Abstract
Objective:
Doxorubicin-mediated chemotherapy has been linked in cancer patients with acute toxic manifestations. This study aimed to assess the protective effect of natural honey against doxorubicin-induced toxicity in mice.
Methods:
Forty male BALB/C mice were divided randomly and equally into four groups of ten mice. Group I was on a normal diet and served as the control. Group II received only honey to investigate its antitoxic effects (oral administration at 10 g/kg for 10 days). Group III was injected with a single dose of doxorubicin (10 mg/kg i.p.). Group IV received both doxorubicin and honey. On day 11, blood was taken from the various groups of mice and the quantitative and qualitative changes were observed in the white blood cells. The levels of hepatic, renal, and cardiac toxicity biomarkers including aspartate aminotransaminase (AST), alanine aminotransaminase (ALT), blood nitrogen urea (BUN), serum creatinine, lactate dehydrogenase (LDH), and creatinine kinase (CK) were also assessed.
Results:
This study revealed that doxorubicin caused acute toxicity, including weight loss, depletion of white blood cells, and altered liver, kidney, and cardiac parameters (P < 0.05). White blood cells in the blood picture from the doxorubicin-injected mice exhibited toxicological changes, including abnormal morphology, disorganized nuclei, dispersed chromatin strands, and granules. The consumption of honey improved the liver, kidney, and heart biomarkers of toxicity in doxorubicin-injected mice (P < 0.05). Honey reduced the levels of ALT, AST, BUN, serum creatinine, LDH, and CK in doxorubicin-injected mice (P < 0.05).
Conclusion:
The findings suggest that the consumption of natural honey can alleviate doxorubicin-induced toxicity in cancer patients.
Keywords: Biochemical markers, cancer, doxorubicin, drug chemotherapy, honey, toxicity
Introduction
Chemotherapy is widely used to treat malignancies by virtue of its ability to kill rapidly dividing tumor cells. Moreover, chemotherapy targets fast-growing cells such as the white blood cells and bone marrow cells. Therefore, the administration of chemotherapy results in immunosuppressive conditions in treated patients.[1,2] Although chemotherapy has been successful in extending the survival of cancer patients, its administration affects the quality of life.
Doxorubicin, also known as adriamycin, is one of the most effective antitumor drugs with strong antineoplastic activity against various types of cancers.[3] However, the use of doxorubicin in medicine has been limited due to its diverse toxicities, including hematopoietic suppression, cardiac toxicity, nausea, vomiting, and alopecia.[4,5] Doxorubicin intercalates the DNA and produces free radicals by forming a compound called semiquinone or through oxidation-reduction enzymes.[6] These free radicals are toxic because they induce the production of hydrogen peroxide, which reacts with lipids and forms lipid peroxides.[7] Lipid peroxides destroy the plasma membrane leading to toxicity of body organs such as the heart, kidney, and liver.[6] In recent years, various drug delivery formulations have been prepared to increase the efficacy and reduce the toxicity of doxorubicin.[8,9] Co-administration of some immune-stimulating molecules have been resulted in the recovery of doxorubicin-induced immunosuppression.[10,11]
The consumption of some natural dietary supplements imparts health benefits and reduces the toxicity of the drugs. For example, drinking camel milk and black tea have been reported to possess health benefits.[12,13] Moreover, the consumption of honey reduced the toxic effects of anticancer drug cyclophosphamide.[14] Honey has been utilized by humans for centuries for nutritional and medicinal purposes.[15,16] It is a natural food product formed from the nectar of flowers by honey bees (Apis mellifera) and has therapeutic, nutritional, and religious value.[16] It is widely used as a therapeutic agent for wound healing, cataracts, cough, sore throat, leg ulcers, and measles.[15,17] The polyphenols in natural honey have antiproliferative properties against different types of cancer, and it has been shown to act as an immune booster in cancer chemotherapy.[18,19] Honey has also been shown to attenuate the toxic effects of tartrazine and melamine in rats.[20,21] Here, we assessed the protective effect of honey against doxorubicin-induced toxicity in a murine model.
Materials and Methods
Reagents
Doxorubicin was obtained from Tocris Biosciences (Bristol, UK). White blood cell counting reagents and the Leishman stain were supplied by Loba Chemie (Mumbai, India). Natural honey was collected freshly from sealed honeycombs in the College of Agriculture Farm, Qassim University, Buraydah, Saudi Arabia. The honey kept in the dark glass bottles and preserved at 10°C.
Mice
Male BALB/C mice were procured from the vivarium of King Saud University, Riyadh, Saudi Arabia. Mice were housed under standard housing conditions (25 ± 2°C temperature and 12 h light/dark cycle) for several weeks to acclimatize them before the start of the experiment. Mice were supplied with standard chow and tap water ad libitum.
Animal grouping and treatment
This work used 12–14-week-old male BALB/C mice. Mice were divided randomly and equally into four groups, and each group contained 10 mice. Group I was on a normal diet and served as a control. Mice in Group II received honey orally at 10 g/kg. Group III was labeled as the treatment group and mice received doxorubicin at a dose 10 mg/kg through an intraperitoneal route as described previously.[8] Group IV was labeled as a protective group and received doxorubicin and honey, where a group of doxorubicin-injected mice was given natural honey orally at a dose of 10 g/kg twice for 10 days.[22] The blood samples were collected from the mice for analysis after the completion of treatment. A schematic of the experimental design is shown in Figure 1.
Figure 1.
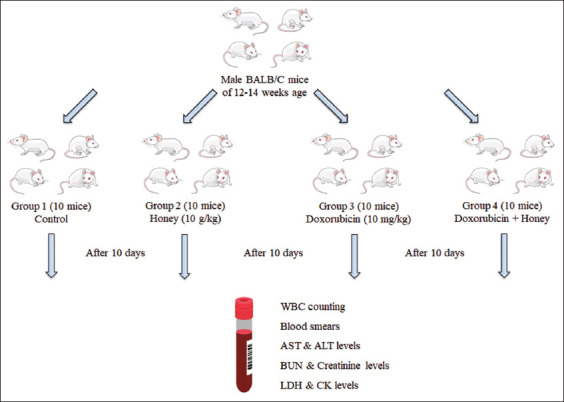
Schematic diagram of the study methodology. AST: Aspartate transaminase; ALT: Alanine transaminase; BUN: Blood nitrogen urea; LDH: Lactate dehydrogenase; CK: Creatinine kinase
Quantitative and qualitative analysis of white blood cells
The protective effect of the natural product honey against doxorubicin-induced leukopenia in male BALB/C mice was determined by evaluating both changes in the quantitative and qualitative in white blood cells. The process of quantitative analysis of white blood cells started on day 11 post-doxorubicin injection in male BALB/C mice. The blood samples were collected through retro-orbital puncture from three male BALB/C mice in all mice groups, and the number of white blood cells was then counted. Analysis of qualitative changes in the white blood cells was performed by preparing the blood smear and then staining these prepared slides with Leishman reagent. The stained slides were washed and dried. The slides were then examined under the microscope to analyze the structural changes in the white blood cells.[8]
Determination of the protective effect of natural honey against doxorubicin-induced toxicity
The protective effect of natural honey against hepatotoxicity induced by doxorubicin was determined in this study by measuring the levels of liver inflammation biomarkers in the blood such as aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT) through commercial kits as described.[23] The effect of natural honey against doxorubicin-induced renal and cardiac toxicity was also determined by estimating the levels of blood urea, serum creatinine, lactate dehydrogenase (LDH), and creatinine kinase (CK).[8]
Statistical analysis
The data were expressed in the current study as mean ± standard error of the mean (SEM). Various groups were compared using a one-way ANOVA followed by Bonferroni’s post hoc test. P < 0.05 was considered statistically significant. Data were graphed and analyzed using Prism software (Version 6.0, San Diego, USA).
Results
Consumption of honey protects white blood cells against doxorubicin-induced toxicity
The protective effect of the natural product honey against doxorubicin-induced toxicity was also evaluated in this study by determining the quantitative and qualitative changes in the white blood cells. The results showed that there was a significant decrease in the number of white blood cells in doxorubicin-injected mice (2827 ± 233/mm3 of the blood) versus the white blood cell count in the control mice (6302 ± 352/mm3 of the blood) on day 10 post-doxorubicin. Mice in the group that received honey following doxorubicin injection have (4400 ± 349 white blood cells per mm3 of the blood): This was significantly higher than in doxorubicin-injected mice (P < 0.05) [Figure 2]. Mice in the group that received honey but no doxorubicin have (6502 ± 878 white blood cells per mm3 of the blood) [Figure 2].
Figure 2.
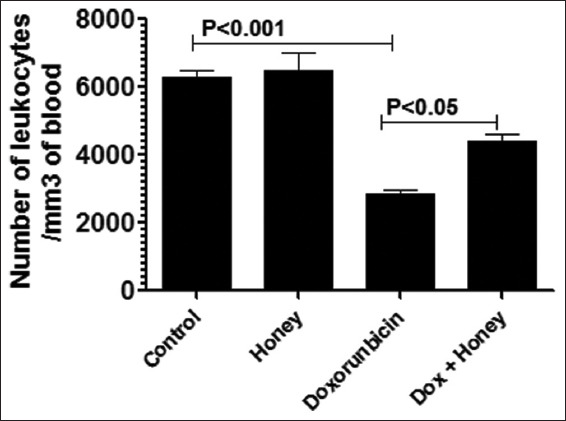
Effect of honey consumption recovery of doxorubicin-induced leukopenia. Honey at a dose of 10 g/kg was given orally to doxorubicin-injected male mice for 10 days. The white blood cells were counted 11 days after doxorubicin in three mice per group. The data are the mean values ± standard error of the mean (SEM); P < 0.05. Dox: Doxorubicin
Doxorubicin induces morphologic changes in white blood cells
There were changes in both the shape and structure of the white blood cells 10 days after doxorubicin injection. The cells from negative control mice exhibited a normal structure [Figure 3a]. Those from doxorubicin-injected mice had an abnormal morphology, disorganized nuclei, dispersed chromatin strands, and granules [Figure 3b]. Furthermore, most white blood cells lost their compactness and integrity, which is important for normal cell function. The white blood cells from the mice that received both doxorubicin and honey had a lower effect of doxorubicin [Figure 3c].
Figure 3.
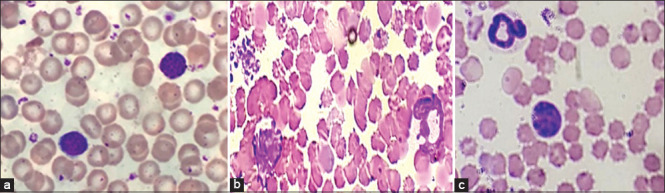
Doxorubicin causes qualitative changes in white blood cells. Using 40× magnification, white blood cells from (a) normal control mice, (b) doxorubicin-injected mice, and (c) doxorubicin-injected mice with honey treatment. Mice treated with doxorubicin at a dose of 10 mg/kg showed disintegrated white blood cells with dispersed chromatin strands and granules (b). Mice treated with doxorubicin and honey showed normal white blood cells and white blood cells undergoing apoptosis (c). Dox: Doxorubicin
Honey consumption ameliorated doxorubicin-induced hepatic toxicity
The effect of honey consumption against doxorubicin-induced hepatic toxicity was determined by measuring the levels of AST and ALT enzymes in the serum samples for both the untreated male mice (control group) and treated male mice. Administration of doxorubicin increased the level of AST to 118.6 ± 8.6 IU/L versus 26 ± 2.50 IU/L in the control mice group (P < 0.001). The honey co-administration remarkably lowered the AST enzyme level to 56.33 ± 6.6 (P < 0.01) [Figure 4a].
Figure 4.
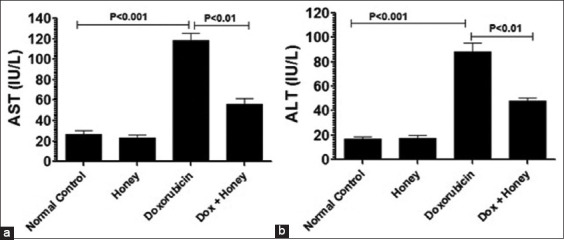
Consumption of honey improves the liver functioning in doxorubicin-injected male mice. On day 11 after doxorubicin injection, the blood sample was drawn from various groups of male mice to determine the levels of (a) aspartate transaminase and (b) alanine transaminase. Data are the mean values ± standard error of the mean; P < 0.01. Dox: Doxorubicin
The ALT levels were also higher in the blood samples of the male mice treated with doxorubicin. The ALT was 88.33 ± 9.62 IU/L in doxorubicin-injected male mice versus 16.7 ± 2.6 IU/L in the normal control mice. Doxorubicin-injected mice treated with honey had a significantly lower ALT level of 48 ± 5.5 IU/L (P < 0.01) [Figure 4b].
Consumption of honey reduces doxorubicin-induced nephrotoxicity
Honey also reduced the doxorubicin-induced nephrotoxicity as assessed by blood nitrogen urea (BUN) and serum creatinine [Figure 5a and b]. Administration of doxorubicin substantially increased the BUN level to 180 ± 22.4 mg/dL versus 22.3 ± 3.1 mg/dL in the control mice group [Figure 5a] (P < 0.001). The consumption of honey resulted in a significant reduction of BUN from 180 ± 22.4 mg/dL to 103.6 ± 13.8 mg/dL (P < 0.05).
Figure 5.
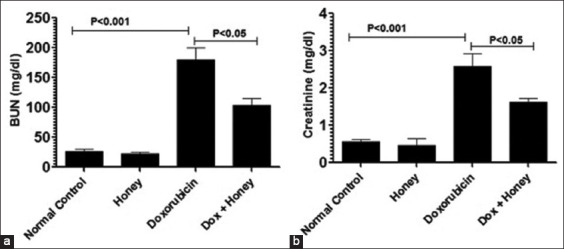
Consumption of honey reduces doxorubicin-induced renal toxicity. On day 11 post-doxorubicin injection, the blood sample was drawn from various groups of male mice to determine the levels of (a) blood nitrogen urea and (b) serum creatinine. Data shown are mean values ± standard error of the mean; P < 0.05. Dox: Doxorubicin
Serum creatinine is another biomarker of kidney dysfunction and was measured in doxorubicin-injected mice with and without honey co-treatment. The serum creatinine level was 2.59 ± 0.36 mg/dL, which is considerably higher than the value of 0.57 ± 0.064 mg/dL seen in male BALB/C mice in the control group [Figure 5b] (P < 0.001). The consumption of honey normalized serum creatinine to 1.61 ± 0.32 mg/dL [Figure 5b] (P < 0.05 vs. no treatment).
Honey lowers the levels of LDH and CK enzymes in doxorubicin-injected male mice
The effect of honey on doxorubicin-induced cardiac toxicity was determined by measuring serum LDH and CK levels. Male mice injected with doxorubicin at 10 mg/kg had LDH levels that increased to 192.7 ± 11.57% [Figure 6a]. This was markedly higher than the LDH value in control mice (P < 0.001). The consumption of natural honey reduced the LDH levels to 152.3 ± 7.5% (P < 0.05).
Figure 6.
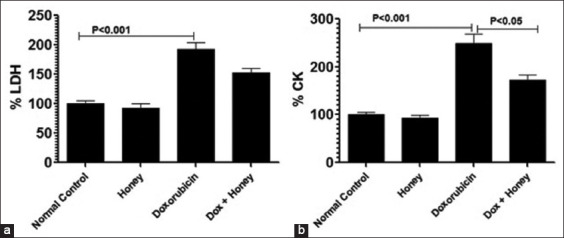
Consumption of honey reduces doxorubicin-induced cardiac toxicity as measured by (a) lactate dehydrogenase and (b) creatinine kinase on day 11 post-doxorubicin injection. Data shown are mean values ± standard error of the mean; P < 0.01. Dox: Doxorubicin
The CK levels were 249 ± 19% higher in doxorubicin-injected mice than the normal control (P < 0.001) but only 172 ± 11.4% higher when co-administered with honey (P < 0.05). The CK levels were comparable in mice from the control group and the honey-treated group [Figure 6b].
Discussion
Doxorubicin is a chemotherapy drug which is commonly used in the treatment of different types of cancers.[24] It can also treat several autoimmune diseases/non-specific immunopathies and can prevent organ rejection due to its immunosuppressive properties.[25,26] This study assessed whether the oral administration of honey could reduce doxorubicin-induced toxicity.
The results of the current study showed that doxorubicin caused severe toxicity, including immunosuppression as well as hepatic, renal, and cardiac toxicity. Consumption of natural honey alleviated the toxic effects of doxorubicin. This is evident from the findings of this study, which showed the recovery of white blood cells in the doxorubicin-injected mice that received honey. The metabolism of doxorubicin generates highly reactive superoxide free radicals that are highly toxic to cells, including white blood cells.[27] Honey contains polyphenols and antioxidants that opposes the effects of doxorubicin-generated free radicals to white blood cells.[19]
Doxorubicin administration is toxic to hepatic cells because of the production of reactive oxygen species, which includes hydroxyl radicals and superoxide anions that lead to lipid peroxidation and tissue damage.[28,29] These results indicated that the administration of doxorubicin caused a substantial elevation of enzymes such as AST and ALT, suggesting liver inflammation and damage. Honey has been shown to have healing properties in previous studies.[15] In this work, honey reduced the serum levels of AST and ALT enzymes in doxorubicin-injected mice that received honey.
The therapeutic use of doxorubicin also led to nephrotoxicity.[30] The increased levels of BUN and creatinine in the doxorubicin-injected mice suggest deterioration of kidney dysfunction. Earlier, the consumption of honey has been shown to protect against cisplatin-induced kidney toxicity through the suppression of inflammation.[31] The results of this study confirmed the protective effect of honey against doxorubicin-induced kidney toxicity in mice.
Doxorubicin induces severe cardiac toxicity through multiple mechanisms which include free radical stress, calcium overloading, and mitochondrial dysfunction.[30] The results of this study suggested that doxorubicin substantially increases the levels of the cardiac toxicity markers LDH and CK in mice (these markers are indicative of cardiac toxicity). Honey is an antioxidant and can significantly ameliorate doxorubicin-induced cardiac toxicity.
The findings of this study can be explained by the fact that reactive superoxide free radicals that generated in the metabolism of doxorubicin are highly toxic to cells. Honey has been shown to contain polyphenols and antioxidants that oppose the effects of doxorubicin-generated free radicals. Therefore, treatment with honey which contains several antioxidants as reported previously can reverse the toxic oxidative damages generated after doxorubicin treatment.[16,32] Furthermore, it has been shown that honey has the capacity to significantly decrease the lipid hydroperoxides concentration generated during the lipid peroxidation process after doxorubicin treatment.[33] This is attributed to the content of polyphenolic compounds found in the honey as shown in previous reports.[18,19] Furthermore, honey has been reported to have antimutagenic activity and a multifactorial process are involved in the antitumoral effects of honey.[16,32]
Conclusions
This study shows that the ingestion of honey after treatment with doxorubicin in mice reduces the toxicological effects of doxorubicin, protects white blood cells, and restores liver, kidney, and heart function. These findings confirm the potency of honey in protecting health due to its contents of some important antioxidants including flavonoids and carotenoids. Further research is needed to evaluate the effectiveness of the honey as an antitoxic agent against other toxic drugs and in humans.
Authors’ Declaration Statements
Ethics approval and consent to participate
All experiments conducted in the current study were strictly performed in accordance with the Guide for the Care and Use of Laboratory Animals. The experiments were approved by the Committee of Health Research Ethics at Qassim University, Saudi Arabia.
Availability of data and material
The data used in this study are available and will be provided by the corresponding author on a reasonable request.
Competing interest
No conflicts of interest are associated with this work.
Funding statement
The author received no financial support for the research or publication of this article.
Authors’ Contributions
100%: Author Fahad A. Alhumaydhi.
Acknowledgment
The author acknowledges the College of Applied Medical Sciences, Qassim University, Saudi Arabia, for providing facilities needed to conduct this study.
References
- 1.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 2.Chu E. Cancer chemotherapy. In: Katzung BG, editor. Basic and Clinical Pharmacology. New York: McGraw-Hill Education; 2018. pp. 948–76. [Google Scholar]
- 3.Nazir T, Taha N, Islam A, Abraham S, Mahmood A, Mustafa M. Monocytopenia;induction by vinorelbine, cisplatin and doxorubicin in breast, non-small cell lung and cervix cancer patients. Int J Health Sci (Qassim) 2016;10:542–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss RB. The anthracyclines:Will we ever find a better doxorubicin? Semin Oncol. 1992;19:670–86. [PubMed] [Google Scholar]
- 5.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–52. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Gewirtz D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–41. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 7.Younus H. Therapeutic potentials of superoxide dismutase. Int J Health Sci (Qassim) 2018;12:88–93. [PMC free article] [PubMed] [Google Scholar]
- 8.Khan MA, Aljarbou AN, Aldebasi YH, Alorainy MS, Khan A. Combination of glycosphingosomes and liposomal doxorubicin shows increased activity against dimethyl-?-benzanthracene-induced fibrosarcoma in mice. Int J Nanomed. 2015;10:6331–8. doi: 10.2147/IJN.S86467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maswadeh HM, Aljarbou AN, Alorainy MS, Rahmani AH, Khan MA. Coadministration of doxorubicin and etoposide loaded in camel milk phospholipids liposomes showed increased antitumor activity in a murine model. Int J Nanomed. 2015;10:2847–55. doi: 10.2147/IJN.S80820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho R, Maccubbin DL, Ujhazy P, Zaleskis G, Eppolito C, Mihich E, et al. Immunological responses critical to the therapeutic effects of adriamycin plus interleukin 2 in C57BL/6 mice bearing syngeneic EL4 lymphoma. Oncol Res. 1993;5:363–72. [PubMed] [Google Scholar]
- 11.Khan MA, Aljarbou AN, Aldebasi YH, Alorainy MS, Rahmani AH, Younus H, et al. Liposomal formulation of glycosphingolipids from Sphingomonas paucimobilis induces antitumour immunity in mice. J Drug Target. 2018;26:709–19. doi: 10.1080/1061186X.2018.1424857. [DOI] [PubMed] [Google Scholar]
- 12.Rasheed Z. Medicinal values of bioactive constituents of camel milk:A concise report. Int J Health Sci (Qassim) 2017;11:1–2. [PMC free article] [PubMed] [Google Scholar]
- 13.Rasheed Z. Molecular evidences of health benefits of drinking black tea. Int J Health Sci (Qassim) 2019;13:1–3. [PMC free article] [PubMed] [Google Scholar]
- 14.Khan MA. Immune potentiating and antitoxic effects of camel milk against cyclophosphamide-induced toxicity in BALB/C mice. Int J Health Sci (Qassim) 2017;11:18–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Richard J. Honey and healing through the ages. J ApiProd ApiMed Sci. 2009;1:2–5. [Google Scholar]
- 16.Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health:A review. J Am Coll Nutr. 2008;27:677–89. doi: 10.1080/07315724.2008.10719745. [DOI] [PubMed] [Google Scholar]
- 17.Manyi-Loh CE, Clarke AM, Ndip N. An overview of honey:Therapeutic properties and contribution in nutrition and human health. Afr J Microbiol Res. 2011;5:844–52. [Google Scholar]
- 18.Badolato M, Carullo G, Cione E, Aiello F, Caroleo MC. From the hive:Honey, a novel weapon against cancer. Eur J Med Chem. 2017;142:290–9. doi: 10.1016/j.ejmech.2017.07.064. [DOI] [PubMed] [Google Scholar]
- 19.Jaganathan SK, Mandal M. Antiproliferative effects of honey and of its polyphenols:A review. J Biomed Biotechnol. 2009;2009:830616. doi: 10.1155/2009/830616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Seeni MN, El Rabey HA, Al-Solamy SM. The protective role of bee honey against the toxic effect of melamine in the male rat kidney. Toxicol Ind Health. 2015;31:485–93. doi: 10.1177/0748233714551765. [DOI] [PubMed] [Google Scholar]
- 21.El Rabey HA, Al-Seeni MN, Al-Sieni AI, Al-Hamed AM, Zamzami MA, Almutairi FM. Honey attenuates the toxic effects of the low dose of tartrazine in male rats. J Food Biochem. 2019;43:e12780. doi: 10.1111/jfbc.12780. [DOI] [PubMed] [Google Scholar]
- 22.Gollu A, Kismet K, Kilicoglu B, Erel S, Gonultas MA, Sunay AE, et al. Effect of honey on intestinal morphology, intraabdominal adhesions and anastomotic healing. Phytother Res. 2008;22:1243–7. doi: 10.1002/ptr.2457. [DOI] [PubMed] [Google Scholar]
- 23.Laskar AA, Khan MA, Rahmani AH, Fatima S, Younus H. Thymoquinone, an active constituent of Nigella sativa seeds, binds with bilirubin and protects mice from hyperbilirubinemia and cyclophosphamide-induced hepatotoxicity. Biochimie. 2016;127:205–13. doi: 10.1016/j.biochi.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch J. An anniversary for cancer chemotherapy. JAMA. 2006;296:1518–20. doi: 10.1001/jama.296.12.1518. [DOI] [PubMed] [Google Scholar]
- 25.Hui-Chou HG, Olenczak JB, Drachenberg CB, Shea SM, Rodriguez ED. Short-term application of doxorubicin chemotherapy immunosuppressive side effects for composite tissue allotransplantation. Ann Plast Surg. 2012;68:215–21. doi: 10.1097/SAP.0b013e3182467f7b. [DOI] [PubMed] [Google Scholar]
- 26.Allison AC. Immunosuppressive drugs:The first 50 years and a glance forward. Immunopharmacology. 2000;47:63–83. doi: 10.1016/s0162-3109(00)00186-7. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee S, Ghosh J, Sil P. Drug metabolism and oxidative stress:Cellular mechanism and new therapeutic insights. Biochem Anal Biochem. 2016;5:255. [Google Scholar]
- 28.Singla S, Kumar NR, Kaur J. In vivo studies on the protective effect of propolis on doxorubicin-induced toxicity in liver of male rats. Toxicol Int. 2014;21:191–5. doi: 10.4103/0971-6580.139808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasheed N, Rasheed Z. Oxidative biomolecular damage:A possible mechanism for systemic autoimmunity. Int J Health Sci (Qassim) 2019;13:1–3. [PMC free article] [PubMed] [Google Scholar]
- 30.Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, et al. Doxorubicin:The good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–85. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 31.Hamad R, Jayakumar C, Ranganathan P, Mohamed R, El-Hamamy MM, Dessouki AA, et al. Honey feeding protects kidney against cisplatin nephrotoxicity through suppression of inflammation. Clin Exp Pharmacol Physiol. 2015;42:843–8. doi: 10.1111/1440-1681.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez-Suarez JM, Tulipani S, Romandini S, Bertoli E, Battino M. Contribution of honey in nutrition and human health:A review. Mediterr J Nutr Metab. 2010;3:15–23. [Google Scholar]
- 33.McKibben J, Engeseth NJ. Honey as a protective agent against lipid oxidation in ground Turkey. J Agric Food Chem. 2002;50:592–5. doi: 10.1021/jf010820a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are available and will be provided by the corresponding author on a reasonable request.


