Abstract
An 83-year-old man was hospitalized for coronavirus disease 2019 (COVID-19) after a 10-day history of a persistent fever. Chest computed tomography showed extensive non-segmental ground glass opacity. Despite the initiation of lopinavir and ritonavir, respiratory failure progressed. Two days of polymyxin B-immobilized fiber column-direct hemoperfusion (PMX-DHP) with adjunctive corticosteroid prevented his respiratory condition from worsening. For rapidly progressive COVID-19 cases, the early use of PMX-DHP may avoid the need for mechanical ventilation by suppressing local inflammation of the lung.
Keywords: COVID-19, SARS-CoV-2, acute respiratory failure, polymyxin B-immobilized fiber column-direct hemoperfusion
Introduction
In December 2019, a local outbreak of pneumonia caused by a novel coronavirus, namely severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan, China. This outbreak then spread all over the world, with more than 5,118,000 confirmed cases and 333,000 deaths as of May 22, 2020 (1). Since there is no proven effective therapy for coronavirus disease 2019 (COVID-19), the illness caused by SARS-CoV-2, many patients have received off-label or compassionate-use therapies, including anti-microbial agents, anti-inflammatory therapies, and convalescent plasma. Polymyxin B-immobilized fiber column-direct hemoperfusion (PMX-DHP) is a blood purification therapy that is used for the treatment of septic shock. However, whether or not the anti-inflammatory mechanism of PMX-DHP is effective against COVID-19 is unclear.
We herein report a case in which PMX-DHP effectively contributed to avoiding the use of mechanical ventilation.
Case Report
One of the passengers on a cruise ship, an 83-year-old Asian man with hypertension, hyperuricemia, and hypothyroidism, developed a fever in January that persisted for 10 days. The result of a real-time reverse transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2 was positive.
On admission, his temperature was 37.4℃, oxygen saturation (SpO2) was 98% on room air, and his respiratory rate was 14 breaths/min. Bilateral patchy shadows were visible on chest radiography. On day 5 of admission, his respiratory condition worsened to SpO2 80%, and 2 L/min oxygen was started. Extensive non-segmental ground glass opacity was observed on chest computed tomography (Fig. 1). On the following day, he was transferred to our hospital, and oral daily administration of lopinavir 800 mg/ritonavir 200 mg (Kaletra; AbbVie, North Chicago, USA) was started. He had a persistent fever with a temperature above 38℃, and the fraction of inspiratory oxygen (FiO2) had to be increased from 0.28 to 0.52 to maintain SpO2 90% 1.5 days after the transfer. He became tachypneic with a respiratory rate of 27 breaths/min, his partial pressure of arterial oxygen (PaO2)/FiO2 ratio decreased to 163 mmHg, and lung shadows deteriorated rapidly within three days (Fig. 2). To halt his respiratory failure and inhibit systemic and local pulmonary inflammation, he was treated with PMX-DHP for three hours a day on days 8 and 9 of admission (Fig. 3).
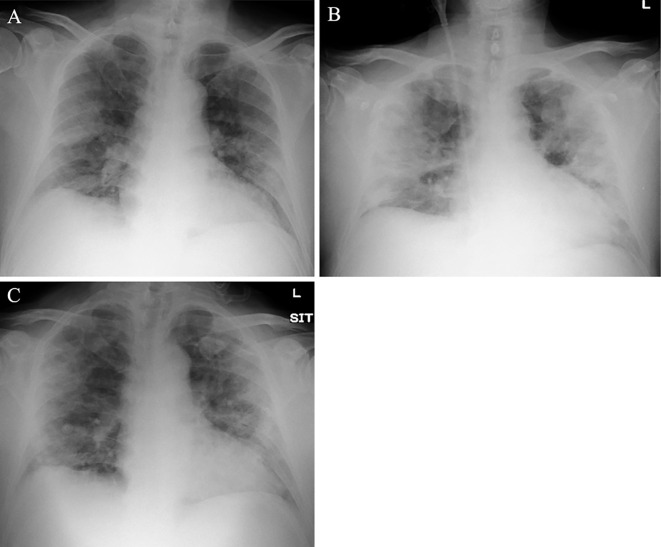
Figure 1.
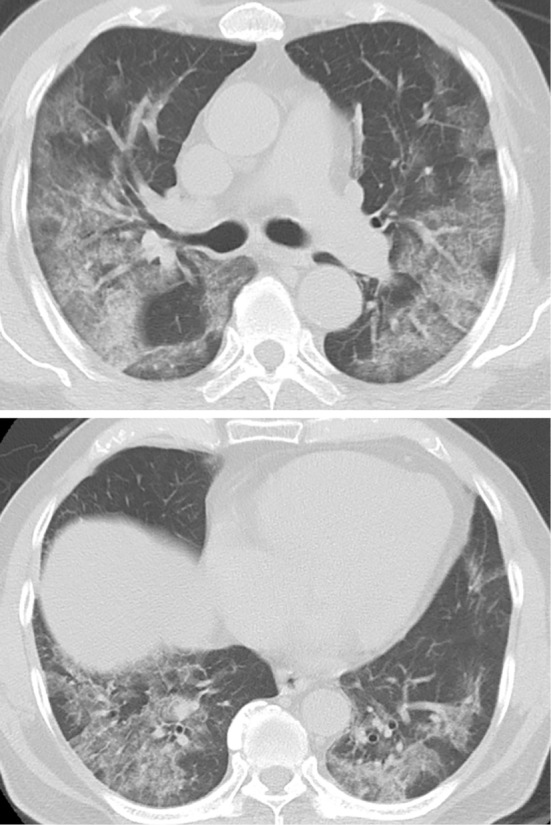
Chest computed tomography shows extensive non-segmental ground glass opacity on day 6 of hospitalization.
Figure 2.
(A) Chest X-ray shows bilateral infiltrative shadows on day 5 of hospitalization. (B) Both lung shadows are increased by day 8. (C) After two sessions of PMX-DHP, the chest X-ray shows fewer infiltrative shadows on day 12.
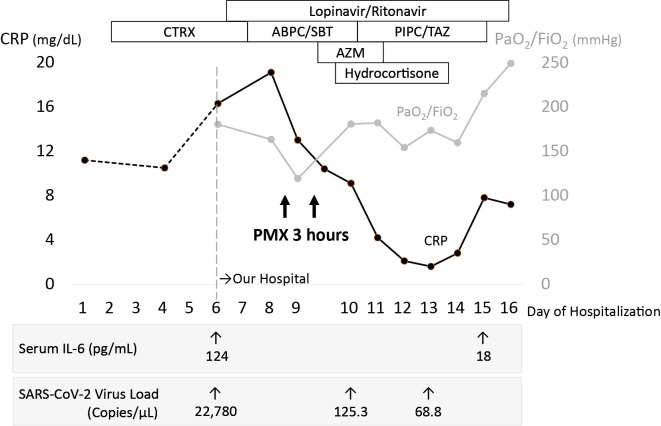
Figure 3.
Timeline of the disease course by the day from hospital admission. After the administration of polymyxin B-immobilized fiber column-direct hemoperfusion (PMX-DHP) followed by short-term steroids, the PaO2/FiO2 ratio is improved, and the serum CRP level is decreased. ABPC/SBT: ampicillin sulbactam, AZM: azithromycin, CRP: C-reactive protein, CTRX: ceftriaxone, FiO2: fraction of inspired oxygen, IL-6: interleukin-6, PaO2: partial pressure of arterial oxygen, PIPC/TAZ: piperacillin tazobactam, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
Immediately after completing two days of PMX-DHP, his temperature decreased, his serum C-reactive protein (CRP) level fell, and his respiratory failure did not progress further. As adjunctive therapy, he received intravenous hydrocortisone 200 mg, followed by continuous administration at 10 mg/hour for 4 days. His temperature remained normal, and his respiratory condition gradually improved until he no longer required oxygen therapy. Chest X-ray showed improvement in the infiltrative shadows on day 12 (Fig. 2). Cytokine assays showed a decrease in interleukin (IL)-6 from 124 pg/mL on day 6 to 18 pg/mL on day 15. Real-time RT-PCR for SARS-CoV-2 showed a marked decrease in the viral load in sputum from 22,780.0 copies/μL on day 6 to 68.8 copies/μL on day 13 (Fig. 3).
Discussion
PMX-DHP is a blood purification therapy that is used for the treatment of septic shock. Its clinical effects are reported to include adsorbing endotoxins, stabilizing hemodynamics, and improving oxygenation (2).
The efficacy of PMX-DHP for improving oxygenation and thus increasing the survival rate in cases of acute respiratory distress syndrome (ARDS)/diffuse alveolar damage (DAD) has also been investigated. Although its mechanism of action is not yet fully understood, reports on the reduction of inflammatory cytokines and the pro-inflammatory mediator high mobility group box 1 (HMGB-1) (3), attenuation of oxidative stress (4), and adsorption removal of activated neutrophils migrating to the lungs (5) have been increasing in number.
In fact, a case has been reported in which PMX-DHP for severe viral pneumonia due to the influenza A (H1N1) pdm09 virus (the characteristic histology of which is known to be DAD) inhibited inflammatory reactions via a decrease in inflammatory cytokines and HMGB-1 and improved oxygenation, which helped save a patient's life (6). In addition to viral pneumonia, PMX-DHP has also been shown to be effective for treating critical conditions with a poor survival due to DAD, such as severe ARDS accompanying non-human immunodeficiency virus pneumocystis pneumonia (7), serious drug-induced lung injury (8), and acute exacerbation of idiopathic pulmonary fibrosis (9). The lung histopathology in one patient who died from ARDS due to COVID-19 was also DAD (10). Therefore, PMX-DHP was used to treat acute respiratory failure caused by SARS-CoV-2 in the present case, even though the patient did not have septic shock.
An investigation of patients who required admission to an intensive-care unit with serious forms of COVID-19 showed that the mortality rate was 81.0% among patients who required endotracheal intubation (11). The present patient was in his 80s, had pre-existing conditions, and could therefore be considered to belong to a cohort with a poor prognosis. During the initial treatment period when he was receiving only anti-viral medication and supportive care, a high fever persisted, and lung opacities spread rapidly, while at the same time his oxygen requirement increased rapidly. This drastically increased the risk of needing mechanical ventilation. PMX-DHP was used with the aim of removing inflammatory cytokines and halting the spread of acute lung injury showing the histological lesions of DAD. In fact, IL-6 and CRP decreased concurrently, and mechanical ventilation was not required. This is an important case in which endotracheal intubation was avoided, and the patient's life was saved. Although systemic steroids were administered for four days, the viral load did not increase.
Regarding the treatment of COVID-19, early introduction of anti-inflammatory therapy is important in preparation for rapid progressive respiratory failure. Although few hospitals can provide PMX-DHP and cover its cost, it may be an important strategy as a multi-faceted, multi-layered, anti-inflammatory treatment regimen to avoid intubation in high-risk patients.
The authors state that they have no Conflict of Interest (COI).
Financial Support
T. Suzuki reports grants from the Japan Agency for Medical Research and Development, AMED (JP19fk0108104 and JP19fk0108110) during the conduct of the study. He also has relevant financial activity outside of the work from the Japan Agency for Medical Research and Development, AMED (JP19fm0208002).
References
- 1.Johns Hopkins University, COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) [Internet]. 2020 [cited 2020 May 22]. Available from: https://coronavirus.jhu.edu/map.html
- 2.Shimizu T, Miyake T, Kitamura N, Tani M, Endo Y. Endotoxin adsorption: direct hemoperfusion with the polymyxin B-immobilized fiber column (PMX). Transfus Apher Sci 56: 682-688, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Abe S, Hayashi H, Seo Y, et al. Reduction in serum high mobility group box-1 level by polymyxin B-immobilized fiber column in patients with idiopathic pulmonary fibrosis with acute exacerbation. Blood Purif 32: 310-316, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Naka T, Shinozaki M, Akizawa T, Shima Y, Takaesu H, Nasu H. The effect of continuous veno-venous hemofiltration or direct hemoperfusion with polymyxin B-immobilized fiber on neutrophil respiratory oxidative burst in patients with sepsis and septic shock. Ther Apher Dial 10: 7-11, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Abe S, Seo Y, Hayashi H, et al. Neutrophil adsorption by polymyxin B-immobilized fiber column for acute exacerbation in patients with interstitial pneumonia; a pilot study. Blood Purif 29: 321-326, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Takeda S, Munakata R, Abe S, et al. Hypercytokinemia with 2009 pandemic H1N1 (pH1N1) influenza successfully treated with polymyxin B-immobilized fiber column hemoperfusion. Intensive Care Med 36: 906-907, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Tachikawa R, Tomii K, Murase K, et al. Therapeutic effect of direct hemoperfusion with a polymyxin B-immobilized fiber column in the treatment of HIV-negative severe pneumocystis pneumonia. Respiration 81: 318-324, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Sato N, Kojima K, Horio Y, et al. Successful treatment of severe amiodarone pulmonary toxicity with polymyxin B-immobilized fiber column direct hemoperfusion. Chest 143: 1146-1150, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Abe S, Azuma A, Mukae H, et al. Polymyxin B-immobilized fiber column (PMX) treatment for idiopathic pulmonary fibrosis with acute exacerbation: a multicenter retrospective analysis. Intern Med 51: 1487-1491, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Xu Z, Shi L, Wang Y, et al. Pathological findings of SARS-COV-2 associated with acute respiratory distress syndrome. Lancet Respir Med 8: 420-422, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-COV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8: 475-481, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]