Abstract
Objective
Liver injury is a notable complication of coronavirus disease 2019 (COVID-19). This study aimed to clarify the clinical features and liver injury in Japanese patients with COVID-19.
Methods
We conducted a multicenter retrospective cohort study. All consecutive patients with COVID-19 who visited or were admitted to our hospital before May 12, 2020, were enrolled. Their demographics, symptoms, laboratory findings, comorbidities, concomitant drugs, treatment, and clinical course were reviewed. We defined liver injury as alanine aminotransferase (ALT) or gamma-glutamyl transferase (GGT) levels over the upper limit of normal.
Results
Twenty-two patients with COVID-19 (median age, 47 years old; men/women, 13/9) were enrolled. Two patients had underlying liver diseases, and two were diagnosed as having COVID-19 without any symptoms. Elevated ALT and GGT levels were found in 12 and 12 patients, respectively, and liver injury was observed in 15 patients (68.2%). Compared with the patients without liver injury, those with liver injury had a significantly higher fever during the clinical course (median, 37.5℃ vs. 38.8℃, p=0.006). A significant correlation was found between the highest serum liver values and the highest body temperature in each patient. Among the 22 patients, 4 required artificial respiratory support, and 2 died thereafter. Liver injury was not associated with the severity or mortality of COVID-19.
Conclusion
Elevated levels of liver enzymes in the Japanese patients with COVID-19 were associated with the highest body temperature during the clinical course but not with the severity or mortality of COVID-19.
Keywords: SARS-CoV-2, coronavirus, COVID-19, liver injury, hepatitis, fever
Introduction
In December 2019, many patients had unexplained pneumonia in Wuhan, China. Shortly after the epidemic, a novel virus was identified as the pathogen (1). While dubbed the 2019 novel coronavirus (2019-nCoV) initially, the pathogen was eventually officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (2). The disease caused by SARS-CoV-2 infection has been termed as coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO). SARS-CoV-2 quickly spread worldwide, so the WHO declared a COVID-19 pandemic on March 11, 2020.
COVID-19 is mainly known as a respiratory disease, although systemic organ failure has been reported, including that of the brain, heart, pancreas, and kidney (3-5). Liver injury is also a notable complication of COVID-19, and elevated aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels ranging from 15% to 53% have been observed (3,6-8).
Whether or not liver injuries significantly influence the outcome remains controversial. Although recent systematic reviews of liver injury cases in patients with COVID-19 suggest that elevated AST, ALT, and total bilirubin levels and reduced albumin levels were associated with severe COVID-19 (9,10), other studies failed to demonstrate an association between liver injury and a poor prognosis (11,12), and cases of severe acute liver injury have been rarely described (8). The pathogenesis of liver injury in patients with COVID-19 is unknown, possibly including the direct cytopathic effect of SARS-CoV-2 infection, immune-mediated damage caused by the provoked inflammatory responses, involvement of other organs, such as myositis, and drug-induced liver injury (13,14). However, data regarding liver injury in patients with COVID-19 are still scarce outside of China.
To our knowledge, no report has investigated the clinical features of SARS-CoV-2-related liver injury in Japanese patients. In this study, we aimed to describe the clinical features, paying special attention to liver injury, in Japanese patients with COVID-19.
Materials and Methods
Study design and participants
We conducted a multicenter retrospective cohort study through a collaboration of three hospitals: Teikyo University Hospital, Teikyo University Mizonokuchi Hospital, and Teikyo University Chiba Medical Center. All consecutive patients who visited or were admitted to the three hospitals until May 12, 2020, were enrolled in this study. All cases were confirmed to have SARS-CoV-2 infection by reverse transcriptase polymerase chain reaction.
This study was approved by the ethics committee of the Teikyo University School of Medicine and the local ethics committee of each center.
Data collection
The following data were reviewed from the patients' electronic medical charts. We retrieved the patients' demographics, including their age and sex, as well as signs and symptoms, including the highest body temperature, cough, dyspnea, diarrhea, nausea, hypogeusia, hyposmia, and percutaneous oxygen saturation (SpO2). We defined liver injury as an elevated ALT or gamma-glutamyl transferase (GGT) level over the upper limit of normal (ULN) at any time during the clinical course. The ULN was set as 42 U/L for ALT and 64 U/L (men) and 34 U/L (women) for GGT. Other laboratory findings, including the white blood cell, lymphocyte cell, and platelet counts; hemoglobin, C-reactive protein, bilirubin, albumin, AST, and alkaline phosphatase (ALP) levels; and estimated glomerular filtration rate, were recorded at the time of maximum ALT or GGT level in the patients with liver injury and at presentation in the patients without liver injury. Comorbidities were reviewed, including underlying liver disease, diabetes, and hypertension, with concomitant drug prescription. Finally, we extracted data on the general and antiviral treatments used for COVID-19, including the administration of antibiotics, antipyretics, ciclesonide, and favipiravir; the severity of COVID-19, defined as the need for artificial respiratory support with a ventilator; and the clinical outcome.
Statistical analyses
Continuous variables are presented as the median [interquartile range (IQR)] and compared using the Mann-Whitney U test. Categorical variables (shown by percentage) were compared using Fisher's exact test. Differences with 2-sided alpha levels of <0.05 were considered statistically significant differences. Statistical analyses were performed using the IBM SPSS Statistics software program, version 24 (IBM, Armonk, USA).
Results
Clinical features of patients with COVID-19
Until May 12, 2020, 22 patients at the 3 hospitals were confirmed to have SARS-CoV-2 infection. The clinical features of these 22 patients are shown in Tables 1 and 2. The median age was 47 years old, ranging from 25 to 85 years old (IQR, 38-59 years old). As underlying liver disease, non-alcoholic fatty liver disease was present in two patients. The highest body temperature was observed at presentation in all the patients, and the median was 38.4℃ (IQR, 37.8℃-39.3℃). Eighteen (81.8%) of the 22 patients complained of respiratory symptoms, such as cough or dyspnea, and the median SpO2 of all patients was 94% at presentation. While gastrointestinal symptoms, hypogeusia, and hyposmia were found in 3, 3, and 4 patients, respectively, 2 of the 22 patients complained of non-clinical symptoms. Regarding the laboratory findings, the white blood cell count was almost normal overall (median, 55×102/μL), but the C-reactive protein level was elevated (median, 4.1 mg/dL). Elevated ALT and GGT levels beyond the ULN were found in 12 patients each (54.5%). Liver injury, defined as either an elevated ALT or GGT level beyond the ULN, was found in 15 patients (68.2%). The serum albumin level was decreased (median, 3.1 g/dL).
Table 1.
Demographics, Comorbidities, Concomitant Drugs, and Symptoms at Presentation of the Patients with COVID-19.
| All (n=22) | Liver injury | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Present (n=15) | Absent (n=7) | ||||||||
| Demographics | |||||||||
| Age (years) | 47 (38–59) | 46 (33–54) | 52 (43–60) | 0.45 | |||||
| Sex (male) | 13 (59) | 11 (73) | 2 (29) | 0.074 | |||||
| Comorbidities and concomitant drugs | |||||||||
| Underlying liver disease | 2 (9) | 2 (13) | 0 | 1.00 | |||||
| Diabetes | 5 (23) | 4 (27) | 1 (14) | 1.00 | |||||
| Hypertension | 5 (23) | 4 (27) | 1 (14) | 1.00 | |||||
| Malignancy | 1 (5) | 1 (7) | 0 | 1.00 | |||||
| Immunomodulators | 2 (9) | 1 (7) | 1 (14) | 1.00 | |||||
| Anticancer agent | 1 (5) | 1 (7) | 0 | 1.00 | |||||
| Signs and symptoms | |||||||||
| Highest body temperature (°C) | 38.4 (37.8–39.3) | 38.8 (38.0–39.3) | 37.5 (37.4–38.1) | 0.006 | |||||
| Respiratory symptoms | 18 (82) | 12 (80) | 6 (86) | 1.00 | |||||
| SpO2 | 94 (90–98) | 95 (90–96.5) | 98 (95–98) | 0.21 | |||||
| GI symptoms | 3 (14) | 3 (20) | 0 | 0.52 | |||||
| Hypogeusia | 3 (14) | 1 (7) | 2 (29) | 0.23 | |||||
| Hyposmia | 4 (18) | 2 (13) | 2 (29) | 0.57 | |||||
| No clinical symptom | 2 (9) | 2 (13) | 0 | 1.00 | |||||
Continuous variables are shown as median (IQR), and categorical variables are shown as number (%). The p values for comparing patients with and without liver injury were calculated using the Mann-Whitney U test and Fisher exact test. SpO2: percutaneous oxygen saturation, GI: gastrointestinal
Table 2.
Laboratory Findings of the Patients with COVID-19.
| All (n=22) | Liver injury | p value | ||||||
|---|---|---|---|---|---|---|---|---|
| Present (n=15) | Absent (n=7) | |||||||
| WBC count (×102/μL) | 55 (43–73) | 58 (50–74) | 45 (39–57) | 0.33 | ||||
| Lymphocytes (×102/μL) | 8.9 (6.9–12.3) | 8.1 (5.5–10.2) | 10.9 (9.2–15.2) | 0.054 | ||||
| Hemoglobin (g/dL) | 13.9 (12.9–15.4) | 13.2 (12.7–15.1) | 14.0 (13.7–15.4) | 0.29 | ||||
| Platelet count (×104/μL) | 22.4 (15.0–30.6) | 21.8 (13.9–32.7) | 23.0 (19.5–28.0) | 0.84 | ||||
| C-reactive protein (mg/dL) | 4.1 (0.4–11.5) | 5.2 (1.8–11.3) | 1.6 (0.2–10.7) | 0.33 | ||||
| Total bilirubin (mg/dL) | 0.71 (0.50–0.90) | 0.67 (0.50–0.88) | 0.80 (0.52–0.88) | 0.74 | ||||
| Albumin (g/dL) | 3.2 (2.7–3.9) | 3.1 (2.6–3.5) | 3.9 (3.4–4.1) | 0.056 | ||||
| Estimated glomerular filtration rate (mL/min) | 75.5 (62.7–95.2) | 74.4 (59.9–90.9) | 85.8 (72.0–100.5) | 0.37 | ||||
Continuous variables are shown as median (IQR), and categorical variables are shown as number (%). The p values for comparing the patients with and without liver injury were calculated using the Mann-Whitney U test and Fisher’s exact test.
In Table 3, the treatments and outcomes of the 22 patients are shown. Before the diagnosis of COVID-19, antibiotics and antipyretics were administered to seven patients each. Acetaminophen was the most frequently administered antipyretic (5/7 patients), and nonsteroidal anti-inflammatory (loxoprofen) and unknown drugs were administered in 1 patient each. As antiviral treatment, ciclesonide and favipiravir were administered to seven and nine patients, respectively. Remdesivir, although officially approved for COVID-19 in Japan on May 7, 2020, was not used as an antiviral agent in these patients. A severe clinical course that required artificial respiratory support with a ventilator was observed in 4 patients (18%), and mortality was reported for 2 patients (9%).
Table 3.
Treatment and Outcome of Patients with COVID-19.
| All (n=22) | Liver injury | p value | ||||||
|---|---|---|---|---|---|---|---|---|
| Present (n=15) | Absent (n=7) | |||||||
| Treatment before diagnosis of COVID-19 | ||||||||
| Antibiotics | 7 (32) | 6 (40) | 1 (14) | 0.35 | ||||
| Antipyretics | 7 (32) | 7 (47) | 0 | 0.051 | ||||
| Treatment of COVID-19 | ||||||||
| Ciclesonide | 7 (32) | 6 (40) | 1 (14) | 0.35 | ||||
| Favipiravir | 9 (41) | 7 (47) | 2 (29) | 0.65 | ||||
| Ventilator | 4 (18) | 3 (20) | 1 (14) | 1.00 | ||||
| Outcome | ||||||||
| Death | 2 (9) | 1 (7) | 1 (14) | 1.00 | ||||
| Hospitalization (days) | 16 (12–21) | 19 (15–26) | 14 (9–15) | 0.13 | ||||
Continuous variables are shown as median (IQR), and categorical variables are shown as number (%). The p values for comparing the patients with and without liver injury were calculated using the Mann-Whitney U test and Fisher’s exact test.
The comparison of clinical features between the patients with and without liver injury
Next, we compared clinical features between the patients with and without liver injury. With regard to the clinical features shown in Table 1, 11 of the 15 patients with liver injury and 2 of the 7 patients without liver injury were men (p=0.047), but the difference was not significant, considering multiple comparisons. Only the highest body temperature was significantly different between the two groups (p=0.006). The body temperature was higher in the patients with liver injury [38.8℃ (IQR, 38.0-39.3℃)] than in those without liver injury [37.5℃ (IQR, 37.4-38.1℃)]. Similarly, no significant differences in laboratory findings were found between the two groups, despite the lymphocyte counts and serum albumin level being lower in the patients with liver injury than in those without it.
In terms of the treatment and outcome, antipyretics were administered before the confirmation of the diagnosis of COVID-19 in 7 (47%) of the 15 patients with liver injury and in none of the patients without liver injury (p=0.029). Antibiotics and treatments for SARS-CoV-2 infection (ciclesonide and favipiravir) were similarly administered between the two patient groups. Of the patients with and without liver injury, three and one had a severe clinical course that required artificial respiratory support, respectively (p=0.75), indicating that liver injury was not associated with a severe clinical course. The mortality and length of hospitalization were comparable between the two groups.
The association between liver injury and the highest body temperature
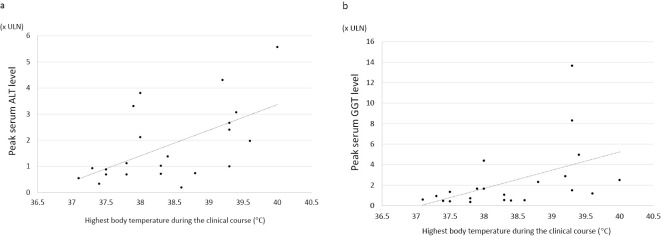
Finally, we examined the association of liver injury with the highest body temperature in all patients (Table 4, Figure a, b). The correlation of the highest ALT value with the highest body temperature during the clinical course was significant (r=0.574, p=0.005; Table 4, Figure a). Similarly, a significant correlation was observed between the highest GGT value and the highest body temperature in the clinical course (r=0.479, p=0.024) (Table 4, Figure b). In addition, the correlations of the highest AST and ALP values were also significant (Table 4).
Table 4.
Associations of the Highest Body Temperature and Liver Enzyme Levels in Patients with COVID-19.
| AST | ALT | ALP | GGT | |||||
|---|---|---|---|---|---|---|---|---|
| R | 0.730 | 0.574 | 0.494 | 0.479 | ||||
| P | <0.001 | 0.005 | 0.019 | 0.024 |
AST and ALP were measured at the time of maximum value of ALT or GGT in patients with liver injury, and at presentation in patients without liver injury. AST: aspartate aminotransferase. Since the upper limit of normal was different in male (64 U/L) and female (34 U/L), serum values of GGT adjusted as xULN were used for the analysis. ALT: alanine aminotransferase, ALP: alkaline phosphatase, GGT: gamma-glutamyl transferase, R: Pearson’s correlation coefficient
Figure.
a: The scatter plot of the highest body temperatures during the clinical course and the peak serum alanine aminotransferase (ALT) levels. Each dot represents each patient’s temperature and ALT level. b: The scatter plot of the highest body temperatures during the clinical course and the peak serum gamma-glutamyl transferase (GGT) level. Each dot represents each patient’s temperature and GGT level. Since the ULN differed between men (64 U/L) and women (34 U/L), the serum value of GGT was shown as xULN.
Discussion
In the present study, we describe the clinical features of patients with SARS-CoV-2 infection in the Japanese population, mainly focusing on liver injury. To our knowledge, this is the first report to demonstrate the association of liver injury with COVID-19 in a Japanese case series, although the number of patients was small.
Liver injury is a common pathological feature of COVID-19, but the incidence of elevated AST and ALT levels varies greatly, ranging from 2.5% to 50.0% and from 2.5% to 61.1%, respectively, as shown in a recent systematic review (15). In the present study, an elevated ALT level was observed in 12 (54.5%) of the 22 patients, which was comparable with the reports of previous studies. Although GGT levels are less frequently reported, the incidence has been reported to range from 13.0% to 22.8% (16-19), while our study indicated elevated GGT levels in 12 patients (54.5%). Liver abnormalities appear to be more prevalent outside of China (20).
The mechanism by which liver injury is induced by COVID-19 remains to be elucidated. The proposed mechanisms include direct cytotoxicity of SARS-CoV-2, immune-mediated damage with excess cytokine release from the inflammatory responses, involvement of other organs, such as myositis, and drug-induced liver injury (13,14). SARS-CoV-2 was thought to bind to the angiotensin-converting enzyme 2 (ACE2) receptor directly, thereby invading cells, where the virus replicates and infect other cells (21). Indeed, the ACE2 receptor is present in the liver or bile duct, and ACE2 expression has been detected in 2.6% of hepatocytes and 59.7% of cholangiocytes, which suggests that SARS-CoV-2 might infect the hepatobiliary system, especially the bile duct, and lead to abnormal liver biochemistries (22). However, if this was the case, intrahepatic cholestasis would be a dominant feature reflecting bile duct injury in patients with COVID-19. This is unlikely, as the results of our study and previous studies indicate that mild to moderate elevation of transaminase levels, not intrahepatic cholestasis, were mainly observed.
We detected a significant association of liver injury with the highest body temperature during the clinical course. The serum peak values of all four liver enzymes were significantly associated with the highest body temperature (Table 4, Figure a, b). Most patients with COVID-19 experience a flu-like syndrome with a high fever and arthralgia, mainly caused by aberrant cytokine production and release (cytokine storm) elicited by the SARS-CoV-2 infection (23). Therefore, these circulating cytokines during infection may cause the transient elevation of transaminase levels, a phenomenon called “bystander hepatitis,” as in other systemic viral infections (13).
However, we should consider drug-induced liver injury as another possibility, as several medications are prescribed for patients with COVID-19. In this regard, ciclesonide and favipiravir administration were not the likely cause of the liver injury in our patients because these treatments were equally administered for SARS-CoV-2 infection. Although antipyretics were administered only in the patients with liver injury, not in those without liver injury, we do not consider antipyretics to be involved in the causality of the liver injury in COVID-19 for the following reasons: Acetaminophen was the most frequently used antipyretic (6/7 cases) in the present study. This drug is well known for its hepatotoxicity and frequently causes an elevation of transaminase levels but rarely increases the GGT level, which was observed in 12 patients (54.5%) in our study.
Another controversial issue is the association of liver injury with the prognosis. At present, the mortality due to COVID-19 is relatively low in Japan compared with that in European and American countries, and unknown ethnicity-specific, genetic, or environmental factors may influence the severity and mortality of the disease. Therefore, the association of liver injury with the severity and mortality of COVID-19 should be determined in Japan, independent of studies in other countries. In the present study, disease severity, defined as the need for ventilation and mortality, was not associated with the presence of liver injury. Nevertheless, the number of patients is too small to draw any firm conclusion on this issue, so further larger-scale studies in Japan are warranted.
The present study has several limitations. First, the number of patients was small, and the statistical power was not enough to detect any significance. Nevertheless, the association of liver injury with a fever, the main finding of this study, was remarkable even with the small number of patients and should not be overlooked. Second, we failed to follow all of the patients' clinical courses until normalization of their liver test results because the patients were discharged before the complete improvement of the liver injury. The need for a longer follow-up of liver injury is another important issue that should be addressed in future studies.
Conclusion
We herein report for the first time the clinical features of Japanese patients with COVID-19 in Japan, mainly focusing on liver injury. We observed a significant association between liver injury and the highest body temperature during the clinical course. These findings provide insight into the pathogenesis of liver injury in COVID-19, important information that is needed in preparation for the “second wave” of COVID-19 in Japan.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We sincerely thank all patients participating in the present study as well as the physicians, nurses, and healthcare workers fighting against COVID-19 in the three hospitals. In addition, we would like to thank Ms. Kayono Unno and Ms. Tomomi Takagi for their secretarial assistance.
References
- 1.Zhu N, Zhang D, Wang W, et al. . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727-733, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group (CSG) of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5: 536-544, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markus HS, Brainin M. COVID-19 and stroke-a global World Stroke Organization perspective. Int J Stroke 15: 361-364, 2020. [DOI] [PubMed] [Google Scholar]
- 5.Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic injury patterns in patients with COVID-19 pneumonia. Gastroenterology. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Q, Huang D, Yu H, et al. . COVID-19: abnormal liver function tests. J Hepatol. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan Z, Chen L, Li J, et al. . Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol 18: 1561-1566, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int 40: 998-1004, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parohan M, Yaghoubi S, Seraj A. Liver injury is associated with severe coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of retrospective studies. Hepatol Res. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youssef M, Hussein M, Attia AS, et al. . COVID-19 and liver dysfunction: a systematic review and meta-analysis of retrospective studies. J Med Virol. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, He W, Yu X, et al. . Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect 80: 639-645, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Li W, Shi X, et al. . Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19). J Intern Med. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 13.Boettler T, Newsome PN, Mondelli MU, et al. . Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fix OK, Hameed B, Fontana RJ, et al. . Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment Pharmacol Ther. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai Q, Huang D, Ou P, et al. . COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 17.Hu LL, Wang WJ, Zhu QJ, Yang L. Novel coronavirus pneumonia-related liver injury: etiological analysis and treatment strategy. Zhonghua Gan Zang Bing Za Zhi 28: 97-99, 2020(in Chinese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 18.Ji D, Qin E, Xu J, et al. . Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 20.Sultan S, Altayar O, Siddique SM, et al. . AGA Institute rapid review of the GI and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou P, Yang XL, Wang XG, et al. . A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270-273, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis GJ, Goor HV. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631-637, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascarella G, Strumia A, Piliego C, et al. . COVID-19 diagnosis and management: a comprehensive review. J Intern Med. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]