The recent coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]), known as coronavirus disease 2019 (COVID-19), is associated with high morbidity and mortality mostly in adults, owing to systemic symptoms, especially pulmonary sequalae.
Interestingly, children seem to have milder symptoms than adults associated with COVID-19. Furthermore, the incidence of allergy or asthma in children infected with COVID-19 seems to be lower than that of the general population giving the impression that these atopic diseases may be protective.1 Such a conclusion is premature, because the increased lung eosinophils found in these diseases may had some protective effect especially because reduced numbers of eosinophils were reported in COVID-19.1 Moreover, medications used for the treatment of these atopic diseases (eg, antihistamines, corticosteroids) may had offered some protection rather than the atopic diseases themselves. Nevertheless, there have been recent reports of childhood multisystem inflammatory syndrome with symptoms resembling toxic shock or Kawasaki syndrome,2 suggesting children may still experience symptoms related to inflammation.
The pulmonary pathological findings associated with COVID-19 seems to result from the release of multiple proinflammatory cytokines, especially interleukin (IL)-6, that can damage the lungs.3 A key source of such cytokines and chemokines is the mast cells, which are ubiquitous in the body, especially the lungs, and are critical for allergic and pulmonary diseases.3 In fact, activated mast cells were recently detected in the lungs of deceased patients with COVID-19 and were linked to pulmonary edema, inflammation, and thromboses.4
Mast cells are typically activated by allergic triggers, but they can also be triggered by pathogen-associated molecular patterns via activation of Toll-like receptors. In addition, mast cells express the renin-angiotensin system, the ectoprotease angiotensin-converting enzyme 2 required for SARS-CoV-2 binding, and serine proteases, including TMPRSS2, required for priming of the corona spike protein.3 Such triggers could lead to secretion of multiple proinflammatory mediators selectively, without release of histamine or tryptase, as we had previously reported in the Journal of Immunology for release of IL-6 in response to IL-1β from cultured human mast cells (Fig 1 ).3 Moreover, we recently reported in the Proceedings of the National Academy of Sciences of the United States of America that human mast cells can be synergistically stimulated by the peptide substance P and IL-33 to release impressive amounts of vascular endothelial growth factor, IL-1β or tumor necrosis factor again without secretion of histamine or tryptase.3
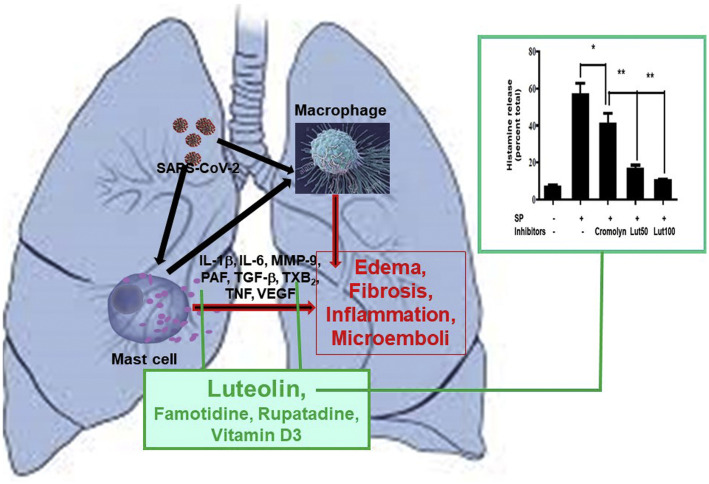
Figure 1.
Mast cells in COVID-19. SARS-CoV-2 stimulates mast cells to release pathogenic mediators, inhibited by luteolin. Luteolin inhibits histamine release. Human mast cells were stimulated with substance P (10 μM, 30 minutes) with or without luteolin (50 or 100 μM) or cromolyn (100 μM) on 30-minute preincubation (the asterisk and double asterisk indicate P < .05 and P < .01, respectively; n = 3). COVID-19, coronavirus disease 2019; IL, interleukin; lut, luteolin; MMP-9, matrix metalloproteinase 9; PAF, platelet-activating factor; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SP, substance P; TGF-β, transforming growth factor beta; TNF, tumor necrosis factor; TXB2, thromboxane B2; VEGF, vascular endothelial growth factor.
In addition to the proinflammatory cytokines and chemokines, activated mast cells could release matrix metalloproteinases (eg, matrix metalloproteinase 9) and transforming growth factor beta, which could contribute to lung fibrosis, including thromboxanes (thromboxane B2) and platelet-activating factor, leading to the recently reported microthromboses in the lungs of deceased patients with COVID-19.4 Moreover, mast cells communicate with endothelial cells, fibroblasts, and macrophages (Fig 1), further stimulating release of proinflammatory, fibrotic, thrombogenic, and vasoactive mediators.
Many recent reports indicate that a considerable number of patients who received positive test results for SARS-CoV-2 are asymptomatic or have mild symptoms. However, increasing anecdotal evidence suggests that many patients who either recovered from or had mild symptoms after COVID-19 exhibit diffuse, multiorgan symptoms months after the infection prompting the Centers for Disease Control and Prevention to name it adult multisystem inflammatory syndrome. These symptoms include malaise, myalgias, chest tightness, brain fog, and other neuropsychiatric symptoms that are quite similar to those presented by patients diagnosed as having mast cell activation syndrome (MCAS).5
It is, therefore, critical that MCAS (International Classification of Diseases, Tenth Revision code D89.42—idiopathic mast cell activation syndrome, not systemic mastocytosis) be suspected, evaluated, and addressed in any patient with COVID-19, who experiences chronic multiorgan symptoms. Given the abovementioned discussion, it would be prudent to consider blocking mast cells and the action of their mediators both prophylactically and symptomatically during the COVID-19 pandemic. Unfortunately, there are no effective clinically available mast cell inhibitors. Disodium cromoglycate (cromolyn) is a weak inhibitor of degranulation (not cytokine release), is very poorly absorbed (<5%) from the intestine, and has rapid tachyphylaxis requiring frequent dose escalations. The natural flavonoid luteolin is a much more potent inhibitor of mast cell release of histamine than cromolyn (Fig 1). Furthermore, luteolin is a potent inhibitor of proinflammatory cytokine and chemokine release from mast cells. Luteolin, especially in the available supplements containing its liposomal form in olive pomace oil to increase oral absorption, could be useful because, in addition to blocking release of pathogenic mediators from mast cells, it might block SARS-CoV-2 binding to target cells.3
Liposomal luteolin could be supplemented with the H1 receptor antagonist rupatadine (not available in the United States except if compounded), which has anti–platelet-activating factor and mast cell–inhibitory actions, and the H2 receptor antagonist, famotidine, which has been reported to be beneficial in COVID-19. In addition, supplementation with vitamin D3 could be useful, because it can reduce allergic inflammation and has been reported to be of benefit in COVID-19.
In conclusion, mast cells could contribute to the pathogenesis of COVID-19 and any postinfectious inflammatory syndromes through the release of proinflammatory, fibrotic, or thrombogenic mediators. Hence, it is reasonable to consider their inhibition at least for prophylaxis if not symptomatic treatment of patients diagnosed as having COVID-19 with mild symptoms. However, there is no published evidence on whether patients with MCAS or systemic mastocytosis may be more susceptible to COVID-19 infection or experience symptoms after infection. Hence, more basic and clinical research should be conducted to evaluate the associations suggested.
Footnotes
Disclosures: The author is the Scientific Director of and shareholder in Algonot, LLC (Sarasota, FL), which markets flavonoid-containing dietary supplements.
Funding: This work was supported by anonymous donations.
References
- 1.Ciprandi G., Licari A., Filippelli G., Tosca M.A., Marseglia G.L. Children and adolescents with allergy and/or asthma seem to be protected from coronavirus disease 2019. Ann Allergy Asthma Immunol. 2020;125(3):361–362. doi: 10.1016/j.anai.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldstein L.R., Rose E.B., Horwitz S.M. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Theoharides T.C. Covid-19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. Biofactors. 2020;46(3):306–308. doi: 10.1002/biof.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motta Junior J.D.S., Miggiolaro AFRDS, Nagashima S. Mast cell degranulation in alveolar septa and SARS-COV-2: a pathogenic pathway linking interstitial edema to immunothrombosis. Front Immunol. 2020;11:574862. doi: 10.3389/fimmu.2020.574862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akin C., Valent P., Metcalfe D.D. Mast cell activation syndrome: proposed diagnostic criteria. J Allergy Clin Immunol. 2010;126(6):1099–1104. doi: 10.1016/j.jaci.2010.08.035. .e4. [DOI] [PMC free article] [PubMed] [Google Scholar]