Abstract
Propionibacterium acnes (P. acnes) is a commensal bacterium indigenous to the skin. Previous reports have suggested that infection with P. acnes causes sarcoidosis, a systemic granulomatous disease. We present the case of a 63-year-old woman who developed subcutaneous nodules. A skin biopsy revealed necrotizing vasculitis and noncaseating granulomas, which are characteristic of sarcoidosis. Immunohistostaining revealed a P. acnes skin infection, which led to the diagnosis of sarcoidosis. Minocycline treatment resolved the infection and improved the patient's symptoms. We herein report a case in which immunohistochemistry was useful in the diagnosis of sarcoidosis.
Keywords: sarcoidosis, P. acnes, necrotizing vasculitis
Introduction
Sarcoidosis is a systemic granulomatous disease of unknown cause that is pathologically characterized by noncaseating granulomas. Cutaneous lesions have been reported in approximately 20-30% of sarcoidosis patients (1). Immunosuppressive agents such as corticosteroids are generally effective for the treatment of sarcoidosis with systemic symptoms (2). However, several studies have reported that minocycline therapy may be a promising option for the treatment of cutaneous sarcoidosis (3).
Sarcoidosis has long been considered to have microbial etiologies because of the histological similarity to infectious granulomatous diseases (4). Propionibacterium acnes (P. acnes) is currently the only microorganism isolated from sarcoid lesions (5,6). Using quantitative polymerase chain reaction, Ishige et al. discovered that P. acnes resided or proliferated specifically in sarcoid lesions (7). To elucidate the pathogenic role of P. acnes, Negi et al. used immunohistochemical methods with novel P. acnes-specific monoclonal antibodies that react with cell membrane-bound lipoteichoic acid (PAB antibody) or ribosome-bound trigger factor protein (TIG antibody) (8). Immunohistochemistry performed with the PAB antibody revealed the presence of P. acnes within sarcoid granulomas, which suggests an etiological link between sarcoidosis and P. acnes. Thus, Eishi proposed that the PAB antibody may be useful for diagnosing sarcoidosis caused by P. acnes, in cases in which its reactivity is evaluated in idiopathic granulomas (9).
We herein report here a case of necrotizing vasculitis related to sarcoidosis that was diagnosed via immunohistochemical staining using P. acnes-specific antibodies and which was treated successfully with minocycline.
Case Report
A 63-year-old Japanese woman, with a past medical history of uveitis that had been treated with corticosteroids for two months, developed arthritis in both the right wrist and left ankle. Laboratory tests showed increased levels of C-reactive protein (CRP, 4.88 mg/dL, normal range: <0.3 mg/dL), an elevated erythrocyte sedimentation rate (ESR, 78 mm/h, normal range: 1-15 mm/h), and rheumatoid factor positivity (64 U/mL, normal range: <15 U/mL). The patient was negative for anti-cyclic citrullinated peptide antibodies (1.1 U/mL, normal range: <4.5 U/mL). Based on these findings, she was diagnosed with early rheumatoid arthritis. Although both bucillamine (100 mg daily) and salazosulfapyridine (1,000 mg daily) were administrated for three months, the arthritis worsened and a subcutaneous nodule appeared on the lower left leg. The patient visited our hospital for further evaluation and treatment.
On admission, a physical examination indicated aphthous stomatitis, swelling in the right elbow, and tenderness in the right ankle. Subcutaneous nodules (max size: 10 mm×10 mm) were observed on the lower legs, with tenderness and partial redness, which were compatible with erythema nodosum. In ophthalmologic studies, no significant findings (including uveitis) were detected. Laboratory tests revealed elevated levels of CRP (1.53 mg/dL), ESR (78 mm/h), but a normal white blood cell count (8,560 cells/mL). The patient's serum calcium (9.4 mg/dL, normal range: 8.2-10.0 mg/dL), angiotensin converting enzyme (9.9 U/L, normal range: 7.0-25.0 U/L), soluble interlukin-2 receptor (362 U/mL, normal range: 122-496 U/mL), and anti-neutrophil cytoplasmic antibody levels were within the normal limits. A tuberculin test and an interferon-γ release assay were negative, which suggested the nodules were not related to underlying tuberculosis. A chest X-ray showed no abnormalities (including bilateral hilar lymphadenopathy). A hand X-ray showed a narrow cleft between articulations on the hand with bilateral wrist pain. Abdominal computed tomography revealed no abnormalities. Magnetic resonance imaging of the legs showed a high intense area on the left thigh under short-tau inversion recovery and Gadolinium diethylenetriaminepentaacetic acid conditions adjacent to subcutaneous nodules, which suggested inflammation of the muscle and fascia due to vasculitis and corresponded with active inflammation of sarcoidosis (10). Neither gallium scintigraphy nor a positron emission tomography test were performed.
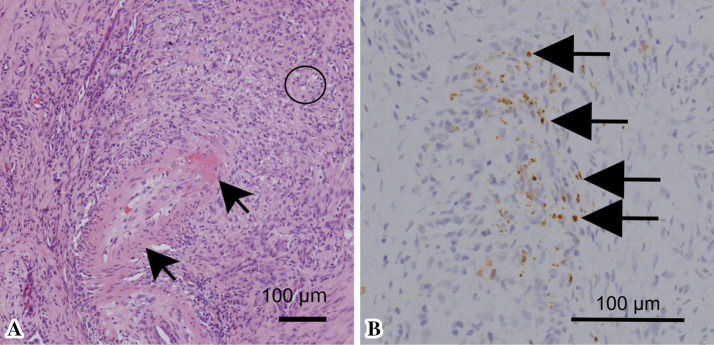
A histological assessment of the affected skin confirmed necrotizing vasculitis and noncaseating granulomas (Figure A). Multinucleated giant cells were not detected in the granulomas. Acid fast staining did not detect any organisms. Immunohistochemical staining with PAB antibodies showed a large number of P. acnes-positive cells in and surrounding both blood vessels and noncaseating granulomas (Figure B). There were so many P. acnes-positive cells that it was difficult to identify the cell types of the positive cells.
Figure.
A: Hematoxylin and Eosin staining revealed necrotizing vasculitis (shown as black arrows, fibrinous extraction was found in the vascular wall) and noncaseating granuloma with epithelioid cells (shown as a circle). B: Immunohistochemical staining using Propionibacterium acnes-specific antibodies revealed the presence of Propionibacterium acnes-positive cells (shown as black arrows).
Based on all the findings, the patient was diagnosed with sarcoidosis and treated with oral minocycline (200 mg daily). After three months of treatment with minocycline, the patient's arthritis improved. Additionally, the subcutaneous nodule was reduced in size and no relapse of nodules was detected. Moreover, the serum levels of CRP decreased to 0.51 mg/dL. The patient's rheumatoid factor level was not followed after the administration of minocycline.
Discussion
We described the first case of cutaneous sarcoidosis with necrotizing vasculitis that was diagnosed via immunohistochemical staining using P. acnes-specific antibodies. Granulomas with necrotizing vasculitis can be found in patients with primary vasculitis (e.g., microscopic polyangiitis, granulomatosis polyangiitis) and secondary vasculitis (e.g., tuberculosis infection, Bechet's disease, sarcoidosis). It can be difficult to diagnose secondary vasculitis because the symptoms are sometimes similar to primary vasculitis. We emphasize here that immunohistochemical staining using P. acnes-specific antibodies is useful for the diagnosis of sarcoidosis-related vasculitis.
The treatment options for cutaneous sarcoidosis include corticosteroids, hydroxychloroquine, immunosuppressants (e.g., methotrexate or azathioprine), tumor necrosis factor alpha (TNF-α) inhibitors and minocycline (1). In previous cases of sarcoidosis accompanied by vasculitis, corticosteroids and/or immunosuppressants are used (11). While these therapies are effective, their long-term use may be associated with problematic side effects, including infection. Minocycline, which is a widely used antibiotic, can be used without severe side effects other than pigmentation. As shown in our case, minocycline therapy may be effective and may be considered as a treatment for cutaneous vasculitis due to P. acnes-associated sarcoidosis.
The mechanism underlying the effects of minocycline in the treatment of sarcoidosis has not been elucidated. Some argue that the usefulness of tetracyclines in sarcoidosis lies in their well-known anti-inflammatory properties, rather than their antibacterial action (3). The therapeutic effect of TNF-α inhibitors has been reported: TNF-α is one of the inflammatory cytokines involved in the formation of granulomas in sarcoidosis (12). On the other hand, cases of TNF-α inhibitor-induced sarcoidosis have been reported (13). Additionally, a case in which vasculitis and sarcoidosis developed simultaneously during TNF-α inhibitor treatment has been reported (14). In that case, the production of neutralizing auto-antibodies against TNF-α inhibitor, which resulted from the activation of T lymphocytes, may be related with sarcoid-like granulomatous disease and leukocytoclastic vasculitis, which are the most common manifestations of cutaneous vasculitis (14). Minocycline has been shown to inhibit T lymphocytes proliferation (15). In our present case, the therapeutic effect of minocycline may be associated with the inhibition of T lymphocyte activation.
In conclusion, immunohistochemical staining with P. acnes-specific monoclonal antibodies might be useful in the diagnosis of sarcoidosis-related vasculitis. Antibiotics such as minocycline can be recommended as an initial treatment in patients with cutaneous necrotizing vasculitis related to sarcoidosis.
Written informed consent for publication was obtained from the patient. According to ethical guidelines for medical science in Japan, case reports are exempt from ethical review because they are not regarded as medical research. Therefore, we have not applied for ethical review concerning this case report.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Dr. Eisaku Ito for performing the pathological examination associated with this report.
References
- 1.Ruocco E, Gambardella A, Langella GG, Lo Schiavo A, Ruocco V. Cutaneous sarcoidosis: an intriguing model of immune dysregulation. Int J Dematol 54: 1-12, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs 68: 1361-1383, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Steen T, English JC. Oral minocycline in treatment of cutaneous sarcoidosis. JAMA Dermatol 149: 758-760, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Lazarus A. Sarcoidosis: epidemiology, etiology, pathogenesis, and genetics. Dis Mon 55: 649-660, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol 40: 198-204, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichikawa H, Kataoka M, Hiramatsu J, et al. Quantitative analysis of propionibacterial DNA in bronchoalveolar lavage cells from patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 25: 15-20, 2008. [PubMed] [Google Scholar]
- 7.Ishige I, Usui Y, Takemura T, Eishi Y. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet 354: 120-123, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Negi M, Takemura T, Guzman J, Uchida K, Furukawa A, Suzuki Y. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod Pathol 25: 1287-1297, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eishi Y. Etiologic link between sarcoidosis and Propionibacterium acnes. Respiratory investigation 51: 56-68, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Sekine T, Amano Y, Hidaka F, et al. Hepatosplenic and muscular sarcoidosis: characterization with MR imaging. Magn Reson Med Sci 11: 83-89, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Dalia T, Liu D, Fraga GR, Springer J. A rare case of sarcoidosis presenting with cutaneous medium-vessel granulomatous vasculitis treated with rituximab. J Clin Rheumatol. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 12.Amber KT, Bloom R, Mrowietz U, Hertl M. TNF-α: a treatment target or cause of sarcoidosis? J Eur Acad Dermatol Venereol 29: 2104-2111, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Isshiki T, Matsuyama H, Sakamoto S, et al. Development of Propionibacterium acnes-associated sarcoidosis during etanercept therapy. Intern Med 58: 1473-1477, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Numakura T, Tamada T, Nara M, et al. Simultaneous development of sarcoidosis and cutaneous vasculitis in a patient with refractory Crohn's disease during infliximab therapy. BMC Pulm Med 16: 30, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloppenburg M, Verweij CL, Miltenburg AM, et al. The influence of tetracyclines on T cell activation. Clin Exp Immunol 102: 635-641, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]