Abstract
Objectives:
Acute kidney injury (AKI) is a major cause of morbidity and mortality. Whether aminophylline administration can prevent or treat AKI among pediatric patients are not clear. This meta-analysis aimed to assess the efficacy and effectiveness of aminophylline for pediatric AKI.
Methods:
We carried out a systematic search of six databases: PubMed, EMBASE/Excerpta Medica, Scopus, Cochrane library, and Google Scholar from January 1995 up till May 2019. Summary measures of risk ratios and standard mean difference were calculated using the random effects model.
Results:
We identified seven papers containing data on aminophylline use in children with AKI. Meta-analysis of single-arm studies indicated no statistically significant difference in mean rate of serum creatinine clearance (−0.39 [−0.80–1.58], P = 0.52), mean urine output (1.99 [−1.43–5.42]; P = 0.25), or mean blood urea nitrogen levels (0.83 [−1.86–3.03], P = 0.54) before and after aminophylline administration. However, among double-arm studies, aminophylline administration in the intervention arm significantly reduced the serum creatinine level as compared to control arm (mean diff = −34 [−55.18–−12.83]; P = 0.002). Mean urine output (−112.68 [−27.43–48.9], P = 0.17), incidence of AKI (RR = 1.05 [0.80–1.37], P = 0.72), and mortality rates (RR = 0.79 [0.42–1.47], P = 0.45) were found to be statistically insignificant.
Conclusions:
Aminophylline administration in children with AKI reduces serum creatinine level without significant adverse effects or effect on the incidence of AKI, urine output, or mortality. Further, large-scale well-planned randomized controlled trials are needed to evaluate its use and its potential long-term effects.
Keywords: Aminophylline, pediatric patients, acute kidney injury, renal outcomes, efficacy
Introduction
Acute kidney injury (AKI) has been defined as the sudden reduction in the kidney functions leading to reduced glomerular filtration rate (GFR). It occurs as a consequence of a few major diseases, major surgeries such as cardiac surgery and sepsis among all age groups. However, the condition is much more serious and difficult to control among children.[1,2] The incidence of AKI ranges from 28 to 51% among children, 52% in infants (<90 days), and 64% in neonates (aged ≤6 weeks) who underwent surgery for congenital heart defects such as biventricular cardiac repairs and cardiopulmonary bypass, resulting in high morbidity and mortality.[3-5] Serum creatinine is a traditional marker of renal function; however, it does not rise before 50% loss of renal function.[6] A minor rise in serum creatinine levels can seriously impact the socioeconomic status of a family by increasing length of hospital stay, greater hospital cost, prolonged need for mechanical ventilation, and increased mortality.[1,3,6,7]
There are two possible pathways of the complications developed by a pediatric patient after the first episode of AKI. One pathway hypothesizes that the survivors after the first attack of AKI would fully recover without having adverse effects in the future.[2,8] However, a recent meta-analysis has confirmed an increased risk of proteinuria, hypertension, and GFR <90 mL/min/1.73 m2 as a long-term complication after the first episode of AKI. This evidence reinforces the need to properly manage first episode of AKI effectively so as to reduce future morbidities.[2,8]
Various classes of drugs have undergone randomized controlled trials (RCTs) to assess their effectiveness in the prevention and/or treatment of AKI in children. Some these medications include diuretics (furosemide), dopamine, fenoldopam, theophylline/aminophylline, and rasburicase. Furosemide is a widely used diuretic for increasing the urine outflow but it has not shown any effect on prevention or treatment of pediatric AKI.[9,10] Similarly, dopamine and another dopamine receptor agonist fenoldopam have initially showed promising results in animal models but failed to prevent or treat AKI in clinical trials among humans.[11] Recently, studies have suggested the potential beneficial effects of methylxanthines such as theophylline and aminophylline in both the prevention and treatment of AKI.[12-24] In animal studies, theophylline – an adenosine receptor antagonist – was found to be protective of kidney injury following ischemia in rodents.[12-13] Furthermore, two meta-analyses have shown that theophylline was useful in the prevention of contrast-induced nephropathy and another meta-analysis noted an over 50% reduction in the risk of contrast-induced nephropathy-related AKI with the use of theophylline.[14-16] Similarly, several case series, case reports, and observational studies have suggested that the non-selective adenosine antagonist aminophylline was beneficial in both the prevention and treatment of pediatric AKI following cardiac surgeries or in critically ill children.[17-25] However, some of the recently published randomized clinical trials have shown inconsistent finding of its benefits.[26,27]
A few systematic reviews and meta-analyses have been conducted to assess the effects of methylxanthines and other medications for the improvement in or reduction of AKI in children.[29-31] However, most of these focused mainly on the effects of theophylline or other pharmacological measures such as dexmedetomidine, acetaminophen, and fenoldopam with limited evaluation of the effects of aminophylline.[29-31] We conducted this systematic review and meta-analysis to explore the present evidence and summarize the effects of aminophylline for the prevention and/or treatment of AKI among pediatric patients aged <18 years. The review specifically assessed the efficacy and safety of aminophylline compared with standard therapy or placebo for pediatric AKI prevention and/or treatment in terms of its effect on serum creatinine clearance rate, urine output, incidence of AKI, and mortality rate.
Methods
We have performed this meta-analysis as per the standard guidelines of Cochrane Collaboration. We followed the Preferred Reporting Items for Systematic Reviews and Meta- analyses and Meta-analysis of Observational Studies in Epidemiology guidelines for reporting systematic review and meta-analysis of RCTs and observational studies.[32,33]
Study population/exposure group
Pediatric patients (between the age of 0 and 18 years) having AKI or at increased risk of developing AKI.
Operational definitions
Increased risk of developing AKI: This term refers to the pediatric patients who did not have AKI preoperatively but had high chances of developing it after a major surgery like cardiac surgery or after sepsis. The definition of AKI in this study was based on the definition used by the included studies.
Interventions to be compared
Intervention arm
Aminophylline administered among pediatric patients who have/had AKI or increased risk of developing AKI.
Control arm
Non-aminophylline arm where either some other drug or standard therapy other than aminophylline was administered or no drug was administered (placebo). We excluded children with preexisting acute or chronic kidney disease and children with seizures and cardiac complications.
Study selection
Types of study
We included all RCTs as well as single-arm trials from January 1995 up till May 2019. Due to limited literature examining the effects of aminophylline, we also included relevant single-arm observational studies and case series.
However, we summarized the findings of single-arm observational studies and case series separately from RCTs to allow for meaningful interpretation of the findings. This approach allowed us to strengthen the evidence base in a research area with scarcity of well-planned double-armed RCTs which are considered to be the highest level of evidence-based studies.
Study selection
The following data sources were searched for all RCTs and prospective cohort control studies: PubMed, EMBASE/Excerpta Medica, SCOPUS, Cochrane Central Register of Controlled Trials, Google Scholar, and reference lists of relevant publications. The search strategies were independently designed and performed by two separate investigators (SA and SB). We used the following MeSH terms or keywords in different combinations and permutations to perform the search and limited our search to studies published from January 1995 to May 2019 without language restriction in the selected databases:
“AKI,” “Acute kidney failure,” “Aminophylline,” “pediatric patients,” and “children.”
The search strategies described above provided a list of studies. The titles and abstracts of all the retrieved studies were screened independently by two of the investigators (SA and SB). The irrelevant studies were discarded in the first screen of the titles and abstracts after removing duplicate articles. Subsequently, the full text of the selected studies was analyzed for the presence of outcome measures of interest.
Primary outcome measures
Serum creatinine clearance rate
Urine output rate
Incidence of developing AKI
Mortality rate.
Data extraction
The data were extracted using a pilot tested pro forma designed for the review. Two of the investigators (SA and SB) independently extracted data. The following information were extracted from the selected studies: First author, publication year, country, number of participants in each group, patient profile, intervention therapy details along with clearance rates of serum creatinine, rate of urine output, incidence of developing AKI, and mortality rates among the intervention and control groups. Outcomes reported in two or more articles were extracted for meta-analysis.
Data analysis
Extracted data were entered and analyzed using Revman 5.3. Before the analysis, data were standardized into equivalent units. Dichotomous variables AKI incidence and mortality rates in the aminophylline and non-aminophylline arms were expressed as rate ratio with 95% confidence interval (CI). For continuous variables such as serum creatinine clearance rate and urine output rate, standardized mean difference and 95% CI were calculated for each study. Pooled estimates were determined using the random effects model. Heterogeneity in the studies was evaluated using the Cochrane Q test and I2 statistic to assess the degree of interstudy variation. I2 values of 0%–24.9%, 25%–49.9%, 50%–74.9%, and 75%–100% were considered as having no, mild, moderate, and significant thresholds for statistical heterogeneity.
Ethical considerations
Ethical approval was not required for this study as it involved data extraction and data summation of the studies, which had already been conducted. These studies had already sought ethical approval and informed consent from the institute and the study participants.
Results
Section A. Study selection and description
The combined literature search identified around 1500 relevant studies, which contained the MeSH terms either in the title or abstract. Following the removal of duplicate studies (126) and a detailed review of the titles and abstracts; we included 18 studies for full-text review. Finally, only seven studies matched the inclusion criteria. The excluded studies were rejected on various grounds described in Figure 1.
Figure 1.

Flowchart showing selection of studies
The eligible studies were conducted from year 2000 till 2016. Out of totals even eligible studies, there was four single-arm trials or case series, as depicted in Table 1.[19,21,22,25] These studies did not have any comparison group and were, therefore, analyzed them separately. These single-arm trials were carried out using varied methodologies like the one done by McLaughlin et al. was a retrospective observational study, whereas Axelrod et al. documented a retrospective cohort study, as depicted in Table 1.[22,26] Lynch et al. and Tamburro et al. documented a case series to evaluating the effectiveness of aminophylline.[21,25] The total sample size was 89 with mean age ranging from 24 weeks to 3.8 years.
Table 1.
Matrix of single-arm studies

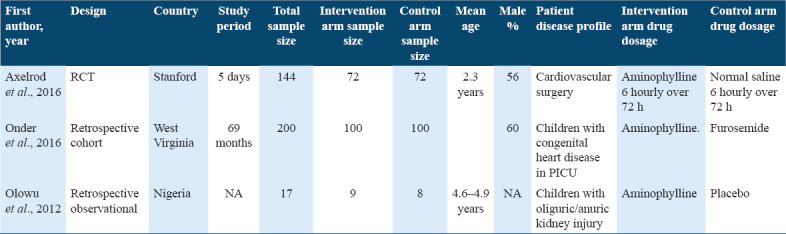
The other three studies had the comparison group but again, followed different methodologies.[23,24,26] Of these two are retrospective cohort studies and one was a RCT. The total sample size for these three studies was 361. All the three studies had similar intervention arm but different control arm as shown in Table 2. Two of the studies were conducted among children who underwent cardiovascular surgery.
Table 2.
Matrix of double-arm studies
Section B: Analysis of single arm studies
Outcome 1: Rate of serum creatinine clearance
The summary mean difference of all studies[19,21,22,25] indicated that there was no significant difference in the mean creatinine clearance rate among pediatric patients before and after the administration of aminophylline (mean difference = 0.39, 95% CI = −0.80-1.58, P = 0.52), Figure 2. There was significant heterogeneity among the selected studies for this outcome measure (τ2 = 1.27, Chi-square = 34.65, df = 3, P < 0.00001, I2 = 91%).
Figure 2.

Forest plot comparing serum creatinine clearance rate before and after aminophylline administration among pediatric patients
Outcome 2: Rate of urine output
Only two studies carried out by Tamburro et al. and McLaughlin et al. documented the rate of urinary output before and after administering aminophylline.[22,25] It was found that there was no significant difference in the mean rate of urinary output among pediatric patients before and after the administration of aminophylline (mean difference = 1.99, 95% CI = −1.43-5.42, P = 0.52), Figure 3. There was also significant heterogeneity among the selected studies for this outcome measure (τ2 = 5.77, Chi-square = 17.15, df = 1, P < 0.00001, I2 = 94%).
Figure 3.

Forest plot comparing urine output rate before and after aminophylline administration among pediatric patients
Outcome 3: Difference in mean blood urea nitrogen (BUN) levels
The summary measures of two studies[21,25] indicated that there was no significant difference in the BUN clearance rate among pediatric patients before and after the administration of aminophylline (mean difference = 0.83, 95% CI = −1.86-3.53, P = 0.54), Figure 4. There was significant heterogeneity among the selected studies for this outcome measure (τ2 = 3.62, Chi-square = 23.14, df = 1, P < 0.00001, I2 = 96%).
Figure 4.

Forest plot comparing mean BUN clearance levels before and after aminophylline administration among pediatric patients
Section C: Analysis of double arm studies
Outcome 1: Comparing mean serum creatinine concentration among intervention and control arm
All the three studies provided data to analyze this outcome measure.[23,24,26] The summary mean difference of all studies indicated that there was significant reduction in the mean serum creatinine concentration among pediatric patients who were administered aminophylline as compared to control group (mean difference = −0.34, 95% CI = −55.18–−12.83, P = 0.002), Figure 5. There was significant heterogeneity among the selected studies for this outcome measure (τ2 = 233.45, Chi-square = 43.67, df = 3, P < 0.00001, I2 = 95%).
Figure 5.

Forest plot comparing change in serum creatinine concentration among aminophylline administration group versus control group among pediatric patients
Outcome 2: Comparing mean urine output among intervention and control arm
All the three studies provided data to analyze this outcome measure.[23,24,26] The summary mean difference of all studies indicated that there was no significant improvement in the urinary output among pediatric patients who were administered aminophylline as compared to control group (mean difference = −112.68, 95% CI = −274.26–48.90, P = 0.17), Figure 6. There was 100% heterogeneity among the selected studies for this outcome measure (Chi-square = 416.08, df = 3, P < 0.00001, I2 = 100%).
Figure 6.

Forest plot comparing mean urinary output among aminophylline administration group versus control group among pediatric patients
Outcome 3: Comparing AKI among intervention and control arm
The summary risk ratio of all studies[23,24,26] indicated that there was no significant difference in the incidence rate of AKI among pediatric patients who were administered aminophylline as compared to control group (RR = 1.05, 95% CI = 0.80–1.37, P = 0.72). Figure 7. There was 56% heterogeneity among the selected studies for this outcome measure (Chi-square = 4.52, df = 2, P = 0.10, I2 = 56%).
Figure 7.

Forest plot comparing acute kidney injury among aminophylline administration group versus control group among pediatric patients
Outcome 4: Comparing incidence of mortality rate among intervention and control arm
The summary risk ratio of all studies[23,24] indicated that there was no significant difference in the mortality rates among pediatric patients who were administered aminophylline as compared to control group (RR = 0.79, 95% CI = 0.42–1.47, P = 0.45), Figure 8. There was no heterogeneity among the selected studies for this outcome measure (Chi-square = 0.56, df = 2, P = 0.45, I2 = 0%).
Figure 8.

Forest plot comparing mortality rate among aminophylline administration group versus control group among pediatric patients
Discussion
Summary of main findings
The present meta-analysis was performed to systematically summarize the available evidence and assess the effectiveness of aminophylline in preventing and/or treating AKI induced by a major surgery or illness in pediatric patients. We found only seven studies which were either single-arm observational studies or clinical trials. Outcomes of the meta-analysis revealed that there were no significant effects of aminophylline use on the incidence of AKI, serum creatinine clearance rate, urine output, and all-cause mortality in the single-arm studies. While in the double-arm studies, except for serum creatinine clearance, similar findings were observed. This approach adopted to perform this review enabled us to pool data from all available studies irrespective of the study design. Hence, this may be considered as a strength of this meta-analysis which has summarized data on the effectiveness of aminophylline use from both clinical trials, observational studies and case series.
In our study, the analysis of single-arm studies or case series suggested that there is no difference in the mean creatinine clearance rate, mean urinary output, and mean BUN levels before and after the administration of aminophylline among pediatric patients. Similarly, except for the mean change in the serum creatinine level, the results were statistically insignificant for other outcomes in double-arm trials.[23,24,26] The findings of this meta-analysis are in line with the individual studies included in this analysis. Furthermore, although the standardized mean difference in urinary output increased following the administration of aminophylline, the increase did not reach statistical significance. This finding may be due to the relatively small sample sizes of the included studies and these studies may not have enough power to detect the real difference or effects. Furthermore, there was substantial heterogeneity among the studies because of varied population attributes due to different age groups, geographical distribution, clinical conditions, different type of surgeries, and, importantly, different times of administering aminophylline. For instance, Axelrod et al. administered aminophylline to the intervention group after 4 h postoperatively following cardiac surgery among children having mean age of 2.3 years,[26] while Onder et al. administered aminophylline intraoperatively during cardiac surgery among pediatric patients.[24]
The findings of this systematic review suggest that the use of aminophylline in children has minimal effect in the prevention or treatment of AKI, creatinine concentrations, urine output, and mortality. Although, some of the findings of the individual studies suggested that the medication may have an effect in improving renal perfusion,[19,21-26] and some of these outcomes, their pooled analysis indicated limited effects on the outcomes. A previous study has suggested that renal tissue response may be age dependent; but this needs to confirmed in future large-scale studies.[28] Pooled analysis from studies having a control group indicates that aminophylline use was associated with significant reduction in the mean serum creatinine concentration.[23,24,26]
The mechanisms of action of aminophylline in lowering serum creatinine clearance and possibly improving renal perfusion could be explained by adenosine receptor inhibition at minimal doses and type IV phosphodiesterase blockade at increased doses. Adenosine plays an important role in tubuloglomerular feedback.[34] With increasing solute load in the renal tubules, energy depletion occurs, accompanied by the release of adenosine. The secreted adenosine stimulates pre-glomerular vasoconstriction leading to a reduction in solute flow and, contributing to, maintaining energy balance. Furthermore, aminophylline-induced phosphodiesterase inhibition lowers the breakdown of cAMP, which promotes renal vasodilation and renal perfusion.[35] Despite the divergent results of the effects of aminophylline, substantial interest still exists in the evaluation of the effect of aminophylline for AKI. The Pharmacology of Aminophylline for AKI in Neonates (PAANS) trial (NCT02276170) is currently underway and aims to explore the role of aminophylline as a treatment for AKI by measuring changes in urine output, creatinine, and other urine biomarkers among neonates.[36]
Limitations
This review has some strengths and limitations. This is an updated systematic review consisting of all studies irrespective of design which summarized the effect of aminophylline for AKI prevention or treatment in children. However, the review has some limitations. First, we found that most of the studies included in the review had small sample sizes and therefore insufficient power to detect differences in the outcomes. Second, there was substantial heterogeneity between the studies. This might have arisen from variations in the population attributes such as different age groups, geographical spread, clinical presentation, type of surgeries, and timing of administration aminophylline.[19,21-26] Finally, none of the studies assessed the long-term effect of use of aminophylline in children with AKI. Despite these limitations, this review has provided evidence to further justify the need for well-designed RCTs such as the ongoing neonates (PAANS) trial to assess the effectiveness of aminophylline for AKI in children.
Conclusions
This meta-analysis showed that aminophylline administration in children with AKI reduces the serum creatinine level without significant adverse effects or effect on the incidence of AKI, urine output, or mortality. Further well-designed trials reporting on all the outcomes are needed to assess the effectiveness of aminophylline in improving the renal outcomes of children with AKI or those at increased risk of developing it.
Author Declaration Statements
Ethical approval
Not applicable.
Statement of informed consent
Not applicable.
Availability of data and materials
Available with the corresponding author and will be produced on reasonable request.
Funding
None.
Interests of competing
None.
Authors’ contributions
All authors equally contributed.
Acknowledgment
None.
References
- 1.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, et al. Evolving importance of kidney disease:From subspecialty to global health burden. Lancet. 2013;382:158–69. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg JH, Coca S, Parikh CR. Long-term risk of chronic kidney disease and mortality in children after acute kidney injury:A systematic review. BMC Nephrol. 2014;15:184. doi: 10.1186/1471-2369-15-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baskin E, Saygili A, Harmanci K, Agras PI, Özdemir FN, Mercan S, et al. Acute renal failure and mortality after open-heart surgery in infants. Ren Fail. 2005;27:557–60. doi: 10.1080/08860220500199035. [DOI] [PubMed] [Google Scholar]
- 4.Blinder JJ, Goldstein SL, Lee VV, Baycroft A, Fraser CD, Nelson D, et al. Congenital heart surgery in infants:Effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. 2012;143:368–74. doi: 10.1016/j.jtcvs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Morgan CJ, Zappitelli M, Robertson CM, Alton GY, Sauve RS, Joffe AR, et al. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr. 2013;162:120–7.e1. doi: 10.1016/j.jpeds.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 6.Yuan SM. Acute kidney injury after pediatric cardiac surgery. Pediatr Neonatol. 2019;60:3–11. doi: 10.1016/j.pedneo.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Zappitelli M, Bernier PL, Saczkowski RS, Tchervenkov CI, Gottesman R, Dancea A, et al. A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int. 2009;76:885–92. doi: 10.1038/ki.2009.270. [DOI] [PubMed] [Google Scholar]
- 8.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury:A systematic review and meta-analysis. Kidney Int. 2012;81:442–8. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho KM, Sheridan DJ. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ. 2006;333:420. doi: 10.1136/bmj.38902.605347.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jetton JG, Rhone ET, Harer MW, Charlton JR, Selewski DT. Diagnosis and treatment of acute kidney injury in pediatrics. Curr Treat Options Pediatr. 2016;2:56–68. [Google Scholar]
- 11.Ricci Z, Luciano R, Favia I, Garisto C, Muraca M, Morelli S, et al. High-dose fenoldopam reduces postoperative neutrophil gelatinase-associated lipocaline and cystatin C levels in pediatric cardiac surgery. Crit Care. 2011;15:R160. doi: 10.1186/cc10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, et al. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–91. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okusa MD, Linden J, Macdonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol. 1999;277:F404–12. doi: 10.1152/ajprenal.1999.277.3.F404. [DOI] [PubMed] [Google Scholar]
- 14.Bagshaw SM, Ghali WA. Theophylline for prevention of contrast-induced nephropathy:A systematic review and meta-analysis. Arch Intern Med. 2005;165:1087–93. doi: 10.1001/archinte.165.10.1087. [DOI] [PubMed] [Google Scholar]
- 15.Dai B, Liu Y, Fu L, Li Y, Zhang J, Mei C. Effect of theophylline on prevention of contrast-induced acute kidney injury:A meta-analysis of randomized controlled trials. Am J Kidney Dis. 2012;60:360–70. doi: 10.1053/j.ajkd.2012.02.332. [DOI] [PubMed] [Google Scholar]
- 16.Ix JH, McCulloch CE, Chertow GM. Theophylline for the prevention of radiocontrast nephropathy:A meta-analysis. Nephrol Dial Transplant. 2004;19:2747–53. doi: 10.1093/ndt/gfh468. [DOI] [PubMed] [Google Scholar]
- 17.Ng GY, Baker EH, Farrer KF. Aminophylline as an adjunct diuretic for neonates a case series. Pediatr Nephrol. 2005;20:220–2. doi: 10.1007/s00467-004-1692-9. [DOI] [PubMed] [Google Scholar]
- 18.Park K, Trout LC, Xu C, Wang M, Tamburro RF, Halstead ES. No requirement for targeted theophylline levels for diuretic effect of aminophylline in critically ill children. Pediatr Crit Care Med. 2018;19:e425–32. doi: 10.1097/PCC.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Axelrod DM, Anglemyer AT, Sherman-Levine SF, Zhu A, Grimm PC, Roth SJ, et al. Initial experience using aminophylline to improve renal dysfunction in the pediatric cardiovascular ICU. Pediatr Crit Care Med. 2014;15:21–7. doi: 10.1097/01.pcc.0000436473.12082.2f. [DOI] [PubMed] [Google Scholar]
- 20.Bakr AF. Prophylactic theophylline to prevent renal dysfunction in newborns exposed to perinatal asphyxia a study in a developing country. Pediatr Nephrol. 2005;20:1249–52. doi: 10.1007/s00467-005-1980-z. [DOI] [PubMed] [Google Scholar]
- 21.Lynch BA, Gal P, Ransom JL, Carlos RQ, Dimaguila MA, Smith MS, et al. Low-dose aminophylline for the treatment of neonatal non-oliguric renal failure-case series and review of the literature. J Pediatr Pharmacol Ther. 2008;13:80–7. doi: 10.5863/1551-6776-13.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaughlin GE, Kashimawo LA, Steele BW, Kuluz JW. Reversal of acute tacrolimus-induced renal vasoconstriction by theophylline in rats. Pediatr Crit Care Med. 2003;4:358–62. doi: 10.1097/01.PCC.0000074269.30004.7E. [DOI] [PubMed] [Google Scholar]
- 23.Olowu WA. Epidemiology, pathophysiology, clinical characteristics and management of childhood cardiorenal syndrome. World J Nephrol. 2012;1:16–24. doi: 10.5527/wjn.v1.i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onder AM, Rosen D, Mullett C, Cottrell L, Kanosky S, Grossman OK, et al. Comparison of intraoperative aminophylline versus furosemide in treatment of oliguria during pediatric cardiac surgery. Pediatr Crit Care Med. 2016;17:753–63. doi: 10.1097/PCC.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamburro RF, Thomas NJ, Ceneviva GD, Dettorre MD, Brummel GL, Lucking SE. A prospective assessment of the effect of aminophylline therapy on urine output and inflammation in critically ill children. Front Pediatr. 2014;2:59. doi: 10.3389/fped.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Axelrod DM, Sutherland SM, Anglemyer A, Grimm PC, Roth SJ. A double-blinded, randomized, placebo-controlled clinical trial of aminophylline to prevent acute kidney injury in children following congenital heart surgery with cardiopulmonary bypass. Pediatr Crit Care Med. 2016;17:135–43. doi: 10.1097/PCC.0000000000000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahbazi S, Alishahi P, Asadpour E. Evaluation of the effect of aminophylline in reducing the incidence of acute kidney injury after cardiac surgery. Anesth Pain Med. 2017;7:e21740. doi: 10.5812/aapm.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merrikhi AR, Ghaemi S, Gheissari A, Shokrani M, Madihi Y, Mousavinasab F. Effects of aminophyllinein preventing renal failure in premature neonates with asphyxia in Isfahan-Iran. J Pak Med Assoc. 2012;62:S48–51. [PubMed] [Google Scholar]
- 29.Bhatt GC, Gogia P, Bitzan M, Das RR. Theophylline and aminophylline for prevention of acute kidney injury in neonates and children:A systematic review. Arch Dis Child. 2019;104:670–9. doi: 10.1136/archdischild-2018-315805. [DOI] [PubMed] [Google Scholar]
- 30.Lee JW, Lee SY, An SH. The effect of theophylline on improvement of renal function in asphyxiated neonates:A systematic review and meta-analysis. Korean J Clin Pharm. 2019;29:115–24. [Google Scholar]
- 31.Bellos I, Iliopoulos DC, Perrea DN. Pharmacological interventions for the prevention of acute kidney injury after pediatric cardiac surgery:A network meta-analysis. Clin Exp Nephrol. 2019;23:782–91. doi: 10.1007/s10157-019-01706-9. [DOI] [PubMed] [Google Scholar]
- 32.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology:A proposal for reporting. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses:The PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Lai EY, Huang Y, Eisner C, Mizel D, Wilcox CS, et al. Renal afferent arteriolar and tubuloglomerular feedback reactivity in mice with conditional deletions of adenosine 1 receptors. Am J Physiol Renal Physiol. 2012;303:F1166–75. doi: 10.1152/ajprenal.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas NJ, Carcillo JA. Theophylline for acute renal vasoconstriction associated with tacrolimus:A new indication for an old therapeutic agent? Pediatr Crit Care Med. 2003;4:392–3. doi: 10.1097/01.PCC.0000075322.56699.83. [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Busse LW. Novel therapies for acute kidney injury. Kidney Int Rep. 2017;2:785–99. doi: 10.1016/j.ekir.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available with the corresponding author and will be produced on reasonable request.


