Abstract
Objectives:
The study aimed to review the literature on the use of ultraviolet-C (UV-C) sterilization to assess its clinical efficacy in reducing risk and transmission of nosocomial infections as well as its associated health safety or hazards.
Methods:
Four main search engines were used to identify potential studies which included: (1) Google Scholar, (2) ScienceDirect, (3) PubMed, and (4) Cochrane. Studies in English and published from 2010 to 2020 were considered. Studies on efficacy were limited to those in unseeded hospital environments, examining environmental disinfection, and with true experimental, randomized controlled trial, or quasi-experimental study designs. No additional criterion was used for safety studies due to the scarcity of literature. In the end, a total of 17 studies were selected. Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed. Risk of bias assessment and manual data extraction and tabulation were done.
Results:
Twelve eligible efficacy studies were identified together with five safety studies. It was found that UV-C irradiation had positive results when used as an adjunct for existing cleaning protocols. The germicidal effect of UV-C is potent against microorganisms including viruses, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant enterococci. Safety study results showed dermal effects of UV-C exposure including DNA lesions, formation of cyclobutane pyrimidine dimers in cells, and effects on the skin’s stratum corneum.
Conclusion:
It was found that UV-C can be utilized as an adjunct to terminal manual cleaning because of its efficacy as a germicidal agent. Further studies must still be done to exact a standard for safe exposure dose, especially for 222 nm germicidal lamps. Direct evidence is needed for any targeted implementation of UV-C during Coronavirus Disease 2019 (COVID-19) pandemic.
Keywords: Ultraviolet-C, environmental sterilization, hospitals, systematic review, coronavirus disease-19
Introduction
Rationale
The hospital environment poses a threat to the health and safety of patients as it is regarded as a source and reservoir of infection. Health-care associated infections (HAIs) not only impact public health but also the economic and social status of patients and their families, because of prolonged hospital stays, possible disabilities, and mortality.[1] Among the major contributors to outbreaks and mortality cases of HAIs include methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci (VRE), carbapenem-resistant Enterobacteriaceae (CRE), and extended-spectrum beta-lactamase producing Escherichia coli.[2] To control the nosocomial spread of infection, hospitals, as advised by the Center for Disease Control and Prevention, use standard sterilization and disinfection which include the use of chemicals such as bleach and alcohol, as well as other enzymatic disinfectants for contaminated equipment and surfaces.[3]
Germicidal ultraviolet (UV) light, an anti-infective strategy which uses wavelengths of light, can kill microorganisms and inactivate viruses. It was previously utilized for controlling tuberculosis outbreaks[4] and the H1N1 influenza virus.[5] However, guidelines released by the World Health Organization (WHO) in 2014 regarding the infection prevention and control of epidemic and pandemic-prone acute respiratory infections (ARI) in healthcare, such as severe acute respiratory syndrome (SARS), reported no recommendation yet for this system due to lack of evidence supporting the ability of UV irradiation to reduce the risk of transmission and infection of specific pathogens causing ARIs from patients to healthcare workers during the delivery of care, with and without the use of other precautions.[6] UV-C, which uses short wavelength of 250–280 nm, is considered the most lethal of wavelengths due to its capability of inactivating microorganisms as it gets strongly absorbed in their nucleic acids. This often leads to the formation of cyclobutane pyrimidine dimers (CPD) in the nucleic acid strands, which might cause defects in cell replication and eventual cell death.[7]
Coronavirus disease 2019 (COVID-19) is a potentially life-threatening disease caused by the single-stranded RNA virus, SARS coronavirus (SARS-CoV-2). One of the main ways it is transmitted to healthy individuals is by touching surfaces which are contaminated with droplets released from infected persons when they cough or exhale. Viral droplets can survive on surfaces for hours even when viral load is reduced.[8] Since the WHO declared the COVID-19 as a global health emergency on January 30, 2020, a spike in the sales of UV-C disinfection systems have been reported.[9] This presents the need to evaluate current evidence to support its possible application for air and surface disinfection in hospitals during the 2019 CoV pandemic. With the emergence of SARS-CoV-2, and other multidrug-resistant microorganisms, the UV-C irradiation may potentially serve as an adjunct to existing cleaning protocols implemented in hospitals. Despite this, UV-C use remains controversial due to associated health risks. Skin and eye irritation has been reported[6,10] and due to the lack of substantial research, has mentioned UV-C to be a reasonably anticipated human carcinogen.[11]
Objectives
This systematic review assessed the clinical efficacy of UV-C sterilization in reducing the risk of transmission and infection of pathogens from patients to healthcare workers and other exposed individuals. It also aimed to examine evidence-based protocols followed for UV-C sterilization and its associated health safety or hazards.
Research question
Is UV-C irradiation used as a sterilization method in hospitals effective against pathogenic microorganism and what are its associated health safety hazards?
Methodology
The review was primarily modeled after the Cochrane template for systematic reviews and followed the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). The review focused on two aspects of UV-C irradiation: (1) efficacy and (2) safety.
Eligibility criteria for studies on UV-C efficacy
Types of studies
The review included true experimental and randomized controlled clinical trials (RCTs) as well as quasi-experimental studies. Observational studies were not eligible for inclusion. Studies were identified from online search engines and databases for publications in peer-reviewed journals.
Types of participants
Selected studies were focused on UV-C irradiation in the hospital environment under operational conditions. All types of hospital units (intensive care unit [ICUs], non-ICUs, and other units providing specialty care) were included in the study. Health-care facility patients were included as study participants for several studies included in the review. Various exposure status and characteristics of interest depending on the factors being observed, for example, incidence of bacterial or viral infection in different studies were considered.
Types of interventions
Studies that assessed the following interventions, regardless of comparator, were all eligible: (1) UV-C surface-disinfecting devices, (2) UV-C germicidal irradiation (UVGI) technologies, (3) UV-C irradiation germicidal lamps, (4) mercury-based, light emitting diodes (LED), pulsed xenon (PX) lamps were included, (5) UV-C irradiation incorporated into disinfection systems as stand-alone technology in the hospital environment as treatment or interventions in various study designs, (6) UV-C irradiation as an adjunct to standard cleaning procedures, and (7) studies that involved UV water disinfection were excluded from the study.
Types of outcome measures
The following clinical outcomes were considered: (1) Hospital acquired infection rates, (2) bacterial or viral infection incidence, (3) bacterial concentrations, and (4) contamination levels.
Eligibility criteria for studies on UV-C safety
Types of studies
Due to the limited number of accessible studies on safety of UV-C irradiation, types of studies were not limited so as to be able to adequately capture the true extent of the existing literature.
Types of participants
Participants were not limited to any specific population. The majority of studies included in the review did not involve humans in a research subject capacity.
Types of interventions
The review selected studies that utilized UV-C germicidal lamps, as well as the following interventions, regardless of comparator: (1) UV-C surface-disinfection devices, (2) UVGI technologies, (3) mercury based, LED, PX lamps were included, (4) UV-C irradiation incorporated into disinfection systems as stand-alone technology in the hospital environment as treatment or interventions in various study designs, (5) UV-C irradiation as an adjunct to standard cleaning procedures, and (6) studies that involved UV water disinfection were excluded from the study.
Types of outcome measures
The following outcomes were considered: (1) Incidence of acute and chronic UV-induced skin inflammation, (2) incidence of acute and chronic ocular effects, and (3) incidence of other cytotoxic effects.
Search methods
A systematic search on the following electronic databases was carried out on May 8, 2020, to identify relevant studies on the efficacy and safety of UV-C irradiation: (1) Cochrane Central Register of Controlled Trials in the Cochrane Library, (2) Google Scholar, (3) ScienceDirect, and (4) PubMed. Other sources were Wiley Online Library, American Journal of Infection Control, and SpringerLink. In addition, the review authors searched the reference lists of the articles retrieved and other relevant papers for eligible articles. Only published studies from January 1, 2010, to May 8, 2020, were considered. To ensure that no misinterpretation and mistranslation occur, and considering the linguistic capacities of the investigators, selected studies were limited to those written in the English language. Full search strategies and all data used to arrive at the results and findings in this study can be found at https://doi.org/10.5281/zenodo.3933425.[12]
Data collection
Study selection
The titles and abstracts of candidate studies identified by the specified search strategy were independently screened. Full text of all reviews that were thought to be potentially eligible for further investigation was obtained and examined. Duplicates were excluded as well as other studies that did not meet the eligibility criteria.
Data extraction and management
Data were obtained through manual perusal of the studies that passed the multiple screening criteria. The collected data were collated and stored in spreadsheets, to be further organized into tables for data presentation.
Study quality assessment
The risk of bias for each study was independently assessed using tools from Effective Practice and Organization of Care/Cochrane, ROBINS-I, and the Joanna Briggs Institute. Multiple tools were used to ensure that the appropriate assessment for the different study designs would be done. The data abstraction and assessment tools can be found at https://doi.org/10.5281/zenodo.3933425.[12] In general, the following domains were assessed: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias. Reviewers graded each potential source of bias as either high, low, or unclear. Discrepancies were settled by discussion. Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach was utilized to examine the overall quality of evidence of included studies. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each outcome. Accordingly, the evidence can be downgraded from “high certainty” by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Expected outcomes
The primary outcome of this study was a comprehensive synthesis of all eligible identified studies containing publication details and the extracted data. Extracted data for efficacy studies included the type of study, test subject, exposure, UV source and wavelength, outcome measure used, and a summary of findings. Extracted data for safety studies included microorganism samples used, outcome measures, findings, and other considerations.
Results
Efficacy
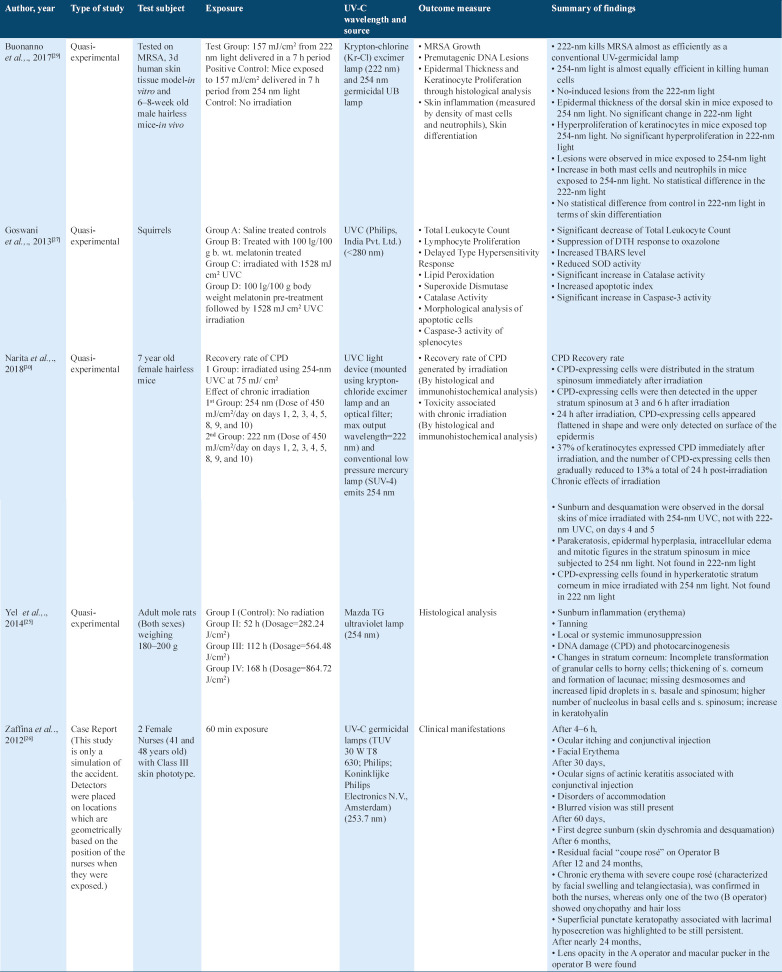
A total of 64 articles were initially identified. After removing duplicates, the articles were reduced down to 59. The articles were further screened down based on the full text, and predetermined eligibility criteria for this study. Among the 12 final articles, seven were quasi-experimental studies, four were uncontrolled, before and after studies, and one was a cluster randomized controlled trial (RCT). The search algorithm is shown in Figure 1.
Figure 1.
Summary of the study selection design on efficacy studies
Safety
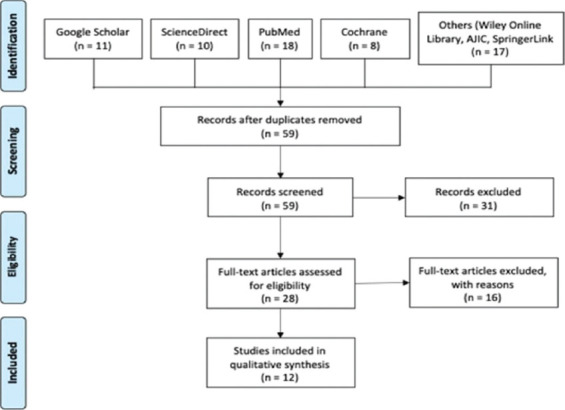
A total of 19 articles were initially identified. There were no duplicates present, so all 19 articles were screened based on full text and predetermined eligibility criteria for this study. Five articles, which are composed of three non-RCTs and two RCTs, were included in the final list. The search algorithm is shown in Figure 2.
Figure 2.
Summary of the study selection design on safety studies
Summary of included studies
Efficacy
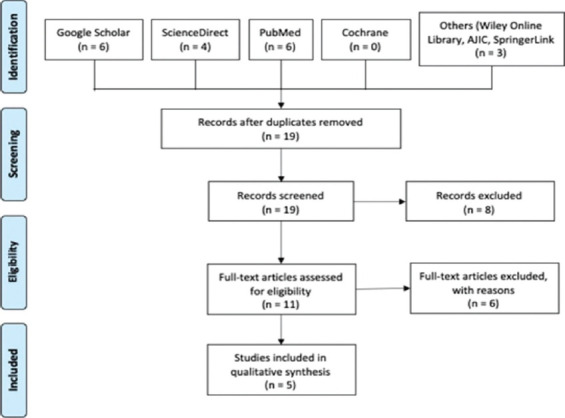
The methodology of the 12 included studies, evaluating the efficacy of various UV-C devices in sterilizing hospital rooms, is summarized in Table 1.[13-24]
Table 1.
Methodology of included efficacy studies
Study design
Seven of the studies included were quasi-experimental in nature, specifically five of them are classified as a non-equivalent control group design[13-16] and two are considered as an interrupted time-series design.[18,19] In the non-equivalent control group design, the researchers compared two different sets: (1) control units or rooms sterilized through the hospital’s own standard cleaning procedures, and (2) intervention units or rooms disinfected by applying the UV device. Whereas, in the interrupted time-series design,[18] there was a comparison between a baseline period for a year and another year wherein continuous UV-C cleaning took place (24 observations in total, and one for each month).
On the other hand, four studies adopted an uncontrolled, before and after study design[20-23] in which the investigators used the same study site or hospital rooms before and after the introduction of the UV device. Any observed differences in the colony-forming unit (CFU) count were assumed to be due to the intervention. Whereas Anderson et al. used an experimental design, particularly a cluster-randomized, crossover trial. Here, the chosen hospital rooms were terminally cleansed with one of four strategies: (1) reference, which includes quaternary ammonium disinfectant except for Clostridium difficile, (2) UV-C, (3) bleach, and (4) bleach and UV-C. The results of their study were the incidence of infection with all target organisms among exposed patients, as well as the incidence of C. difficile infection among exposed patients in the intention-to-treat population.[24]
Ultimately, each of the 12 studies were done to evaluate the value and benefit of the UV device in killing various organisms in surfaces as well as in the air in comparison with manual cleaning disinfectants only.
Study setting
Eleven studies conducted their research at a single hospital site only. Seven of these were done in the USA,[15-19,21,22] one in Canada,[13] one in the Western Cape of South Africa,[14] one in Japan,[20] and one in Ecuador.[23] Whereas the study conducted in the southeastern United States was able to utilize multiple hospital sites, a total of nine hospitals.[24]
Hospital units targeted for UV sterilization varied remarkably across the 12 studies. Four studies applied the device primarily for rooms of patients[17,21,22,24] while three studies evaluated the device over different hospital units, including ICUs, hallway and biohazard rooms,[18] hematology and bone marrow transplant units,[17] operating rooms, neo-ICU, and microbiology laboratory.[23] One study used the device for hallway bathrooms,[13] one for high risk feed preparation areas,[14] one entirely for operating rooms,[15] one specifically for toddler units,[19] and one solely for internal care units.[20]
UV device and timing of disinfection
Out of the 12 studies, half of these made use of the PX UV device,[14-17,20,23] manufactured by Xenex Disinfection Services, San Antonio, Texas, USA. The other five employed a UV-C device created by different manufacturers (Tru D SmartUVC, Memphis, Tennesse, USA;[24] Sanuvox, Montreal, Canada;[13] American Green Technology, South Bend, Indiana, USA;[18] Clorox Healthcare, Oakland, California, USA;[19] and Skytron, Grand Rapids, Michigan, USA).[22] Whereas only one utilized the Sterilray (Somersworth, New Hampshire, USA) device that makes use of far-UV radiation, which has more photon energy than UV-C.[21]
With regard to declaration of interests, three studies reported that they had authors employed by the manufacturer,[15,18,23] two studies received research grants or funding from the manufacturer,[15,16] and one study had authors receive consulting fees from the manufacturer of the device.[24]
The disinfection procedures were predominantly scheduled after the discharge of a patient or transfer to ward. In one study, the feed preparation areas were sterilized every day, whereas in another the operating rooms were exclusively sanitized every night.[15] A separate study utilized an automated UV device every 30 s of no motion in two hallway bathrooms,[14] while another study focused on cleaning of chosen units 2 or 3 times per week only.[19] Additional information about the disinfection protocols, including the number and length of cycles applied per room, specific location of the device inside the units, additional process measures, as well as the manual cleaning disinfectants that were used for every study, can be found at https://doi.org/10.5281/zenodo.3933425.[12]
Safety
Five selected studies pertaining to the safety of UV-C exposure describing possible health hazards and effects are summarized in Table 2.[25-30]
Table 2.
Summary of selected UV-C safety articles
Risk of bias
An overview of the study level judgments for all included studies on UV-C efficacy is presented in Figure 3 and the characteristics of each study can be found at https://doi.org/10.5281/zenodo.3933425.[12] Blank sections in this graph are due to the use of different risk of bias criteria appropriate for each type of study design.
Figure 3.
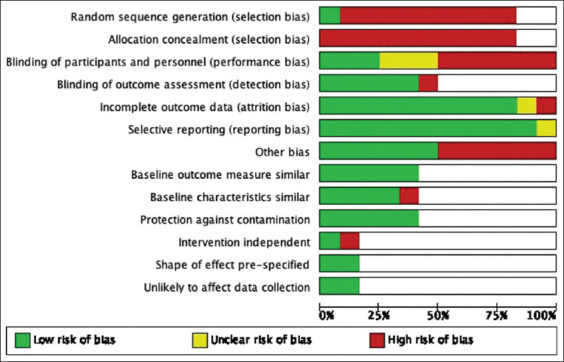
Risk of bias graph: Review authors’ judgment about each risk of bias item presented as percentages across all included studies on the efficacy of ultraviolet-C irradiation
The majority of the studies exhibited a high risk of bias mainly related to non-randomized methods of allocation. In one study,[24] randomization was adequately performed through the use of random number generator and algorithm, whereas the remaining studies were judged to have high risk of selection bias due to non-randomization. Allocation concealment was not performed in all trials. Three studies Dippenaar and Smith, Penno et al., Villacís et al.,[14,22,23] applied blinding. Three trials Cooper et al., El Haddad et al., Pavia et al.,[13,15,19] did not report whether blinding was used or not while the remainder did not apply blinding.
In terms of attrition bias, Anderson et al.[24] reported missing data due to unavailability of records arising from changes in electronic health record systems during the duration of the study. The proportion of missing records was significant relative to the overall sample size; hence, the reviewers assessed this study as being at high risk of attrition bias. Meanwhile, the risk of bias was unclear in Jinadatha et al.[16] because of limited reporting of outcomes and no mention of missing data.
Other potential sources of bias were identified across studies and were considered high risk. In Anderson et al.[24] ascertainment bias might have been introduced due to changes in culturing practices of the clinicians involved during the course of standard care. A potential source of bias in Haddad et al.[15] was the difference in case types and frequency of cases in the operating rooms which may have influenced the impact of PX-UV use between cases on the reduction of room turnover time and pathogen transmission to patients. The differences in the technique of terminal disinfection among various staff were potentially the source of bias in Villacis et al.[23] while the time needed for the hospital staff to become fully proficient in the recommended protocol for the use of the UV-C in Pavia et al.[19] suggested a confounding effect which may have resulted in bias.
As demonstrated in Figure 4, all four non-RCTs on UV-C irradiation safety were at low risk of bias in terms of blinding, protection against contamination and selective outcome reporting. However, it is important to note that these studies lack randomization and allocation concealment. Meanwhile, the decision to include the case report was premised on the reviewers’ assessment (can be found at https://doi.org/10.5281/zenodo.3933425)[12] that it presented clear patient demographic, history, clinical condition, diagnostics, and possible adverse events. However, pre- and post-interventions were not clearly defined.
Figure 4.
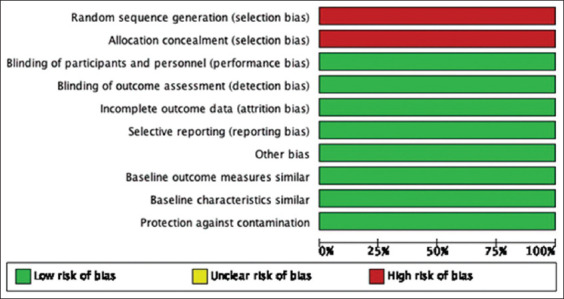
Risk of bias graph: Review authors’ judgment about each risk of bias item presented as percentages across all included non-randomized controlled clinical trials on the safety of ultraviolet-C irradiation
Effects of interventions
Efficacy
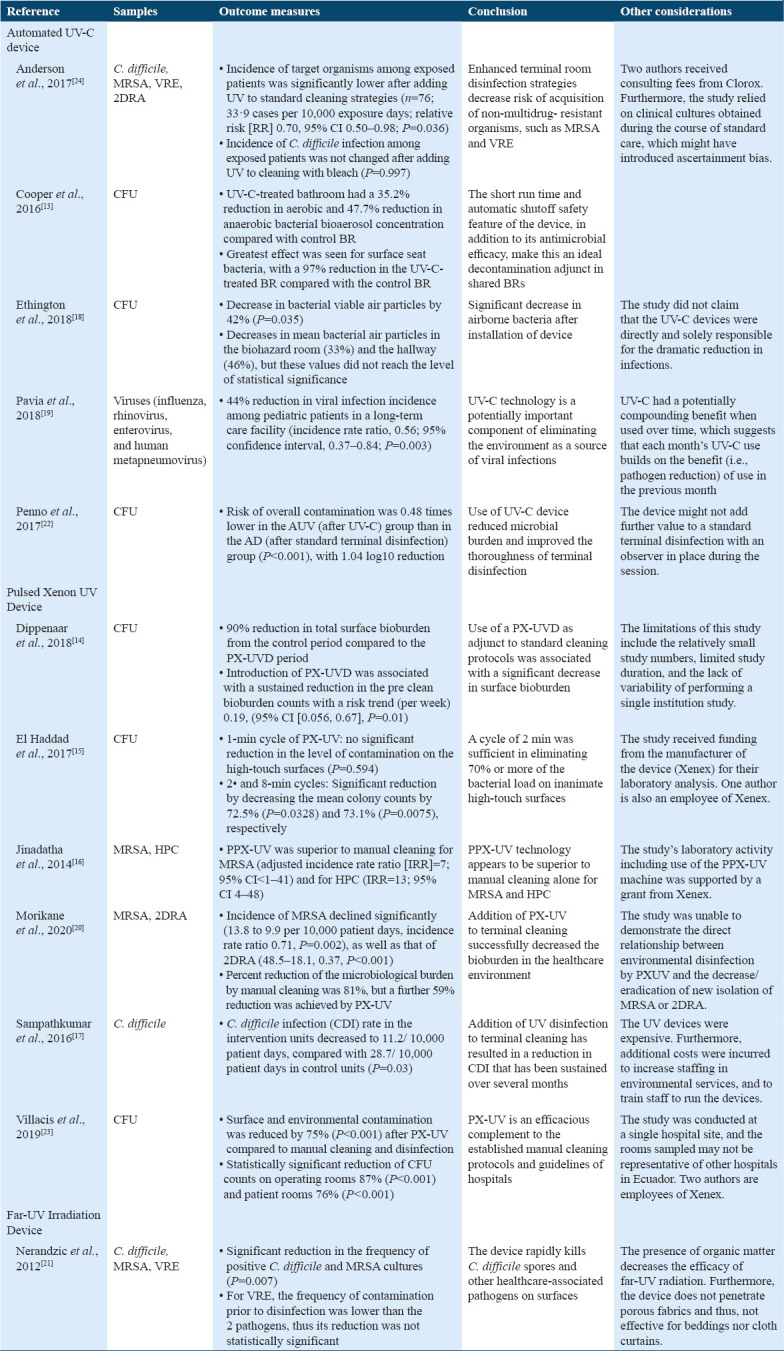
With the heterogeneity of the methodology and reported outcomes of the chosen studies, quantitative analysis was deemed inapplicable. Rather, a qualitative synthesis of the results of the included research is shown in Table 3.[13-24]
Table 3.
Results of included efficacy studies
DISCUSSION
Summary of main findings
UV-C light destroys pathogens by inactivating its DNA. When the DNA absorbs a high photon energy, such as that of UV-C, its peptide and disulfide bonds break and become permanently damaged.[21] UV-C irradiation is effective in the reduction of different microorganisms including different hospital endemic strains particularly C. difficile, MRSA, and VRE,[14,16,17,20-24] as well as different fungi and virus such as Ebola virus, influenza, rhinovirus, enterovirus, and human metapneumovirus.[15,19] It is safe to consider that UV-C light disinfection is an effective germicidal agent against different microorganisms, reducing infection rates, and contamination. However, there are no studies in disinfection using UV-C against SARS-CoV-2 found in the literature. It is important to note that pathogen concentration does not significantly affect the efficacy of UV-C and different surfaces have similar reduction rates with the use of UV-C, except for steel.[31] In addition, there is reduction in efficacy when distance is increased between target surface and UV-C light device, when the surface is not in-line-of-sight of the UV-C light device and when there is the presence of organic matter. On the other hand, an increased efficacy is noted in the reduction of different microorganisms when the inoculum is spread out on a larger surface area.[31] These factors can be considered to create strategies to increase efficacy and efficiency of the UV-C disinfection process.
The use of UV-C light as a disinfecting tool seems to be most effective as an adjunct to already existing terminal cleaning standard operating procedures. This disinfecting process even outperformed active hydrogen peroxide in the removal of MRSA, VRE, and C. difficile.[18] UV-C disinfection is especially useful as an adjunct in the disinfection process of surfaces with a high microbial burden where there is frequent occupant use. These places may be harder to clean manually as it takes a longer time and disinfection may be harder. In addition, UV-C as an adjunct has the upper hand compared to manual terminal cleaning as this is dependent on cleaner’s education and efficiency. Moreover, purely manual terminal cleaning presents a risk of contamination through cleaning materials used and potential transfer of micro-organisms. It also poses a risk for microbial resistance and increased labor.[20]
Some considerations that may arise with the use of UV-C as a disinfecting tool are its efficacy as a stand-alone procedure seeing as some studies have not seen significant results without the isolated use of UV-C in the reduction of infections.[14] This suggests that other factors are at play and that UV-C is most helpful as a supplement to the standard manual terminal cleaning practices. Other concerns include, largely, the lack of standardization in irradiation dose (irradiance and exposure time) and the distance between surfaces for different UV-C light devices such as automated UVGI and hand-held UV-C light devices.
The effects of the use of UV-C light devices beyond its proven germicidal function include a slew of damages such as erythema, tanning, missing desmosomes, and changes in the stratum corneum. Exceptional findings include DNA damage, formation of lacunae and cytoplasmic debris, thickening of the stratum corneum, increase in keratohyalin, and vacuole formation in stratum granulosum.[25-27] These effects depend on exposure time lengths, number of cycles, and irradiance intensity. Current guidelines for exposure to UV-C radiation should not exceed 30 J/m2 at 270 nm for the eyes and skin. At 254 nm, the maximum exposure limit is set at 60 J/m2.[28]
In general, conventional UV-C light devices used as a disinfecting tool utilize 254 nm UV-C. Findings show that this particular wavelength induces cellular damage in the DNA of microorganisms, effectively killing it and reducing surface and air bioburden. This occurs specifically by inducing CPD formation in cells. In humans, this wavelength induces the formation of mutagenic and cytotoxic damages to the DNA, possibly leading to photocarcinogenesis. DNA lesions may cause epidermal hyperplasia which is a strong correlator of UV-B effects rather than chronic irradiation, regardless of wavelength.[29,30] This suggests that conventional 254 nm UV-C light devices do not produce isolated UV-C light. In fact, <10% of light emitted by conventional germicidal lamps are not at the 254 nm wavelength.[29,30]
On the other hand, newer studies suggest that the 222 nm wavelength has the same bactericidal effects as the conventional 254 nm UV-C without the hazardous effects. The 222 nm irradiation causes apoptotic cell death that has a protective function against photocarcinogenesis. However, this mechanism is not yet well understood, and its chronic effects are not yet explored.[29,30] The safe use of the 222 nm UV-C in disinfection is largely because it cannot penetrate mammalian nuclei and does not even reach the stratum corneum because of its short wavelength.[30] Furthermore, the presence of melatonin seems to have a protective function against UV-C irradiation, whether that melatonin protects against UV-C or possibly UV-B emitted in germicidal lamps is something to be explored.[27]
This review was carried out to determine whether the use of UV-C light in the disinfecting processes is effective and whether it poses risks. UV-C light, indeed, is effective in the reduction of infections from both surfaces and the air. Single and chronic irradiation from these devices, however, pose a risk through photocarcinogenesis and other dermal damages.[32] To maximize the positive effects of UV-C germicidal light devices, current terminal end manual cleaning should be supplemented with a standard effective dose of UV-C. In addition, UV-C disinfecting processes should explore the use of isolated 222 nm UV-C to reduce safety issues.
In a hospital setting, UV-C can be employed to disinfect the air of bacterial particles with the use of the upper room UV-C lights. This strategy may be useful for reducing HAIs. However, studies pertaining to the efficacy of air decontamination decreasing HAIs outside laboratory testing are still to be explored.[18] Another strategy using UV-C is to install them in shared toilet rooms, especially in wards as toilet flushing may produce bioaerosols.[13] Furthermore, the use of UV-C in hospital rooms, particularly in operating rooms where there is a rapid bed turnover rate, may create an efficient disinfecting system, decreasing time, and labor needed to prepare rooms for the next patient especially in times of emergencies.[15] Along with reducing incidence of different pathogens on patient room surfaces, the use of UV-C also shows a sustained reduction of bioburden on surfaces even before cleaning[14] and a reduction in developing drug resistance.[16,17] These may explain the reduction of HAIs in rooms where previous occupants were infected with multi-drug resistant organisms.[24] These studies focus only on hospital room surfaces, toilets and the air and were not able to test the efficacy of UV-C on medical instruments and equipment that may also have a high bioburden. Its efficacy on different surfaces including medical instruments and equipment as well as possible damages to the integrity of materials is something to be explored.
In the context of the COVID-19 global pandemic, the use of UV-C disinfection for surfaces and air should be explored as only sterilization of personal protective equipment reuse has been studied.[33-35] However, it seems that UV-C has the potential to effectively inactivate SARS-CoV-2. Studies on UV-C inactivating SARS-CoV-1 present the possibility because of its close genomic identity with SARS-CoV-2.[36,37] Leveraging on this possibility, upper room UV-C light devices may be installed in COVID-19 isolation rooms to provide a no-touch disinfecting system and portable UV-C light devices may also be employed to disinfect isolation rooms after occupancy. Strategies like these may reduce time and labor in cleaning and disinfecting. Most importantly, it may reduce the risk of disease transmission from patient to health workers.
For the main interest of this review, several evidence of varying methodological quality across different outcomes was identified. Although a number of studies addressing the efficacy of UV-C irradiation in reducing hospital associated infection rates were included, the overall certainty of evidence collected was low, with the highest quality of evidence coming from a single RCT that studied the efficacy of mercury UV-C disinfection in reducing HAIs and colonization. The reasons for downgrading the certainty of the evidence were due to limitations in study design, imprecision due to wide confidence intervals, and high risk of bias among studies. In this review, there was considerable uncertainty as the majority of the included studies were before and after studies that had inconsistent effects on different hospital acquired infection rates. Limitations with this type of study include difficulty in controlling confounding variables that may influence both the pre- and post-intervention periods which could lead to an overestimation of the efficacy of UV-C irradiation. In addition, there was high risk of bias across studies, predominantly attributable to non-randomized methods of allocation. Insufficient randomization or allocation concealment put studies at risk of selection bias.[38] The unpredictability of conditions occurring in a live setting like a hospital is high, thus, it is important to blind the evaluating observers to treatment allocation and treatment supervision. Blinding is important in disqualifying confounders that may sweep in after the allocation has taken place; the lack thereof may result in an overestimation of the effects. It is also important to note that sample sizes of some of the included studies were generally small, which might compromise the value of the outcomes resulting in an underpowered study. Unlike the studies on the efficacy of UV-C irradiation, limited studies regarding its safety were included in this review. Similarly, overall certainty of the evidence collected was low due to limitations in study design.
Limitations
All relevant studies were identified and included in this review. However, only a limited number of studies addressing UV-C safety were included due to the lack of prior research that fit the pre-specified inclusion criteria. The main limitation was that a quantitative synthesis was not possible due to substantial heterogeneity in the methodology and reported outcomes of the included studies. For the studies on the UV-C efficacy, clinical diversity was observed largely from differences in the type of health-care setting, protocols used for both UV-C and standard disinfection, and outcome measures between studies. The type of subjects, amount of UV-C exposure, and outcome measures demonstrated in the studies concerning UV-C safety were greatly varied as well. Studies also presented with different degrees of bias, suggesting methodological diversity. It was only possible to provide a qualitative synthesis, which, nonetheless, provides a conclusion that will help guide both future researchers and policy makers.
Conclusions
UV-C can be utilized as an adjunct to terminal manual cleaning protocols in hospitals because of its efficacy as a germicidal agent. It could be particularly useful in high-traffic, high-touch places, and surfaces where bioburden is high. In addition to its efficacy, it also takes up less time and less manpower. However, further studies must be done to exact a standard for safe exposure dose especially for 222 nm germicidal lamps. More information and studies should be made in the context of UV-C disinfection and COVID-19 infection. Direct evidence is sorely needed for the implementation of UV-C against said virus. Overall, the use of UV-C as a disinfecting tool can outweigh its safety issues with the standardization of dose and possible use of 222 nm UV-C irradiation.
Ethics Approval and Consent to Participate
The study does not need ethical approval nor consent to participate as there is no human participation. The approval to conduct the study is found in the methodology section.
Availability of Data and Material
The data and other materials used to derive at the findings and conclusion in this study can be found at: https://doi.org/10.5281/zenodo.3933425.
Competing Interest
The authors declare that there are no conflicts of interest in the conduct of this study.
Funding Statement
The study was not funded by any funding agency nor required funding.
Authors’ Contribution
ALH conceptualized the study. CCRR, JLAR, DBS, and LEGS reviewed the literature. CCRR, JTPS, KIBT, GST, and PCR initially wrote the manuscript. All revised and approved the final manuscript.
Acknowledgment
None.
References
- 1.Donkor ES. Nosocomial pathogens:An in-depth analysis of the vectorial potential of cockroaches. Trop Med Infect Dis. 2019;4:14. doi: 10.3390/tropicalmed4010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sydnor ER, Perl TM. Hospital epidemiology and infection control in acute-care settings. Clin Microbiol Rev. 2011;24:141–73. doi: 10.1128/CMR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Guideline for Disinfection and Sterilization in Healthcare Facilities. Georgia, United States: Centers for Disease Control and Prevention; 2008. [Last accessed on 2020 May 08]. Available from: https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines.pdf . [Google Scholar]
- 4.World Health Organization. WHO Guidelines on Tuberculosis Infection Prevention and Control:2019 Update. Geneva: World Health Organization; 2019. [Last accessed on 2020 Jul 08]. Available from: https://www.who.int/tb/publications/2019/guidelines-tuberculosis-infection-prevention-y2019/en . [PubMed] [Google Scholar]
- 5.McDevitt JJ, Rudnick SN, Radonovich LJ. Aerosol susceptibility of influenza virus to UV-C light. Appl Environ Microbiol. 2012;78:1666–9. doi: 10.1128/AEM.06960-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaidzu S, Sugihara K, Sasaki M, Nishiaki A, Igarashi T, Tanito M. Evaluation of acute corneal damage induced by 222-nm and 254-nm ultraviolet light in Sprague-Dawley rats. Free Radic Res. 2019;53:611–7. doi: 10.1080/10715762.2019.1603378. [DOI] [PubMed] [Google Scholar]
- 7.Yin R, Dai T, Avci P, Jorge AE, de Melo WC, Vecchio D, et al. Light based anti-infectives:Ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr Opin Pharmacol. 2013;13:731–62. doi: 10.1016/j.coph.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–7. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang K. Scientists Consider Indoor Ultraviolet Light to Zap Coronavirus in the Air. 2020. [Last accessed on 2020 May 27]. Available from: https://www.nytimes.com/2020/05/07/science/ultraviolet-light-coronavirus.html .
- 10.Trevisan A, Piovesan S, Leonardi A, Bertocco M, Nicolosi P, Pelizzo MG, et al. Unusual high exposure to ultraviolet-C radiation. Photochem Photobiol. 2006;82:1077–9. doi: 10.1562/2005-10-27-ra-728. [DOI] [PubMed] [Google Scholar]
- 11.National Toxicology Program. 14th Report on Carcinogens. 2016. [Last accessed on 2020 Jul 08]. Available from: http://www.ntp.niehs.nih.gov/ntp/roc/twelfth/roc12.pdf.2019 .
- 12.Hilario AL, Ramos CC, Roque JL, Sarmiento D, Suarez LE, Sunio JT, et al. Use of UV-C Sterilization in Hospitals:A Systematic Review on Efficacy and Safety. United States: Zenodo; 2020. [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper J, Bryce E, Astrakianakis G, Stefanovic A, Bartlett K. Efficacy of an automated ultraviolet C device in a shared hospital bathroom. Am J Infect Control. 2016;44:1692–4. doi: 10.1016/j.ajic.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dippenaar R, Smith J. Impact of pulsed xenon ultraviolet disinfection on surface contamination in a hospital facility's expressed human milk feed preparation area. BMC Infect Dis. 2018;18:91. doi: 10.1186/s12879-018-2997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Haddad L, Ghantoji SS, Stibich M, Fleming JB, Segal C, Ware KM, et al. Evaluation of a pulsed xenon ultraviolet disinfection system to decrease bacterial contamination in operating rooms. BMC Infect Dis. 2017;17:672. doi: 10.1186/s12879-017-2792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jinadatha C, Quezada R, Huber TW, Williams JB, Zeber JE, Copeland LA. Evaluation of a pulsed-xenon ultraviolet room disinfection device for impact on contamination levels of methicillin-resistant Staphylococcus aureus. BMC Infect Dis. 2014;14:187. doi: 10.1186/1471-2334-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sampathkumar P, Folkert C, Barth JE, Nation L, Benz M, Hesse A, et al. A trial of pulsed xenon ultraviolet disinfection to reduce Clostridioides difficile infection. Am J Infect Control. 2019;47:406–8. doi: 10.1016/j.ajic.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Ethington T, Newsome S, Waugh J, Lee LD. Cleaning the air with ultraviolet germicidal irradiation lessened contact infections in a long-term acute care hospital. Am J Infect Control. 2018;46:482–6. doi: 10.1016/j.ajic.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Pavia M, Simpser E, Becker M, Mainquist WK, Velez KA. The effect of ultraviolet-C technology on viral infection incidence in a pediatric long-term care facility. Am J Infect Control. 2018;46:720–2. doi: 10.1016/j.ajic.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Morikane K, Suzuki S, Yoshioka J, Yakuwa J, Nakane M, Nemoto K. Clinical and microbiological effect of pulsed xenon ultraviolet disinfection to reduce multidrug-resistant organisms in the intensive care unit in a Japanese hospital:A before-after study. BMC Infect Dis. 2020;20:82. doi: 10.1186/s12879-020-4805-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nerandzic MM, Cadnum JL, Eckart KE, Donskey CJ. Evaluation of a hand-held far-ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens. BMC Infect Dis. 2012;12:120. doi: 10.1186/1471-2334-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penno K, Jandarov RA, Sopirala MM. Effect of automated ultraviolet C-emitting device on decontamination of hospital rooms with and without real-time observation of terminal room disinfection. Am J Infect Control. 2017;45:1208–13. doi: 10.1016/j.ajic.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Villacís JE, Lopez M, Passey D, Santillán MH, Verdezoto G, Trujillo F, et al. Efficacy of pulsed-xenon ultraviolet light for disinfection of high-touch surfaces in an Ecuadorian hospital. BMC Infect Dis. 2019;19:575. doi: 10.1186/s12879-019-4200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson DJ, Chen LF, Weber DJ, Moehring RW, Lewis SS, Triplett PF, et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the benefits of enhanced terminal room disinfection study):A cluster-randomised, multicentre, crossover study. Lancet. 2017;389:805–14. doi: 10.1016/S0140-6736(16)31588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yel M, Güven T, Türker H. Effects of ultraviolet radiation on the stratum corneum of skin in mole rats. J Radiat Res Appl Sci. 2014;7:506–11. [Google Scholar]
- 26.Zaffina S, Camisa V, Lembo M, Vinci MR, Tucci MG, Borra M, et al. Accidental exposure to UV radiation produced by germicidal lamp:Case report and risk assessment. Photochem Photobiol. 2012;88:1001–4. doi: 10.1111/j.1751-1097.2012.01151.x. [DOI] [PubMed] [Google Scholar]
- 27.Goswami S, Sharma S, Haldar C. The oxidative damages caused by ultraviolet radiation Type C (UVC) to a tropical rodent Funambulus pennanti:Role of melatonin. J Photochem Photobiol B. 2013;125:19–25. doi: 10.1016/j.jphotobiol.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 28.International Commission on Non-Ionizing Radiation Protection. Guidelines on limits of exposure to ultraviolet radiation of wavelengths between 180 nm and 400 nm (incoherent optical radiation) Health Phys. 2004;87:171–86. doi: 10.1097/00004032-200408000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Buonanno M, Ponnaiya B, Welch D, Stanislauskas M, Randers-Pehrson G, Smilenov L, et al. Germicidal efficacy and mammalian skin safety of 222-nm UV light. Radiat Res. 2017;187:483–91. doi: 10.1667/RR0010CC.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narita K, Asano K, Morimoto Y, Igarashi T, Nakane A. Chronic irradiation with 222-nm UVC light induces neither DNA damage nor epidermal lesions in mouse skin, even at high doses. PloS One. 2018;13:e0201259. doi: 10.1371/journal.pone.0201259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber DJ, Rutala WA, Anderson DJ, Chen LF, Sickbert-Bennett EE, Boyce JM. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination:Focus on clinical trials. Am J Infect Control. 2016;44:77–84. doi: 10.1016/j.ajic.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.International Commission on Illumination. UV-C Photocarcinogenesis Risks from Germicidal Lamps:Technical Report. Vienna, Austria: International Commission on Illumination; 2010. [Last accessed on 2020 Jul 08]. Available from: http://www.files.cie.co.at/ci187-2010%20(free%20copy%20March%202020).pdf . [Google Scholar]
- 33.Wharton K, Rieker BM. N95 respirator decontamination and reuse:Current state of the evidence. AANA J. 2020. [Last accessed on 2020 Jul 08]. Available from: https://www.aana.com/docs/default-source/aana-journal-web-documents-1/wharton-r.pdf?sfvrsn=1fd9a761_4 .
- 34.World Health Organization. Rational use of Personal Protective Equipment for Coronavirus Disease (COVID-19) and Considerations During Severe Shortages:Interim Guidance. Geneva: World Health Organization; 2020. [Last accessed on 2020 Jul 08]. Available from: https://www.apps.who.int/iris/handle/10665/331695 . [Google Scholar]
- 35.Derraik JG, Anderson WA, Connelly EA, Anderson YC. Rapid evidence summary on SARS-CoV-2 survivorship and disinfection, and a reusable PPE protocol using a double-hit process. MedRxiv. 2020 [Google Scholar]
- 36.Dietz L, Horve PF, Coil DA, Fretz M, Eisen JA, Van Den Wymelenberg K. 2019 Novel coronavirus (COVID-19) pandemic:Built environment considerations to reduce transmission systems. mSystems. 2020;5:e00245–20. doi: 10.1128/mSystems.00245-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.International Commission on Non-Ionizing Radiation Protection. UV-C lamps and SARS-CoV-2. 2020. [Last accessed on 2020 Jul 08]. Available from: https://www.icnirp.org/en/activities/news/news-article/sars-cov-2-and-uvc-lamps.html .
- 38.Nunan D, Heneghan C, Spencer EA. Catalogue of bias:Allocation bias. BMJ Evid Based Med. 2018;23:20–1. doi: 10.1136/ebmed-2017-110882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and other materials used to derive at the findings and conclusion in this study can be found at: https://doi.org/10.5281/zenodo.3933425.