia exert their effects via, in part, gut-brain axis. However, the direct evidence to link dietary inflammation and schizophrenia in a context of gut-brain axis is limited so far.
A case-control study conducted in Bahrain aimed to examine the association between dietary inflammatory potential and schizophrenia using DII [65]. This study was performed using a 1:1 ratio matched design including 120 cases and 120 controls. Dietary intakes were estimated using a semi-quantitative food frequency questionnaire (FFQ), which can assess frequency and amounts of food consumption over a long period of time. Based on the FFQ results, each case and control's DII and energy-adjusted DII (E-DII) were calculated. The E-DII scores were significantly elevated in cases with schizophrenia compared with controls indicating that schizophrenia patients were more likely to have pro-inflammatory diets. Dietary intakes relevant to pro-inflammatory components such as energy, protein, carbohydrates, total fat, saturated fat, and cholesterol were elevated in 4th quartile of E-DII scores compared to 1st quartile. On the other hand, intakes of fiber, vitamins A and C, β-carotene, folic acid, Mg and Zn known as anti-inflammatory component tended to be increased in the 1st quartile of E-DII.
A population-scale study was conducted to explore the difference between severe mental illness population and general population on diet and inflammation using data from UK Biobank [66]. In this study, total eligible subjects for severe mental illness including schizophrenia, major depressive disorder and bipolar disorder were 69,843, and 262 subjects were classified as schizophrenia population. To compare severe mental illness and general population, 54,010 control data were also selected. Dietary intakes were calculated by computerized 24-hour recall method Oxford WebQ. Schizophrenia population among different types of severe mental illness had the biggest difference in dietary intakes in comparison with general population. Intakes of total energy, carbohydrates, protein, total fat, saturated fat, and sugar which are known as most pro-inflammatory components were higher in schizophrenia subjects compared to controls. Furthermore, statistical significance was observed in the finding that DII scores were increased in schizophrenia population.
Research related to DII and cognitive function was conducted using older Korean adult population [58]. DII and E-DII were calculated based on dietary intakes assessed by 24-hour dietary recall with trained interviewers. Cognitive function was assessed using Korean-adjusted version of the Mini-Mental State Examination (K-MMSE), which is a screening tool that estimates the cognitive status for elderly population. E-DII scores were positively correlated with the K-MMSE scores suggesting that participants classified as normal condition had relatively higher anti-inflammatory diets rather than those who were diagnosed with boundary, mild, and moderate cognitive impairment. To investigate the relationship between DII and metabolic syndrome, the analysis using Health Examinees Cohort Study data from the Korean Genome and Epidemiology Study was conducted [67]. Participants over age of 40 years were selected, and definition of Modified National Cholesterol Education Program Adult Treatment Panel III from American Heart Association and National Heart, Lung, and Blood Institute were used to diagnose metabolic syndrome. It was observed that DII scores were significantly increased in metabolic syndrome group, meaning that participants with metabolic syndrome consume more pro-inflammatory foods than general population. The DII scores were significantly increased as the number of metabolic syndrome components increased, but only in women (p for trend < 0.01). Moreover, Korean version of DII (K-DII) was developed considering that Korean dietary pattern features not only high carbohydrates consumption because of rice, main meal in Korea but also soup or stew and side dishes. K-DII aimed to calculate the inflammatory potentials in Korean dietary pattern and to apply it in clinical settings in South Korea. Validation of K-DII was performed to analyze the association between K-DII and the incidence of metabolic syndrome [68]. The group of higher K-DII had significantly increased risk of metabolic syndrome compared to lower K-DII group after adjusting age and sex (odds ratio, 1.204; 95% confidence interval [CI], 1.123-1.290; p = 0.002) [68]. Above this, DII has been applied to many researches that investigate the correlation between inflammation in diet and diseases such as colorectal cancer and osteosarcopenic obesity in postmenopausal women [69,70].
Efforts to demonstrate the inflammatory potentials in schizophrenia population so far have focused mainly on the alterations of pro-inflammatory cytokine levels. However, few studies have been conducted to investigate the dietary inflammation in schizophrenia population using DII in Korea and even worldwide. Further researches should be carried out how dietary inflammatory potentials elicit the pathogenesis of neuropsychiatric disorders using DII as an indicator of assessing dietary inflammation.
ANTI-INFLAMMATORY NUTRITIONAL INTERVENTION FOR SCHIZOPHRENIA
Omega-3 fatty acids
Nutrients such as omega-3 fatty acids and vitamins having anti-inflammatory properties have been suggested to improve the symptoms of schizophrenia. Omega-3 fatty acids play a pivotal role in inflammation by suppressing of nuclear factor kappa B, which is a regulator of inflammatory responses [71], and exhibit anti-inflammatory potentials by reducing the levels of pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α [72]. PUFAs are main components consisting of phospholipid structure of cell membrane. Structural abnormalities of cell membrane phospholipid can cause many types of mental illness [73]. Many studies have shown that docosahexaenoic acid (DHA) and α-linolenic acid (ALA) levels were reduced in the cell membranes of red blood cells of patients with schizophrenia [73]. Eicosapentaenoic acid (EPA) and DHAs can also be produced from essential fatty acid, ALA. EPA does not exist in the cell membrane of neuronal cells; however, one pilot study has revealed that EPA is superior to DHA in decreasing Positive and Negative Syndrome Scale (PANSS) scores [74]. Improvements in PANSS ratings were significantly correlated with EPA, DHA, and arachidonic acid concentrations in erythrocyte membrane especially in EPA-treated subjects [74]. Omega-3 fatty acids treatment for 12 weeks decreased plasma TNF-α levels which were accompanied by the decline in triglycerides (TG) levels in schizophrenia patients with metabolic syndrome [75]. In a placebo-controlled trials of patients with schizophrenia, PANSS score was reduced in allocated to ethyl-EPA group [76]. In a randomized controlled study, decline in severity of symptoms and relapse rate was observed in young patients with first-episode schizophrenia who were randomly assigned to omega-3 fatty acids treatment for almost 6 months [77]. In addition to the traditional symptoms of schizophrenia, recent findings have focused other complications or side effects of the usage of antipsychotic drugs. Omega-3 fatty acids improved cognitive functions in subjects with schizophrenia and metabolic syndrome, which was accompanied by increased brain-derived neurotrophic factor and reduced levels of pro-inflammatory cytokines CRP, IL-6 and TNF-α [41].
Although ameliorative effects of omega-3 fatty acids for symptoms of schizophrenia have been demonstrated, inconsistent findings also exist. Even relatively high amount (3 g per day) of omega-3 fatty acids for 16 weeks did not show any significant effects on the symptoms of schizophrenia [78]. A possible reason for the inconsistencies in the effects is because of characteristics of the study subjects; 1) patients suffered from schizophrenia over 20 years and had symptoms despite taking new anti-psychotic drugs or clozapine may not respond well to the supplementation of omega-3 fatty acids, and 2) patients who exert the positive effects of EPA may be younger and their duration of schizophrenia may be shorter than those participated in the study with no effects from omega-3 fatty acids supplementations. Nevertheless, findings so far support the notion that omega-3 fatty acids ameliorate symptoms of schizophrenia, in part, by anti-inflammatory potentials (Table 1).
Table 1. Research evidence regarding the effects of omega-3 fatty acids on schizophrenia.
| References | Study design | Treatment | Main findings |
|---|---|---|---|
| [74] | Randomized double-blind placebo-controlled pilot study with 30 new or relapsed schizophrenic patients with no medication | 2 g/day of EPA for 3 mo | • Lower PANSS scores |
| [79] | Randomized placebo-controlled, dose-ranging study, 45 outpatients with persistent schizophrenic symptoms and meeting DSM-IV criteria for schizophrenia, prescribed with antipsychotics (clozapine, typical and atypical drugs) | 1, 2, and 4 g/day of E-EPA for 12 wk | • Improve PANSS scores, positive/negative/general symptoms, and MADRS scores |
| • Positive relationship between rating scales and EPA, DHA, and AA levels in red blood cell membrane | |||
| [78] | Randomized double blind placebo-controlled trial with 87 patients with schizophrenia and schizoaffective disorder with residual symptoms | 3 g/day of E-EPA for 16 wk | • No differences between groups |
| [76] | Randomized, parallel-group, double-blind, placebo-controlled, fixed-dose, add-on study with 40 patients diagnosed with schizophrenia according to DSM-IV criteria | 3 g/day of E-EPA supplementation for 12 wk | • Reduce PANSS scores |
| [77] | Randomized placebo-controlled parallel group add-on, one-center trial with 82 patients with first episode schizophrenia according to ICD-10 | 2.2 g/day of n-3 PUFAs for 26 wk | • Decline in severity of symptoms and relapse |
| [75] | Randomized placebo-controlled trial, 80 patients with both schizophrenia and metabolic syndrome, received olanzapine only for a long period | Fish oil containing 720 mg EPA and 480 mg DHA for 12 wk | • Reduce TG levels |
| • Decrease plasma TNF-α levels | |||
| • Significant correlation between declines in TNF-α and decreases in TG |
DHA, docosahexaenoic acid; DSM, The Diagnostic and Statistical Manual of Mental Disorders; EPA, eicosapentaenoic acid; E-EPA, ethyl-eicosapentaenoic acid; ICD-10, the 10th revision of the International Statistical Classification of Diseases and Related Health Problems; MADRS, Montgomery-Asberg Depression Rating Scale; PANSS, Positive and Negative Syndrome Scale; PUFA, polyunsaturated fatty acid; TG, triglyceride; TNF, tumor necrosis factor.
Vitamins, other nutrients, foods and dietary patterns
Vitamin D is involved with the relationship between inflammation-related markers such as CRP, IL-6, TNF-α, and the occurrence of schizophrenia at adolescence and early life as well as symptom severity from first episode psychosis and chronic state of the disease [80]. Low vitamin D levels or vitamin D deficiency have been observed in schizophrenia population, which may influence brain development and its function [81]. The serum levels of vitamin D had negative correlation with symptom severity of psychosis. The higher the serum vitamin D levels, the lower the total PANSS and PANSS negative symptoms scores [82]. In a randomized, placebo-controlled trial examining the effects of vitamin D on chronic schizophrenia patients [83], 8 weeks of vitamin D supplementation significantly improved serum vitamin D concentrations and mean changes in Montreal Cognitive Assessment scores, which is a sensitive tool to detect cognitive impairment of schizophrenia [84]. Vitamin D supplementation for psychotic patients has been spotlighted because vitamin D is assured its safety, and relatively cheap and accessible rather than other substances. There has been an ongoing study in order to design practical approach and easily apply it to clinical field for first episode psychosis schizophrenia population [85]. In a randomized, placebo-controlled trial conducted in Iran, co-supplementation of vitamin D and probiotics had significant effects on improving total PANSS scores and inflammatory markers, hs-CRP as well as metabolic indicators including fasting plasma glucose, insulin, TG, very low density lipoprotein-cholesterol, and total-cholesterol [86]. This study demonstrated the synergistic potentials of vitamin D and probiotics suggesting that probiotics might improve inflammation via gut-brain axis by modulating gut microbiota.
Vitamin C has anti-oxidative properties that defense against damages from inflammation [87]. Male Korean schizophrenia patients had low dietary intakes of vitamin C, which was associated with increased risk for schizophrenia [88]. A prospective placebo-controlled study of vitamin C treatment (200 mg/day) for 8 weeks reported significant effects on the improvement of ascorbic acid levels and brief psychiatric rating scale (BPRS) score, a tool to promptly estimate and diagnose phase of psychopathology [89]. In addition, co-supplementation of vitamins C, E, and omega-3 fatty acids lowered several psychiatric scores including BPRS, Scale for the Assessment of Negative Symptoms, Simpson Angus Scale, and Barnes Akathisia Rating Scale [90]. Compared with previous studies with omega-3 fatty acids, analyses from interventions studies with vitamin C have focused on indices of oxidative stress rather than inflammatory markers.
Resveratrol (RSV) is a polyphenolic compound existing in grapes, berries, and red wine, and has received attention for anti-inflammatory function. RSV treatment (200 mg per day) for 4 weeks did not lead notable results on 19 male schizophrenia patients, except for the decline in the levels of TG in RSV treatment group [91]. Zortea et al. [92] also investigated the effect of RSV on cognition; however, 1 month of RSV treatment (200 mg/day) did not have any positive results on cognition. There were no differences between RSV and placebo groups on symptoms, inhibitory control, interference measurements, working memory, and attention.
The traditional Mediterranean diet is defined as dietary pattern that has high intakes of olive oil and plant foods including vegetables, fruits, legumes, low to moderate intakes of dairy products, and low intakes of animal foods such as red meat and poultry [93]. In a large-scale prospective survey, the high levels of adherence to Mediterranean diet lead to decline in inflammatory markers including CRP, IL-6, and TNF-α [94]. Attempts to investigate the role of Mediterranean diet on schizophrenia have been tried [95,96]; however, evidence is limited and unclear so far. Ketogenic diet is composed of low-carbohydrates and high-fat, and has got attention because of its effect on weight loss. Ketogenic diet induces the alterations of ratio of neurotransmitters, especially γ-aminobutyric acid (GABA) and glutamate in brain [97]. The altered ratio of GABA and glutamate could be a potent therapeutic mechanism because these neurotransmitters get involved in the regulation of dopamine levels [98]. In addition, ketogenic diet has anti-inflammatory properties in that its pretreatment is associated with the reduced levels of TNF-α in plasma and IL-1β in brain [99]. Dietary Approaches to Stop Hypertension (DASH) diet is originally designed to improve the control of blood pressure. The DASH diet could be described as high intakes of vegetables, fruits, whole grains, poultry and fish as well as low intakes of saturated fat, red meat, sweet beverages, and refined grains. A meta-analysis revealed that the levels of serum hs-CRP, a systemic inflammatory marker, were suppressed by the DASH diet [100]. So far evidence regarding the relationship between specific dietary pattern and schizophrenia has not been yet enough to suggest any dietary guideline linking the inflammation-mediating effects. Representative findings regarding the effects of vitamins, other nutrients, and food components on schizophrenia were summarized in Table 2.
Table 2. Research evidence regarding the effects of specific nutrients and food components on schizophrenia.
| References | Study design | Treatment | Main findings |
|---|---|---|---|
| [89] | Prospective, double-blind, placebo-controlled trial with 40 schizophrenia patients received atypical anti-psychotic drugs including olanzapine, quetiapine, and ziprasidone | 500 mg/day of vitamin C for 8 wk | • Increase ascorbic acid levels and brief psychiatric rating scale scores |
| [91] | Randomized, double-blind controlled trial with 19 male schizophrenia patients | Resveratrol (200 mg/day) for 4 wk | • Decline in TG levels |
| [83] | Randomized, double-blind, placebo-controlled clinical trial with 47 schizophrenia patients who are prescribed with clozapine over 18 weeks, vitamin D levels < 75 nmol/L, and PANSS > 70 | Oral vitamin D (14,000 IU) for 8 wk | • Increase the levels of vitamin D |
| • Tend to improve cognitive function | |||
| • No differences in psychotic, depressive or metabolic indicators | |||
| [86] | Randomized, double-blind, placebo-controlled trial with 60 schizophrenia patients with disease duration over 2 years, PANSS > 50, took chlorpromazine, anti-cholinergic medication at least 6 months | Vitamin D (50,000 IU) and 8 × 109 CFU/day of probiotics for 12 wk | • Improve PANSS scores |
| • Reduce hs-CRP levels | |||
| • Improve metabolic parameters (including fasting plasma glucose, insulin, and TG), and vitamin D levels |
hs-CRP, high-sensitivity C-reactive protein; PANSS, Positive and Negative Syndrome Scale; TG, triglyceride.
CONCLUSION
The association between schizophrenia and inflammation, its possible etiological mechanism, has been supported by findings from epidemiological and experimental studies. Although it would not assert that inflammation is the definite mechanism that leads to schizophrenia, inflammation could be one of possible mechanisms for the onset of schizophrenia and the aggravation of its pathophysiology. The cost for treating schizophrenia is quite high, and many studies report that schizophrenia patients have poor dietary patterns. Schizophrenia patients are also likely to be overweight or obese, or to have metabolic syndrome due to the side effects of anti-psychotic drugs and poor dietary pattern. Therefore, recommendation of anti-inflammatory nutrients or foods as a part of balanced diet is beneficial in the long run to decrease the side effects of medication. Therefore, agents having anti-inflammatory potentials are helpful to ameliorate the symptom severity of diseases and to decrease risks triggering the onset of schizophrenia. Researches investigating the relationship between inflammation and schizophrenia so far performed mainly with tablets or a type of supplements, not with real foods or specific dietary patterns. Therefore, further researches should be conducted using anti-inflammatory nutrients, real foods, or specific dietary patterns. Moreover, considerable efforts should be focused on the identification of underlying mechanisms for the anti-inflammatory effects of specific dietary interventions. Because previous findings were limited to report relationship between a few inflammatory markers and symptoms of schizophrenia, studies to identify underlying mechanisms relating to signaling networks of cytokines and microglia activation needed to identify molecular targets of anti-inflammatory diets or agents.
Footnotes
Funding: This research was supported by research grants from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1F1A1065326) and Seoul Women's University (2020-0227). The funder had no role in study design, data collection, and analysis and interpretation, decision to publish, or preparation of the manuscript.
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho SJ, Kim J, Kang YJ, Lee SY, Seo HY, Park JE, Kim H, Kim KN, Lee JY, Sohn JH. Annual prevalence and incidence of schizophrenia and similar psychotic disorders in the Republic of Korea: a national health insurance data-based study. Psychiatry Investig. 2020;17:61–70. doi: 10.30773/pi.2019.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imamura A, Morimoto Y, Ono S, Kurotaki N, Kanegae S, Yamamoto N, Kinoshita H, Tsujita T, Okazaki Y, Ozawa H. Genetic and environmental factors of schizophrenia and autism spectrum disorder: insights from twin studies. J Neural Transm (Vienna) 2020;127:1501–1515. doi: 10.1007/s00702-020-02188-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21:100. doi: 10.1007/s11920-019-1091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 7.Nakazawa K, Sapkota K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol Ther. 2020;205:107426. doi: 10.1016/j.pharmthera.2019.107426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19:15–33. doi: 10.1002/wps.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Hei GR, Yang Y, Liu CC, Xiao JM, Long YJ, Peng XJ, Yang Y, Zhao JP, Wu RR. Increased appetite plays a key role in olanzapine-induced weight gain in first-episode schizophrenia patients. Front Pharmacol. 2020;11:739. doi: 10.3389/fphar.2020.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manu P, Dima L, Shulman M, Vancampfort D, De Hert M, Correll CU. Weight gain and obesity in schizophrenia: epidemiology, pathobiology, and management. Acta Psychiatr Scand. 2015;132:97–108. doi: 10.1111/acps.12445. [DOI] [PubMed] [Google Scholar]
- 11.Lu ML, Wang TN, Lin TY, Shao WC, Chang SH, Chou JY, Ho YF, Liao YT, Chen VC. Differential effects of olanzapine and clozapine on plasma levels of adipocytokines and total ghrelin. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:47–50. doi: 10.1016/j.pnpbp.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Jakobsen AS, Speyer H, Nørgaard HC, Karlsen M, Hjorthøj C, Krogh J, Mors O, Nordentoft M, Toft U. Dietary patterns and physical activity in people with schizophrenia and increased waist circumference. Schizophr Res. 2018;199:109–115. doi: 10.1016/j.schres.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Speyer H, Christian Brix Nørgaard H, Birk M, Karlsen M, Storch Jakobsen A, Pedersen K, Hjorthøj C, Pisinger C, Gluud C, Mors O, Krogh J, Nordentoft M. The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry. 2016;15:155–165. doi: 10.1002/wps.20318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303–1310. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- 15.Müller N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull. 2018;44:973–982. doi: 10.1093/schbul/sby024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller BJ, Herzig KH, Jokelainen J, Karhu T, Keinänen-Kiukaanniemi S, Järvelin MR, Veijola J, Viinamäki H, Päivikki Tanskanen, Jääskeläinen E, Isohanni M, Timonen M. Inflammation, hippocampal volume, and cognition in schizophrenia: results from the Northern Finland Birth Cohort 1966. Eur Arch Psychiatry Clin Neurosci. 2020 doi: 10.1007/s00406-020-01134-x. [DOI] [PubMed] [Google Scholar]
- 17.Sochocka M, Diniz BS, Leszek J. Inflammatory response in the CNS: friend or foe? Mol Neurobiol. 2017;54:8071–8089. doi: 10.1007/s12035-016-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai HQ, Catts VS, Webster MJ, Galletly C, Liu D, O'Donnell M, Weickert TW, Weickert CS. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25:761–775. doi: 10.1038/s41380-018-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fond G, Lançon C, Korchia T, Auquier P, Boyer L. The role of inflammation in the treatment of schizophrenia. Front Psychiatry. 2020;11:160. doi: 10.3389/fpsyt.2020.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mongan D, Ramesar M, Föcking M, Cannon M, Cotter D. Role of inflammation in the pathogenesis of schizophrenia: a review of the evidence, proposed mechanisms and implications for treatment. Early Interv Psychiatry. 2020;14:385–397. doi: 10.1111/eip.12859. [DOI] [PubMed] [Google Scholar]
- 21.Francesconi LP, Victorino AT, Salah IA, Cordova VH, Dias da Rosa E, Oliveira L, Jacobus RV, Belmonte-de-Abreu PS, Ceresér KM. Proinflammatory and anti-inflammatory biomarkers in schizophrenia and influence of simvastatin on the interleukin-6. Int Clin Psychopharmacol. 2019;34:84–88. doi: 10.1097/YIC.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 22.Momtazmanesh S, Zare-Shahabadi A, Rezaei N. Cytokine alterations in schizophrenia: an updated review. Front Psychiatry. 2019;10:892. doi: 10.3389/fpsyt.2019.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barichello T, Simoes LR, Quevedo J, Zhang XY. Microglial activation and psychotic disorders: evidence from pre-clinical and clinical studies. Curr Top Behav Neurosci. 2020;44:161–205. doi: 10.1007/7854_2018_81. [DOI] [PubMed] [Google Scholar]
- 24.Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol. 2000;59:137–150. doi: 10.1093/jnen/59.2.137. [DOI] [PubMed] [Google Scholar]
- 25.van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Wierzba-Bobrowicz T, Lewandowska E, Lechowicz W, Stepień T, Pasennik E. Quantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol. 2005;43:81–89. [PubMed] [Google Scholar]
- 27.Soulet D, Rivest S. Microglia. Curr Biol. 2008;18:R506–8. doi: 10.1016/j.cub.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 28.Felger JC, Treadway MT. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology. 2017;42:216–241. doi: 10.1038/npp.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treadway MT, Cooper JA, Miller AH. Can't or won't? Immunometabolic constraints on dopaminergic drive. Trends Cogn Sci. 2019;23:435–448. doi: 10.1016/j.tics.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72–93. doi: 10.1016/j.neubiorev.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howes OD, McCutcheon R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry. 2017;7:e1024. doi: 10.1038/tp.2016.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Hakeim HK, Al-Rammahi DA, Al-Dujaili AH. IL-6, IL-18, sIL-2R, and TNFα proinflammatory markers in depression and schizophrenia patients who are free of overt inflammation. J Affect Disord. 2015;182:106–114. doi: 10.1016/j.jad.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 33.Frydecka D, Misiak B, Pawlak-Adamska E, Karabon L, Tomkiewicz A, Sedlaczek P, Kiejna A, Beszłej JA. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265:449–459. doi: 10.1007/s00406-014-0533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lesh TA, Careaga M, Rose DR, McAllister AK, Van de Water J, Carter CS, Ashwood P. Cytokine alterations in first-episode schizophrenia and bipolar disorder: relationships to brain structure and symptoms. J Neuroinflammation. 2018;15:165. doi: 10.1186/s12974-018-1197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartwig FP, Borges MC, Horta BL, Bowden J, Davey Smith G. Inflammatory biomarkers and risk of schizophrenia: a 2-sample mendelian randomization study. JAMA Psychiatry. 2017;74:1226–1233. doi: 10.1001/jamapsychiatry.2017.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He X, Ma Q, Fan Y, Zhao B, Wang W, Zhu F, Ma X, Zhou L. The role of cytokines in predicting the efficacy of acute stage treatment in patients with schizophrenia. Neuropsychiatr Dis Treat. 2020;16:191–199. doi: 10.2147/NDT.S218483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim YK, Suh IB, Kim H, Han CS, Lim CS, Choi SH, Licinio J. The plasma levels of interleukin-12 in schizophrenia, major depression, and bipolar mania: effects of psychotropic drugs. Mol Psychiatry. 2002;7:1107–1114. doi: 10.1038/sj.mp.4001084. [DOI] [PubMed] [Google Scholar]
- 38.Low D, Ginhoux F. Recent advances in the understanding of microglial development and homeostasis. Cell Immunol. 2018;330:68–78. doi: 10.1016/j.cellimm.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Laskaris LE, Di Biase MA, Everall I, Chana G, Christopoulos A, Skafidas E, Cropley VL, Pantelis C. Microglial activation and progressive brain changes in schizophrenia. Br J Pharmacol. 2016;173:666–680. doi: 10.1111/bph.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saitgareeva AR, Bulygin KV, Gareev IF, Beylerli OA, Akhmadeeva LR. The role of microglia in the development of neurodegeneration. Neurol Sci. 2020 doi: 10.1007/s10072-020-04468-5. [DOI] [PubMed] [Google Scholar]
- 41.Tang W, Wang Y, Xu F, Fan W, Zhang Y, Fan K, Wang W, Zhang Y, Zhang C. Omega-3 fatty acids ameliorate cognitive dysfunction in schizophrenia patients with metabolic syndrome. Brain Behav Immun. 2020;88:529–534. doi: 10.1016/j.bbi.2020.04.034. [DOI] [PubMed] [Google Scholar]
- 42.Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- 43.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Cagnin A, Kassiou M, Meikle SR, Banati RB. In vivo evidence for microglial activation in neurodegenerative dementia. Acta Neurol Scand Suppl. 2006;185:107–114. doi: 10.1111/j.1600-0404.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- 45.Azizi E, Zavaran Hosseini A, Soudi S, Noorbala AA. Alteration of serum levels of cytokines in schizophrenic patients before and after treatment with risperidone. Iran J Allergy Asthma Immunol. 2019;18:262–268. doi: 10.18502/ijaai.v18i3.1119. [DOI] [PubMed] [Google Scholar]
- 46.Cazzullo CL, Sacchetti E, Galluzzo A, Panariello A, Adorni A, Pegoraro M, Bosis S, Colombo F, Trabattoni D, Zagliani A, Clerici M. Cytokine profiles in schizophrenic patients treated with risperidone: a 3-month follow-up study. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:33–39. doi: 10.1016/s0278-5846(01)00221-4. [DOI] [PubMed] [Google Scholar]
- 47.Feng Z, Zhang Y, You X, Zhang W, Ma Y, Long Q, Liu Z, Hao W, Zeng Y, Teng Z. Effects of risperidone on blood levels of interleukin-6 in schizophrenia: a meta-analysis. Medicine (Baltimore) 2020;99:e19694. doi: 10.1097/MD.0000000000019694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song X, Fan X, Li X, Zhang W, Gao J, Zhao J, Harrington A, Ziedonis D, Lv L. Changes in pro-inflammatory cytokines and body weight during 6-month risperidone treatment in drug naïve, first-episode schizophrenia. Psychopharmacology (Berl) 2014;231:319–325. doi: 10.1007/s00213-013-3382-4. [DOI] [PubMed] [Google Scholar]
- 49.Elewa HF, Hilali H, Hess DC, Machado LS, Fagan SC. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26:515–521. doi: 10.1592/phco.26.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337–352. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levkovitz Y, Mendlovich S, Riwkes S, Braw Y, Levkovitch-Verbin H, Gal G, Fennig S, Treves I, Kron S. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71:138–149. doi: 10.4088/JCP.08m04666yel. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Zheng H, Wu R, Zhu F, Kosten TR, Zhang XY, Zhao J. Minocycline adjunctive treatment to risperidone for negative symptoms in schizophrenia: association with pro-inflammatory cytokine levels. Prog Neuropsychopharmacol Biol Psychiatry. 2018;85:69–76. doi: 10.1016/j.pnpbp.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Zheng H, Wu R, Kosten TR, Zhang XY, Zhao J. The effect of minocycline on amelioration of cognitive deficits and pro-inflammatory cytokines levels in patients with schizophrenia. Schizophr Res. 2019;212:92–98. doi: 10.1016/j.schres.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Nitta M, Kishimoto T, Müller N, Weiser M, Davidson M, Kane JM, Correll CU. Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophr Bull. 2013;39:1230–1241. doi: 10.1093/schbul/sbt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalinkovich A, Pouyrovsky M, Nasyrova R, Livshits G. Resolution of chronic inflammation as a new adjunctive approach in schizophrenia treatment. Brain Behav Immun. 2020;88:867–869. doi: 10.1016/j.bbi.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 56.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kotemori A, Sawada N, Iwasaki M, Yamaji T, Shivappa N, Hebert JR, Ishihara J, Inoue M, Tsugane S JPHC FFQ Validation Study Group. Validating the dietary inflammatory index using inflammatory biomarkers in a Japanese population: a cross-sectional study of the JPHC-FFQ validation study. Nutrition. 2020;69:110569. doi: 10.1016/j.nut.2019.110569. [DOI] [PubMed] [Google Scholar]
- 58.Shin D, Kwon SC, Kim MH, Lee KW, Choi SY, Shivappa N, Hébert JR, Chung HK. Inflammatory potential of diet is associated with cognitive function in an older adult Korean population. Nutrition. 2018;55-56:56–62. doi: 10.1016/j.nut.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canto-Osorio F, Denova-Gutierrez E, Sánchez-Romero LM, Salmerón J, Barrientos-Gutierrez T. Dietary inflammatory index and metabolic syndrome in Mexican adult population. Am J Clin Nutr. 2020;112:373–380. doi: 10.1093/ajcn/nqaa135. [DOI] [PubMed] [Google Scholar]
- 60.Farhangi MA, Vajdi M. The association between dietary inflammatory index and risk of central obesity in adults: an updated systematic review and meta-analysis. Int J Vitam Nutr Res. 2020;90:535–552. doi: 10.1024/0300-9831/a000648. [DOI] [PubMed] [Google Scholar]
- 61.Wirth MD, Shivappa N, Steck SE, Hurley TG, Hébert JR. The dietary inflammatory index is associated with colorectal cancer in the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Br J Nutr. 2015;113:1819–1827. doi: 10.1017/S000711451500104X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitra S, Natarajan R, Ziedonis D, Fan X. Antioxidant and anti-inflammatory nutrient status, supplementation, and mechanisms in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:1–11. doi: 10.1016/j.pnpbp.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 63.Zhu DM, Liu Y, Zhang AG, Chu ZX, Wu Q, Li H, Ge JF, Dong Y, Zhu P. High levels of vitamin D in relation to reduced risk of schizophrenia with elevated C-reactive protein. Psychiatry Res. 2015;228:565–570. doi: 10.1016/j.psychres.2015.05.051. [DOI] [PubMed] [Google Scholar]
- 64.Li S, Zhuo M, Huang X, Huang Y, Zhou J, Xiong D, Li J, Liu Y, Pan Z, Li H, Chen J, Li X, Xiang Z, Wu F, Wu K. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi: 10.7717/peerj.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jahrami H, Faris MA, Ghazzawi HA, Saif Z, Habib L, Shivappa N, Hébert JR. Increased dietary inflammatory index is associated with schizophrenia: results of a case-control study from Bahrain. Nutrients. 2019;11:1867. doi: 10.3390/nu11081867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Firth J, Stubbs B, Teasdale SB, Ward PB, Veronese N, Shivappa N, Hebert JR, Berk M, Yung AR, Sarris J. Diet as a hot topic in psychiatry: a population-scale study of nutritional intake and inflammatory potential in severe mental illness. World Psychiatry. 2018;17:365–367. doi: 10.1002/wps.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim HS, Kwon M, Lee HY, Shivappa N, Hébert JR, Sohn C, Na W, Kim MK. Higher pro-inflammatory dietary score is associated with higher hyperuricemia risk: results from the case-controlled Korean Genome and Epidemiology Study_Cardiovascular Disease Association Study. Nutrients. 2019;11:1803. doi: 10.3390/nu11081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Na W, Kim M, Park S, Lee M, Sohn C. Development and validation of Korean inflammtory index (K-DII) for metabolic disease patients: by using the Health Examinee Cohort (2012-2014) Korean J Hum Ecol. 2017;26:369–381. [Google Scholar]
- 69.Cho YA, Lee J, Oh JH, Shin A, Kim J. Dietary inflammatory index and risk of colorectal cancer: a case-control study in Korea. Nutrients. 2016;8:469. doi: 10.3390/nu8080469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park S, Na W, Sohn C. Relationship between osteosarcopenic obesity and dietary inflammatory index in postmenopausal Korean women: 2009 to 2011 Korea National Health and Nutrition Examination Surveys. J Clin Biochem Nutr. 2018;63:211–216. doi: 10.3164/jcbn.18-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45:1105–1115. doi: 10.1042/BST20160474. [DOI] [PubMed] [Google Scholar]
- 72.Albracht-Schulte K, Kalupahana NS, Ramalingam L, Wang S, Rahman SM, Robert-McComb J, Moustaid-Moussa N. Omega-3 fatty acids in obesity and metabolic syndrome: a mechanistic update. J Nutr Biochem. 2018;58:1–16. doi: 10.1016/j.jnutbio.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peet M. Omega-3 polyunsaturated fatty acids in the treatment of schizophrenia. Isr J Psychiatry Relat Sci. 2008;45:19–25. [PubMed] [Google Scholar]
- 74.Peet M, Brind J, Ramchand CN, Shah S, Vankar GK. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr Res. 2001;49:243–251. doi: 10.1016/s0920-9964(00)00083-9. [DOI] [PubMed] [Google Scholar]
- 75.Xu F, Fan W, Wang W, Tang W, Yang F, Zhang Y, Cai J, Song L, Zhang C. Effects of omega-3 fatty acids on metabolic syndrome in patients with schizophrenia: a 12-week randomized placebo-controlled trial. Psychopharmacology (Berl) 2019;236:1273–1279. doi: 10.1007/s00213-018-5136-9. [DOI] [PubMed] [Google Scholar]
- 76.Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry. 2002;159:1596–1598. doi: 10.1176/appi.ajp.159.9.1596. [DOI] [PubMed] [Google Scholar]
- 77.Pawełczyk T, Grancow M, Kotlicka-Antczak M, Trafalska E, Gębski P, Szemraj J, Żurner N, Pawełczyk A. Omega-3 fatty acids in first-episode schizophrenia - a randomized controlled study of efficacy and relapse prevention (OFFER): rationale, design, and methods. BMC Psychiatry. 2015;15:97. doi: 10.1186/s12888-015-0473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry. 2001;158:2071–2074. doi: 10.1176/appi.ajp.158.12.2071. [DOI] [PubMed] [Google Scholar]
- 79.Peet M, Horrobin DF E-E Multicentre Study Group. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res. 2002;36:7–18. doi: 10.1016/s0022-3956(01)00048-6. [DOI] [PubMed] [Google Scholar]
- 80.Doğan Bulut S, Bulut S, Görkem Atalan D, Berkol T, Gürçay E, Türker T, Aydemir Ç. The relationship between symptom severity and low vitamin D levels in patients with schizophrenia. PLoS One. 2016;11:e0165284. doi: 10.1371/journal.pone.0165284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McGrath JJ, Burne TH, Féron F, Mackay-Sim A, Eyles DW. Developmental vitamin D deficiency and risk of schizophrenia: a 10-year update. Schizophr Bull. 2010;36:1073–1078. doi: 10.1093/schbul/sbq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lally J, Ajnakina O, Singh N, Gardner-Sood P, Stubbs B, Stringer D, Di Forti M, David AS, Smith S, Murray RM, Howes OD, Gaughran F. Vitamin D and clinical symptoms in First Episode Psychosis (FEP): a prospective cohort study. Schizophr Res. 2019;204:381–388. doi: 10.1016/j.schres.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 83.Krivoy A, Onn R, Vilner Y, Hochman E, Weizman S, Paz A, Hess S, Sagy R, Kimhi-Nesher S, Kalter E, Friedman T, Friedman Z, Bormant G, Trommer S, Valevski A, Weizman A. Vitamin D supplementation in chronic schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled clinical trial. EBioMedicine. 2017;26:138–145. doi: 10.1016/j.ebiom.2017.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fisekovic S, Memic A, Pasalic A. Correlation between MoCA and MMSE for the assessment of cognition in schizophrenia. Acta Inform Med. 2012;20:186–189. doi: 10.5455/aim.2012.20.186-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaughran F, Stringer D, Berk M, Smith S, Taylor D, Whiskey E, Landau S, Murray R, McGuire P, Gardner-Sood P, Wojewodka G, Ciufolini S, Jordan H, Clarke J, Allen L, Krivoy A, Stubbs B, Lowe P, Arbuthnott M, Rathod S, Boardman A, Firdosi M, McGrath JJ. Vitamin D supplementation compared to placebo in people with First Episode psychosis - Neuroprotection Design (DFEND): a protocol for a randomised, double-blind, placebo-controlled, parallel-group trial. Trials. 2020;21:14. doi: 10.1186/s13063-019-3758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghaderi A, Banafshe HR, Mirhosseini N, Moradi M, Karimi MA, Mehrzad F, Bahmani F, Asemi Z. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry. 2019;19:77. doi: 10.1186/s12888-019-2059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown HE, Roffman JL. Vitamin supplementation in the treatment of schizophrenia. CNS Drugs. 2014;28:611–622. doi: 10.1007/s40263-014-0172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim EJ, Lim SY, Lee HJ, Lee JY, Choi S, Kim SY, Kim JM, Shin IS, Yoon JS, Yang SJ, Kim SW. Low dietary intake of n-3 fatty acids, niacin, folate, and vitamin C in Korean patients with schizophrenia and the development of dietary guidelines for schizophrenia. Nutr Res. 2017;45:10–18. doi: 10.1016/j.nutres.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Dakhale GN, Khanzode SD, Khanzode SS, Saoji A. Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology (Berl) 2005;182:494–498. doi: 10.1007/s00213-005-0117-1. [DOI] [PubMed] [Google Scholar]
- 90.Sivrioglu EY, Kirli S, Sipahioglu D, Gursoy B, Sarandöl E. The impact of omega-3 fatty acids, vitamins E and C supplementation on treatment outcome and side effects in schizophrenia patients treated with haloperidol: an open-label pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1493–1499. doi: 10.1016/j.pnpbp.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 91.Zortea K, Franco VC, Francesconi LP, Cereser KM, Lobato MI, Belmonte-de-Abreu PS. Resveratrol supplementation in schizophrenia patients: a randomized clinical trial evaluating serum glucose and cardiovascular risk factors. Nutrients. 2016;8:73. doi: 10.3390/nu8020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zortea K, Franco VC, Guimarães P, Belmonte-de-Abreu PS. Resveratrol supplementation did not improve cognition in patients with schizophrenia: results from a randomized clinical trial. Front Psychiatry. 2016;7:159. doi: 10.3389/fpsyt.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salas-Salvadó J, Becerra-Tomás N, García-Gavilán JF, Bulló M, Barrubés L. Mediterranean diet and cardiovascular disease prevention: What do we know? Prog Cardiovasc Dis. 2018;61:62–67. doi: 10.1016/j.pcad.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Koloverou E, Panagiotakos DB, Pitsavos C, Chrysohoou C, Georgousopoulou EN, Grekas A, Christou A, Chatzigeorgiou M, Skoumas I, Tousoulis D, Stefanadis C ATTICA Study Group. Adherence to Mediterranean diet and 10-year incidence (2002-2012) of diabetes: correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes Metab Res Rev. 2016;32:73–81. doi: 10.1002/dmrr.2672. [DOI] [PubMed] [Google Scholar]
- 95.Costa R, Teasdale S, Abreu S, Bastos T, Probst M, Rosenbaum S, Ward PB, Corredeira R. Dietary intake, adherence to Mediterranean diet and lifestyle-related factors in people with schizophrenia. Issues Ment Health Nurs. 2019;40:851–860. doi: 10.1080/01612840.2019.1642426. [DOI] [PubMed] [Google Scholar]
- 96.Joseph J, Depp C, Shih PB, Cadenhead KS, Schmid-Schönbein G. Modified Mediterranean diet for enrichment of short chain fatty acids: potential adjunctive therapeutic to target immune and metabolic dysfunction in schizophrenia? Front Neurosci. 2017;11:155. doi: 10.3389/fnins.2017.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Calderón N, Betancourt L, Hernández L, Rada P. A ketogenic diet modifies glutamate, gamma-aminobutyric acid and agmatine levels in the hippocampus of rats: a microdialysis study. Neurosci Lett. 2017;642:158–162. doi: 10.1016/j.neulet.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 98.Steiniger B, Kretschmer BD. Glutamate and GABA modulate dopamine in the pedunculopontine tegmental nucleus. Exp Brain Res. 2003;149:422–430. doi: 10.1007/s00221-003-1382-z. [DOI] [PubMed] [Google Scholar]
- 99.Dupuis N, Curatolo N, Benoist JF, Auvin S. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. 2015;56:e95–8. doi: 10.1111/epi.13038. [DOI] [PubMed] [Google Scholar]
- 100.Soltani S, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin Nutr. 2018;37:542–550. doi: 10.1016/j.clnu.2017.02.018. [DOI] [PubMed] [Google Scholar]