Abstract
The selective arterial calcium stimulation test (SACST) is one of the most useful localization tests for insulinoma but can cause false-positive and/or unexpected multi arterial positive results that hamper clinical decisions. There are also several adverse effects, such as nausea and hypoglycemia, at the conventional dose (0.025 mEq/kg) of calcium injection. We herein report five consecutive insulinoma cases in which low-dose (0.005-0.007 mEq/kg) calcium injection for SACST led to successful insulinoma localization. No adverse effects of SACST were observed. In conclusion, a low-dose SACST can be a favorable option as an insulinoma localization test in terms of accuracy and safety.
Keywords: calcium stimulation, insulinoma, low-dose, selective arterial calcium stimulation test, selective arterial secretagogue injection test
Introduction
Insulinoma is a pancreatic neuroendocrine neoplasm that causes hypoglycemia due to insulin oversecretion (1,2). Localization of insulinoma is critical for curative surgery (3,4). Selective arterial secretagogue injection tests, especially the selective arterial calcium stimulation test (SACST), are the most useful localization tests for insulinoma (4-6). Although the calcium dose for the SACST has been conventionally set at 0.025 mEq/kg, it is often adjusted empirically by physicians. However, the SACST has been reported to have some clinical difficulties in localizing insulinoma, with reports of false positives and/or unexpected multiarterial positive results, and may also cause adverse reactions, such as nausea (7), hypoglycemia (7,8), and hypercalcemia (9) due to the high calcium dose of 0.025 mEq/kg.
Accordingly, lower doses of calcium injection for the SACST have been proposed (8,10). In fact, 0.0025 mEq/kg and 0.0065 mEq/kg calcium injections for the SACST were reported to have sensitivities of 91% and 75%, respectively, and a specificity of 84% (10). In another study, the sensitivities of 0.025 mEq/kg and 0.00625 mEq/kg calcium injections were 86% and 71%, respectively (8). However, these results, which were obtained in a small number of cases, have varied, and the effectiveness and safety of these low-dose SACST protocols have not been fully examined. In addition, the usefulness of SACST in cases with multiple tumors and the adequacy of low-dose calcium injection remains unclear.
We herein report five consecutive insulinoma cases that underwent a low-dose SACST at 0.005-0.007 mEq/kg.
Patients and Methods
We conducted a retrospective single-center study at Kyoto University Hospital between January 2008 and September 2018. This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Kyoto University Hospital (local identifier: R1789).
We used a slow continuous intravenous infusion of normal saline (1-2 mL/min) during the procedure. We rapidly infused 8.5% calcium gluconate, followed by flushing with 5-mL normal saline. In this study, a low-dose SACST was defined as a calcium dose of <0.010 mEq/kg. The actual calcium dose for the SACSTs among the subjects ranged from 0.005 to 0.007 mEq/kg. As previously reported (4,5), a positive SACST result was defined as a more than 2-fold increase in the serum insulin level from baseline within 120 seconds after calcium infusion. Positive responses in the gastroduodenal artery (GDA) and superior mesenteric artery (SMA) were taken to indicate the presence of an insulin-secreting tumor in the head and body of the pancreas, whereas a positive response in the splenic artery (SPA) was taken to indicate a tumor in the body and tail of the pancreas (11); however, anatomical variations among the cases were considered, based on arteriography.
Surgical localization and postoperative recovery from hypoglycemia were used as the gold standard for successful localization of insulin-secreting tumors. The criteria for cured insulinoma were the disappearance of hypoglycemia and hypoglycemic symptoms, confirmation of negative results in a post-operative fasting test, and improved hyperinsulinemia during the oral glucose tolerance test. Through a chart review, we identified eight adult insulinoma patients who underwent SACST in our hospital, including five consecutive patients who underwent a low-dose SACST for localizing insulinoma (Table 1, 2).
Table 1.
Clinical Characteristics of the Cases.
| Case No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age (years) | 36 | 27 | 51 | 43 | 61 |
| Sex | Female | Female | Male | Female | Male |
| Height (cm) | 159 | 160 | 171 | 155 | 155 |
| Body weight (kg) | 55.0 | 49.2 | 64.7 | 46.5 | 93.4 |
| Body mass index (kg/m2) | 21.8 | 19.2 | 22.1 | 19.4 | 38.9 |
| Hereditary mutations | No | No | No | No | MEN 1 |
| Image modalities used for pre-SACST evaluation | CECT, EUS | MRI, EUS | EUS | CECT, DOTATOC-PET/CT | CECT, MRI |
| Pancreatic tumors on pre-SACST evaluation | Solitary | Solitary | Solitary | Multiple | Multiple |
| Tumor localization by pre-SACST evaluation | Head | Body, Tail | Distal pancreas | Body, Tail | Neck, Body, Tail |
| Corrected serum calciuma (mg/dL) | 8.5 | 8.4 | 8.9 | 9.0 | 6.8 |
| eGFR (mL/min/1.73 m²) | 139.7 | 77.4 | 68.0 | 65.8 | 75.1 |
| Injected calcium doses | |||||
| for the SACST (mEq/kg) | 0.007 | 0.005 | 0.005 | 0.005 | 0.005 |
| IST localization after the SACST | Head | Body, Tail | Body, Tail | Head, Body, Tail | Body, Tail |
| Hypoglycemia related with SACST | No | No | No | No | No |
| Adverse events related with SACST | No | No | No | No | No |
ameasured within a week before the SACST.
For cases with serum albumin level <4.0 g/dL, the corrected serum calcium level was calculated by the formula, serum calcium+[4.0 -serum albumin (g/dL)].
MEN: multiple endocrine neoplasia, SACST: selective arterial calcium stimulation test, CECT: contrast-enhanced computed tomography, EUS: endoscopic ultrasonography, MRI: magnetic resonance imaging, DOTATOC: 68Ga-labeled 1,4,7,10-tetraazacyclododecane-N,N',N,”N”-tetraacetic acid-D-Phe1-Tyr3-octreotide, PET: positron emission tomography, eGFR: estimated glomerular filtration rate, IST: insulin-secreting tumor
Table 2.
the Frequencies of False-positive, False-negative, and Adverse Effects in Conventional and Low-dose SACST.
| Reference | Ca injection (mEq/kg) |
False-positive | False-negative | Adverse effects | ||||
|---|---|---|---|---|---|---|---|---|
| Present study | 0.025 | 0/3(0%) | 0/3(0%) | 1/3(33%) | ||||
| 0.005-0.007 | 0/5(0%) | 0/5(0%) | 0/5(0%) | |||||
| 10 | 0.0025 | 3/19(16%) | 1/11(9%) | No data** | ||||
| 0.00625 | 2/8(25%) | No data** | ||||||
| 8 | 0.025 | 0/2*(0%) | 0/2*(0%) | 1/3(33%) | ||||
| 0.00625 | 1/5(20%) | 0/5(0%) | 0/5(0%) | |||||
| 14 | 0.025 | 1/13(8%) | 1/13(8%) | 1/13(8%) | ||||
| 6 | 0.025 | 2/45(4%) | 5/45(11%) | No data | ||||
| 19 | 0.02-0.025 | 6/11(55%) | 0/6(0%) | No data | ||||
| 11 | 0.025 | No data | 3/25(12%) | 0/25(0%)# | ||||
| Total | Conventional dose | 9/74(12%) | 9/94(10%) | 3/44(7%) | ||||
| Low-dose (<0.010) | 4/29(14%) | 3/29(10%) | 0/10(0%) |
* Patients who have not undergone surgery after SACST are excluded.
** The flushing and tingling related to SACST has occurred, but the frequency has not reported.
# No adverse effects have occurred aside from the mild and transient sensation of warmth in the epigastrium during SACST.
The number of each report corresponds to the reference number. SACST: selective arterial calcium stimulation test, Ca: calcium
Statistical analyses by the Mann-Whitney U test were performed using the JMP software program, version 14.0.1 (SAS Institute, Cary, USA). P-values <0.05 were considered statistically significant. The data were presented as the median [interquartile range (IQR)].
Case Reports
Case 1
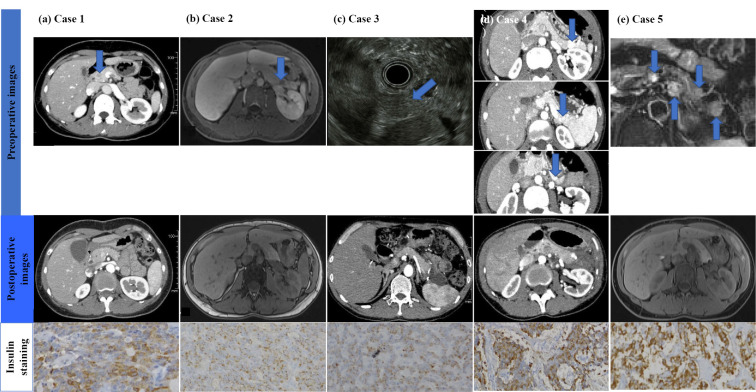
A 36-year-old woman with recurring episodes of loss of consciousness was admitted to our hospital. Her serum glucose level was 40 mg/dL, and her serum insulin level was 9.2 μU/mL. Abdominal computed tomography (CT) demonstrated a 10-mm tumor in the head of the pancreas (Fig. 1a). The SACST was performed using an injected calcium dose of 0.007 mEq/kg, resulting in a significant selective increase in insulin in the SMA and GDA (Fig. 2a). There were no adverse effects associated with the test. The mass in the head of the pancreas was enucleated, following the pathological confirmation of an insulinoma (Fig. 1a). No hypoglycemia has occurred since the surgery.
Figure 1.
Images of the pancreatic tumors of the present cases. Upper panel: (a) Case 1. Contrast-enhanced computed tomography (CECT) of the abdomen showed a tumor in the head of the pancreas (arrow). (b) Case 2. Magnetic resonance imaging (MRI) of the abdomen demonstrated a tumor in the body and tail of the pancreas (arrow). (c) Case 3. EUS revealed a tumor in the distal pancreas (arrow). (d) Case 4. CT of the abdomen showed tumors in the body and tail of the pancreas (arrows). (e) Case 5. MRI of the abdomen revealed tumors in the body and tail of the pancreas (arrows). Lower panel: immunohistochemical images of insulin staining (×100).
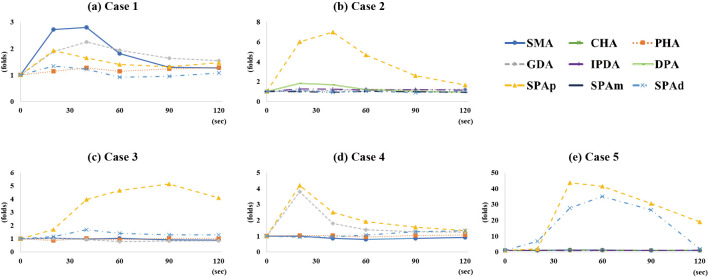
Figure 2.
Results of selective arterial calcium stimulation test (SACST) test of the present cases. (a) The results for the superior mesenteric artery (SMA) and gastroduodenal artery (GDA) are positive in Case 1. (b) The result for the proximal splenic artery (SPAp) is positive in Case 2. (c) A positive response is observed in the SPAp in Case 3. (d) Positive responses are observed in the GDA and SPAp in Case 4. (e) Positive responses are observed in the SPAp and in the distal portion of the SPA (SPAd) in Case 5.
Case 2
A 27-year-old woman was diagnosed with insulinoma (Fig. 1b), and her presentation was detailed in a previous report (9). The SACST was performed using an injected calcium dose of 0.005 mEq/kg and showed a significant increase in the insulin level in the proximal part of the SPA (SPAp) (Fig. 2b). There were no adverse effects associated with the test. Distal pancreatectomy was performed and revealed an insulinoma in the tail of the pancreas on a pathological examination. No hypoglycemia has occurred since the surgery.
Case 3
A 51-year-old woman presented with mood swings. On a laboratory examination, she was found to have hyperinsulinemic hypoglycemia, with plasma glucose at 30 mg/dL, serum insulin at 3.5 μU/mL, and serum C-peptide at 2.3 ng/mL. Although CT failed to demonstrate any pancreatic tumor, endoscopic ultrasonography identified an 11-mm tumor in the distal pancreas (Fig. 1c). The SACST was performed using an injected calcium dose of 0.005 mEq/kg and resulted in a significant increase in the insulin level in the SPAp. There were no adverse effects associated with the test. Distal pancreatectomy was performed and revealed a tumor with positive staining for insulin on a pathological examination. No hypoglycemia has occurred since the surgery.
Case 4
A 43-year-old woman presented with recurring hypoglycemia with tremors and sweating. A fasting test for approximately 8 hours revealed hyperinsulinemic hypoglycemia, with a plasma glucose level of 37 mg/dL, serum insulin level of 24.5 μU/mL, and serum C-peptide leve of 0.45 ng/mL. She had no family history of hypoglycemia, multiple endocrine neoplasia type 1 (MEN1), or MEN1-related disorders. Subsequent genomic DNA polymerase chain reaction testing revealed no pathogenic mutations in the whole exon of the MEN1 gene. Abdominal CT demonstrated three tumors in the body and tail of the pancreas (Fig. 1d). 68Ga-labeled 1,4,7,10-tetraazacyclododecane-N,N′,N,′′N′′-tetraacetic acid-D-Phe1-Tyr3-octreotide positron emission tomography/CT demonstrated a significant accumulation in two of the three tumors but revealed no other tumors in the head of the pancreas. The SACST was performed using an injected calcium dose of 0.005 mEq/kg and revealed more than a two-fold increase in the insulin level in the GDA and SPAp (Fig. 2d). There were no adverse effects associated with the test.
The SACST suggested insulin-secreting tumors not only in the body and tail of the pancreas but also in the head of the pancreas. After carefully considering the risks and benefits of total pancreatectomy, distal pancreatectomy was performed based on the patient's strong request. Histopathology revealed that all three tumors were insulin-positive (Fig. 1d). Furthermore, many small tumors were grossly observed in the entirely resected pancreas, suggesting the possibility of residual tumors in the head of the pancreas. After the surgery, the hyperinsulinemic hypoglycemia persisted, and CT revealed a residual tumor (Fig. 1d).
Case 5
A 61-year-old man was diagnosed with MEN1 based on the presence of primary hyperparathyroidism and prolactinoma in addition to an MEN1 mutation. Abdominal magnetic resonance imaging demonstrated a tumor in the pancreatic neck and five tumors in the body and tail of the pancreas (Fig. 1e). Thereafter, he developed hypoglycemia. A fasting test revealed hyperinsulinemic hypoglycemia, with a plasma glucose level of 39 mg/dL, serum insulin level of 8 μU/mL, and serum C-peptide level of 2.36 ng/mL. The SACST was performed using an injected calcium dose of 0.005 mEq/kg and showed a significant increase in the insulin levels in the SPAp and the distal part of the SPA (Fig. 2e). There were no adverse effects associated with the test. Distal pancreatectomy was performed, and a pathological examination revealed that four of the five tumors were insulin-positive (Fig. 1e). No hypoglycemia has occurred since the surgery.
The comparison between positive and negative arterial stimulations in the SACST
In the analysis of all the arterial stimulations in the 5 cases above, venous sampling in the positive and negative arterial stimulations showed 2.25-43.7-(median 4.67, IQR 24.93) and 1.00-1.92-(median 1.18, IQR 0.30) fold increases, respectively, in the serum insulin levels from the baseline (p<0.01). In the positive arterial stimulations of 4 of the cases, the maximum rate of increase in the serum insulin level was observed within 60 seconds after injection; only 1 case (Case 3) showed a maximum rate at 120 seconds (Fig. 2). Furthermore, in the analysis of the results that were confirmed pathologically (Fig. 3), the venous sampling in the positive and negative arterial stimulations showed 2.25-43.7-(median 5.14, IQR 32.17) and 1.01-1.92-(median 1.23, IQR 0.29) fold increases, respectively, in the serum insulin levels from baseline (p<0.01).
Figure 3.
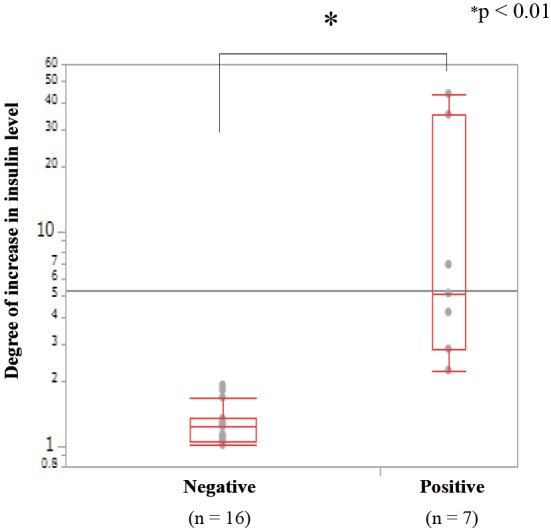
The comparison of the increase in the insulin levels in the venous samples between positive and negative arterial stimulations. The increase in the insulin levels in the positive arterial stimulations (n=7) is significantly higher than that in the negative arterial stimulations (n=16) and was compatible with the findings of the pathological examinations.
Discussion
Surgical resection is the only curative treatment for insulinoma. To determine the precise area of resection in the pancreas, accurate preoperative tumor localization is essential (4,12,13). Compared with other localization tools, the SACST has been reported to be one of the most sensitive diagnostic procedures for localizing insulinoma (14-16). However, the SACST is associated with several adverse effects, including nausea, hypotension, and hypoglycemia, in some cases, especially after a conventional dose of calcium injection (0.025 mEq/kg). Hypoglycemia during the SACST has been reported to occur in about 2% of cases (14); therefore, the calcium dose for the SACST has often been adjusted empirically by physicians (7,8). Furthermore, the clinical risk of repeated calcium injections had been a concern, especially in cases with obesity and renal impairment, which can be affected by MEN1 (17,18). Although empirical adjustments of the injected calcium dose have been performed based on body weight and the renal function, even in the conventional protocol (11), a low-dose SACST (i.e., injected calcium dose of 0.00625 mEq/kg) has recently been proposed to avoid adverse effects (8).
In the conventional-dose SACST, a risk of false positive results remains a potential issue, and the frequency of such results is not negligible (6,14,19). The frequency of multiple positive arteries in conventional SACST has been 31% to 53% in previous reports (6,14). To our knowledge, the frequencies of false-positive results among previous reports has been 0-55%, although this frequency depends in part on the skill of each physician and institute (Table 2). One of the main reasons for false-positive results is a high frequency of complicated vascular anastomoses around the pancreas (19). More than 90% of the vascular anastomoses between the head and tail of the pancreas can be involved in the flow of calcium from the infused artery into the pancreas. Furthermore, repeated calcium injections can lead to an increased flow back into other areas and increase the calcium stimulation even in normal arterial territories (19). In this context, reducing the dosage of injected calcium can theoretically reduce the risk of false positive results.
As previously reported, compared with the conventional dose, the low-dose SACST had comparable effectiveness but without adverse effects (8). However, the usefulness of the SACST at lower doses has not been examined, and the optimal low dose has not been set, with such discussions made in only a few reports (8,10). In our low-dose SACST cases, no adverse events, including hypoglycemia, were observed after the low-dose SACST. As previously suggested, the low-dose SACST may reduce the incidence of adverse effects, including hypoglycemia, during the procedure (8). Furthermore, no false-positive results were recorded among the five cases managed with the low-dose SACST.
For the SACST at lower doses, there remain some concerns as to whether or not low-dose calcium can sufficiently stimulate extracellular and intracellular calcium concentrations, which is required for insulin secretion from insulinoma cells (20). In fact, an insulinoma case that required a higher dose of calcium injection for a positive response in the SACST was previously reported (21). Therefore, the lowest dose of calcium injection that can be used for the SACST remains to be established by further studies; however, false-negative results in the low-dose SACST were not observed in at four of the five present cases, suggesting that the dose used here was reasonable.
In addition, the effectiveness and clinical details of the SACST for cases with multiple tumors should be described. In our case series, all five cases showed apparent hyperinsulinemic hypoglycemia, with an almost normal renal function (Table 1). Furthermore, all cases except for case 4 showed a complete recovery from hypoglycemia after surgery, and the insulinomas localized by the low-dose SACST were demonstrated pathologically. Only one case (case 4) had an unidentified residual insulin-secreting tumor in the head of the pancreas. In the two patients who had multiple insulin-secreting tumors, multiple positive arteries were observed in the SACST; one case was diagnosed as MEN1, and the other had no MEN1-related disorders or family history of MEN1-related diseases. The increase in the serum insulin levels from baseline in the venous samples for the positive and negative arterial stimulations suggested a sufficient localization capacity of the low-dose SACST (0.005-0.007 mEq/kg). While which patients are optimal candidates of low-dose SACST is difficult to determine based solely on the present findings, patients with one or more tumors identified by non-invasive imaging, obesity, and/or MEN1 might be potential subjects, as shown in this case series and based on the suggestions of previous reports (9,22).
Finally, 4 of our 5 cases achieved the maximum rates of increase in the serum insulin level within 60 seconds after low-dose calcium injection. In the conventional-dose SACST, the timing of the evaluation for insulinoma localization has varied among reports, although a recent report recommended performing an evaluation within 120 seconds rather than within 60 seconds (23). The optimal timing of the evaluation for insulinoma localization by the low-dose SACST should be further evaluated.
Several limitations associated with the present study warrant mention. This is a single-center consecutive case series report comprising only five insulinoma cases. Nevertheless, our report may provide an opportunity to revise the calcium dose for the SACST with the goal of reducing adverse events without altering the diagnosis ability. The results suggest that 0.005 mEq/kg may be an adequate dose for calcium injection for the low-dose SACST, as a lower-dose (<0.005 mEq/kg) SACST might show less sensitivity than a conventional-dose test (Table 2). In order to establish the usefulness of low-dose calcium injection as the first choice for the SACST protocol, and to set the lowest adequate dose of calcium injection for a low-dose SACST, further large-scale studies, including a randomized controlled or a cross-over study that compares conventional- (0.025 mEq/kg) and low-dose (especially 0.005 mEq/kg) SACSTs, are warranted. However, these might be challenging to perform because of the rarity of insulinoma cases. In addition, a further study is required for patients with insulinoma who lack hyperinsulinemic hypoglycemia, as all five cases in our report had hyperinsulinemic hypoglycemia.
In summary, the low-dose SACST (0.005-0.007 mEq/kg) demonstrated adequate localization performance without adverse effects in our consecutive insulinoma cases. Our results suggest that the low-dose SACST can be a useful choice for the localization of insulinoma and can avoid the adverse effects associated with excessive calcium load during the procedure.
The authors state that they have no Conflict of Interest (COI).
Tomonobu Hatoko and Takaaki Murakami contributed equally to this work.
References
- 1.Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol 50: 58-64, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Service FJ, McMahon MM, O'Brien PC, Ballard DJ. Functioning insulinoma-incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc 66: 711-719, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Imamura M. Recent standardization of treatment strategy for pancreatic neuroendocrine tumors. World J Gastroenterol 16: 4519-4525, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murakami T, Yabe D, Inagaki N. Case 23-2018: A man with episodes of confusion and hypoglycemia. N Engl J Med 379: 1881-1882, 2018. [DOI] [PubMed] [Google Scholar]
- 5.Imamura M, Shimada Y, Kato M, Doi R, Okada N, Hashimoto M. Usefulness of selective arterial calcium injection test and secretin test in patients with insulinoma. J Hep Bil Pancr Surg 1: 530-534, 1994. [Google Scholar]
- 6.Guettier JM, Kam A, Chang R, et al. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab 94: 1074-1080, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leow MK, Loh KC, Kon WY, Wong DE, Tan BS, Soon PC. Clinical utility of selective intra-arterial calcium-stimulated hepatic venous sampling in regionalisation of insulinomas-the Singapore experience. Ann Acad Med Singapore 32: 86-91, 2003. [PubMed] [Google Scholar]
- 8.O'shea D, Rohrer-Theurs AW, Lynn JA, Jackson JE, Bloom SR. Localization of insulinomas by selective intraarterial calcium injection. J Clin Endocrinol Metab 81: 1623-1627, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Egusa G, Oki K, Yoshii Y, et al. Increased calcium ion levels following systemic circulation after the selective arterial calcium injection test. Pancreas 46: e34-e35, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Braatvedt G, Jennison E, Holdaway IM. Comparison of two low-dose calcium infusion schedules for localization of insulinomas by selective pancreatic arterial injection with hepatic venous sampling for insulin. Clin Endocrinol 80: 80-84, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Doppman JL, Chang R, Fraker DL, et al. Localization of insulinomas to regions of the pancreas by intra-arterial stimulation with calcium. Ann Intern Med 123: 269-273, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Murakami T, Yamashita T, Yabe D, et al. Insulinoma with a history of epilepsy: still a possible misleading factor in the early diagnosis of insulinoma. Intern Med 56: 3199-3204, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelini L, Bezzi M, Tucci G, et al. The ultrasonic detection of insulinomas during surgical exploration of the pancreas. World J Surg 11: 642-647, 1987. [DOI] [PubMed] [Google Scholar]
- 14.Morera J, Guillaume A, Courtheoux P, et al. Preoperative localization of an insulinoma: selective arterial calcium stimulation test performance. J Endocrinol Invest 39: 455-463, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Morganstein DL, Lewis DH, Jackson J, et al. The role of arterial stimulation and simultaneous venous sampling in addition to cross-sectional imaging for localization of biochemically proven insulinoma. Eur Radiol 19: 2467-2473, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Wiesli P, Brändle M, Schmid C, et al. Selective arterial calcium stimulation and hepatic venous sampling in the evaluation of hyperinsulinemic hypoglycemia, potential and limitations. J Vasc Interv Radiol 15: 1251-1256, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Lourenço DM, Coutinho FL, Toledo RA, Montenegro FL, Correia-Deur JE, Toledo SP. Early-onset, progressive, frequent, extensive, and severe bone mineral and renal complications in multiple endocrine neoplasia type 1-associated primary hyperparathyroidism. J Bone Miner Res 25: 2382-2391, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Murakami T, Usui T, Nakamoto Y, et al. Challenging differential diagnosis of hypergastremia and hyperglucagonemia with chronic renal failure, report of a case with Multiple Endocrine Neoplasia Type 1. Intern Med 56: 1375-1381, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi T, Honda H, Yasumori K, et al. Selective intra-arterial injection of calcium for localizing of insulinomas: proposed new criteria. Nihon Igaku Hoshasen Gakkai Zasshi (J Japan Radiol Soc) 55: 952-956, 1995(in Japanese). [PubMed] [Google Scholar]
- 20.Kato M, Doi R, Imamura M, Furutani M, Hosotani R, Shimada Y. Calcium-evoked insulin release from insulinoma cells is mediated via calcium-sensing receptor. Surgery 122: 1203-1211, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura Y, Doi R, Kohno Y, et al. High-dose calcium stimulation test in a case of insulinoma masquerading as hysteria. Endocrine 19: 127-130, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Doppman JL, Miller DL, Chang R, Shawker TH, Gorden P, Norton JA. Insulinomas: localization with selective intraarterial injection of calcium. Radiology 178: 237-241, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Ueda K, Ito T, Kawabe K, et al. Should the selective arterial secretagogue injection test for insulinoma localization be evaluated at 60 or 120 seconds? Intern Med 56: 2985-2991, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]