Abstract
Primary lymphoma of the breast is a rare disease, accounting for about 0.5% of all primary breast tumors. Often found as a solitary indolent mass, it is difficult to distinguish from breast cancer on imaging and is often diagnosed for the first time based on histological findings. Diffuse large B-cell lymphoma is the most common histological subtype. A 48-year-old woman visited our hospital because of a painless mass in the left breast. Breast cancer was suspected based on the imaging findings. A core needle biopsy was performed, and the invasive ductal carcinoma was diagnosed. Partial mastectomy and sentinel lymph node biopsy were performed. The resected specimen was finally diagnosed as diffuse large B-cell lymphoma based on immunohistochemical staining. The patient was treated with R-CHOP and intrathecal injection of methotrexate. The patient remains alive without recurrence 4 years later. Awareness of primary breast lymphoma is essential for accurate and timely diagnosis and avoidance of unnecessary surgery.
Keywords: Primary breast lymphoma, Breast, Immunohistochemistry
Abbreviations: CNB, core needle biopsy; PBL, primary breast lymphoma
Introduction
Malignant lymphoma includes both nodular lymphoma, which develops within the lymph nodes, and extranodal lymphoma, which develops outside the lymph nodes. It is classified into Hodgkin's lymphoma and non-Hodgkin's lymphoma, the latter of which is further divided into B-cell and T/NK-cell types. About 2% of extranodal malignant lymphomas originate in the mammary gland [1,2]. Primary breast lymphoma (PBL) is rare, accounting for about 0.5% of primary breast tumors [3], [4], [5]. The reported mean age at diagnosis varies between 60 and 65 years [6]. Often found as a solitary indolent mass, PBL may present as multiple masses or diffuse mammary enlargement. The mass often feels softer than breast cancer on palpation. PBL is difficult to distinguish from cancer on imaging and is often diagnosed for the first time based on histological findings. Here we report a case of PBL and a review of the literature.
Case report
A 48-year-old woman came to our hospital because of a painless mass in the left breast. On palpation, a relatively soft tumor measuring 40 mm in diameter was detected in the upper inner quadrant of the left breast. No axillary lymph nodes were palpable.
Mammography findings were normal (category 1) and showed no mass or calcification (Fig. 1). Ultrasonography revealed an irregular hypoechoic mass of 23 mm in diameter with angular and indistinct margins (Fig. 2). Whole-body CT performed for staging showed a contrast-enhancing tumor of 22 mm in diameter. There was no axillary lymphadenopathy, and no lesions were detected in other organs. Magnetic resonance imaging revealed an irregular heterogeneously enhancing mass with irregular margins with a diameter of 37 mm. Fast initial-phase and washout delayed-phase enhancement kinetics suggested invasive ductal carcinoma, such as scirrhous carcinoma (Fig. 3). Early deep staining and internal washout in the equilibrium phase suggested invasive ductal carcinoma, such as scirrhous carcinoma.
Fig. 1.
Mammography findings are normal (category 1) and show no mass or calcification. (a: mediolateral oblique view; b: craniocaudal view).
Fig. 2.
Ultrasonography shows an irregular hypoechoic mass of 23 mm in diameter with angular and indistinct margins. The imaging findings are suggestive of a malignant tumor.
Fig. 3.
Magnetic resonance imaging shows an irregular heterogeneously enhancing mass with irregular margins. The tumor shows strong enhancement from an early stage, and the maximum diameter of the tumor is 37 mm.
Breast cancer was suspected based on the imaging findings, and an ultrasound-guided core needle biopsy was performed.
Histological examination revealed large atypical epithelial cells arranged in a cord/alveolar pattern with proliferation and infiltration. The diagnosis was invasive ductal carcinoma (Fig. 4).
Fig. 4.
Histological examination shows large atypical epithelial cells arranged in a cord/alveolar pattern with proliferation and infiltration. The diagnosis is invasive ductal carcinoma.
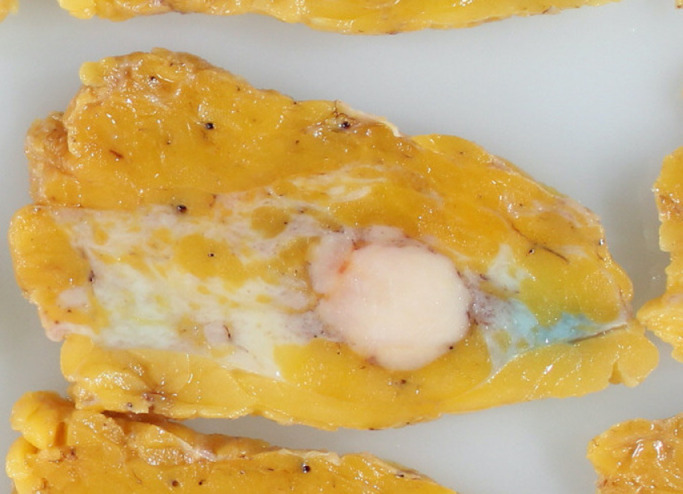
Partial mastectomy and sentinel lymph node biopsy were performed. A white tumor with a clear border measuring 30 mm was found in the specimen (Fig. 5). Histologically, a dense proliferation of atypical cells with a high N/C ratio and rounded nuclei were observed. Immunostaining was performed because poorly differentiated cancer cells or malignant lymphoma were suspected.
Fig. 5.
A white tumor of 30 mm in diameter with a clear border is found in the specimen.
Immunostaining for breast cancer was negative for ER, PgR, and HER2, with an MIB-1 labeling index of 90%. The results were positive for CD20, CD79a, and Bcl2 and negative for CD3, CD10, and S100, confirming a final diagnosis of diffuse large B-cell lymphoma (DLBCL) (Fig. 6). The patient received R-CHOP and intrathecal injection of methotrexate. No radiation therapy was administered. The patient is alive without recurrence 4 years after the operation.
Fig. 6.
Histologically, dense proliferation of atypical cells with rounded nuclei and a high N/C ratio is observed (a, b). Immunostaining for breast cancer is negative for ER (c), PgR (d), and HER2 (e), with an MIB-1 labeling index of 90% (f). Results are positive for CD20 (g) and negative for CD3 (h) and cytokeratin (i), confirming a final diagnosis of diffuse large B-cell lymphoma.
Discussion
PBL is a relatively rare disease. It is reported to account for 0.17% of all malignant breast tumors in Japan, 0.4%-0.5% of those in the United States, 0.38%-0.7% of non-Hodgkin's lymphomas, and 1.7%-2.2% of extranodal lymphomas. It is often found as a solitary indolent mass. In general, the duration of illness is short and the tumor is characterized by rapid growth. Nipple and skin contraction and nipple discharge are rare. Bilateral involvement is described in about 11% of cases [7]. The most common site of this lesion in the breast is the upper outer quadrant (40%), followed by the upper inner quadrant (20%), the lower outer quadrant (17%), and the nipple/areolar area (14%) [8,9].
The usual B symptoms seen in lymphoma, such as fever, weight loss, and night sweats, are very rare in PBL. Mean age at diagnosis is 60-65 years.
Most patients are female, and few male cases have been reported.
Non-Hodgkin's DLBCL is the most common histopathological subtype of PBL, followed by follicular and MALT (mucosa-associated lymphoid tissue) lymphoma. Breast lesions with Hodgkin's disease or T-cell lymphoma are rare [10,11]. Although malignant lymphoma is a systemic disease and it is difficult to pinpoint the primary site as the mammary gland, Wiseman and Liao [12] proposed the following three diagnostic criteria for PBL: (1) the mammary gland tissue and tumor tissue have a close relationship; (2) an appropriate histopathological evaluation has been performed; and (3) no malignant lymphoma is found at sites other than the mammary gland at the first visit, although ipsilateral axillary lymph node metastases may be present. This case met these criteria and was diagnosed as malignant lymphoma of the mammary gland.
Imaging features of PBL are nonspecific and may resemble those of other breast malignancies or even a benign tumor [13]. Mammography often shows a smooth marginal tumor with clear or unclear boundaries. PBL is not accompanied by calcification, architectural distortion, nipple retraction, or spicula. Characteristic findings on ultrasound are decreased internal echogenicity and increased posterior acoustic enhancement, reflecting the high cell density.
However, the findings for PBL are essentially the same as those for lymphoma in other organs. In many cases, there are coexisting low and high internal echoes with variable enhancement of backward echoes. On dynamic MRI, PLB often demonstrates fast early-phase enhancement reflecting hypervascularity. Furthermore, diffusion-weighted images, which reflect cell density, show restricted diffusion.
Diagnosis of malignant lymphoma on imaging is difficult but malignant disease can be suspected.
Histological examination is also important. A characteristic feature of malignant lymphoma is a monotonous appearance of many tumor cells without epithelial connections and granular lymphoglandular bodies with degenerating cytoplasm in the background. In addition, tumor cells have finer nuclear chromatin and a significantly higher N/C ratio than epithelial-derived cancer cells, and strong nuclear irregularities, such as constrictions and breaks in the nucleus, are observed. Differential diagnoses for high-grade lymphomas such as DLBCL include solid invasive ductal carcinoma and epithelial malignancies such as lobular carcinoma. A common finding is monotonous tumor cells with marked nuclear irregularity that appear in a scattered manner. The distinction is that in the solid type of invasive ductal carcinoma, the cytoplasm is richer and the N/C ratio is lower than in lymphoma, and epithelium-bound clumps are observed in the specimen. In lobular carcinoma, the cytoplasm is wide and cell-to-cell adhesion is mild, but characteristic findings of beaded arrays and small glandular spaces in the cytoplasm are more common. However, the polymorphic form of lobular carcinoma may be difficult to distinguish because the atypical form is strongly seen and immunohistochemistry is useful.
Given that low-grade lymphoma consists mainly of small-to-medium-sized atypical lymphocytes, the differential diagnoses include intramammary lymph nodes and granulomatous mastitis. Malignant lymphoma is composed of round monotonous cells with mild constriction of the nucleoli in the nucleus whereas small mature lymphocytes with no atypia are common in mammary gland lymph nodes, which also contain various lymphocytes, such as medium-sized lymphocytes and immunoblasts. Macrophages that phagocytose nuclear fragments are also found occasionally, and the characteristic feature is that the cells that emerge are rich in variety. It is important to compare and carefully observe the characteristics and findings of such cells in order to distinguish between breast cancer and PLB.
Histologically, the important points that distinguish breast cancer from malignant lymphoma are (1) conspicuous apoptosis; (2) marked nuclear rays due to crushing; and (3) polymorphism in tumor cells (marked large and small anomalies and nuclear incisions, constrictions, polynuclear cells, and megakaryocytic cells). Based on the above, careful diagnosis is necessary.
Unfortunately, we were unable to reach the correct diagnosis before surgery in this case. Although there are no imaging findings specific to PBL, the radiologist must consider this rare malignancy and inform the pathologist that it is included in the differential diagnosis.
Treatment of PLB includes surgery, radiation therapy, chemotherapy, and immunotherapy, either alone or in combination. However, there are still no established treatment guidelines. Currently, most patients with PLB receive chemotherapy and radiation therapy, and a declining number of patients undergo surgery [14].
Radical mastectomy has recently been shown to be ineffective and may delay the initiation of chemotherapy. CHOP or CHOP-like anthracycline-based chemotherapy combined with rituximab is now considered the standard treatment for most patients with DLBCL of the breast [5,15]. This may be followed by irradiation of the breast and regional lymph nodes [16].
PBL is an aggressive tumor with a 5-year overall survival rate of 50%-60% and spreads mainly by extranodal invasion [17]. Higher CNS recurrence rates have also been reported in PBL-DLBCL, and addition of CNS-directed treatment is widely considered to be essential [18,19].
Conclusion
Accurate histological diagnosis of PBL is necessary to avoid unnecessary surgery. To that end, it is important to first suspect the presence of malignant lymphoma. Although it is difficult to make the diagnosis based on image findings alone, it is necessary to convey to pathologists that PBL is one of the differential diagnoses. It is important to carefully examine each imaging test and consider malignant lymphoma as a differential diagnosis. We report this case in the hope that others can learn from our experience and avoid similar pitfalls in the future.
Ethics approval and patient consent
Ethical approval is not required at our institution for publication of case reports.
Patient consent: Written informed consent for publication was obtained from the patient who is the subject of this report.
Footnotes
Competing interest: None.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Giardini R, Piccolo C, Rilke F. Primary non-Hodgkin's lymphomas of the female breast. Cancer. 1992;69(3):725–735. doi: 10.1002/1097-0142(19920201)69:3<725::aid-cncr2820690320>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 2.Sabate JM, Gomez A, Torruba S, Camins S, Roson N, De Las Heras P. Lymphoma of the breast; clinical and radiological features with pathologic correlation in 28 patients. Breast J. 2002;8(5):294–304. doi: 10.1046/j.1524-4741.2002.08509.x. [DOI] [PubMed] [Google Scholar]
- 3.Hugh JC, Jackson FI, Hanson J, Poppema S. Primary breast lymphoma. An immunohistologic study of 20 new cases. Cancer. 1990;66(12):2602–2611. doi: 10.1002/1097-0142(19901215)66:12<2602::aid-cncr2820661224>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Ribrag V, Bibeau F, EI Weshi A, Frayfer J, Fadel C, Cebotaru C. Primary breast lymphoma: a report of 20 cases. Br J Haematol. 2001;115(2):253–256. doi: 10.1046/j.1365-2141.2001.03047.x. [DOI] [PubMed] [Google Scholar]
- 5.Avile A, Delgado S, Nambo MJ, Neri N, Murillo E, Cleto S. Primary breast lymphoma: results of a controlled clinical trial. Oncology. 2005;69(3):256–260. doi: 10.1159/000088333. [DOI] [PubMed] [Google Scholar]
- 6.Jeon HJ, Akagi T, Hoshida Y, Hayashi K, Yoshino T, Tanaka T. Primary non-Hodgkin lymphoma of the breast. An immunohistochemical study of seven patients and literature review of 152 patients with breast lymphoma in Japan. Cancer. 1992;70(10):2451–2459. doi: 10.1002/1097-0142(19921115)70:10<2451::aid-cncr2820701011>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Joks M, Mysliwiec K, Lewandowski K. Primary breast lymphoma—a review of the literature and report of three cases. Arch Med Sci. 2011;7(1):27–33. doi: 10.5114/aoms.2011.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg NK, Bagul NB, Rubin G, Shah EF. Primary lymphoma of the breast involving both axillae with bilateral breast carcinoma. World J Surg Oncol. 2008;6:52. doi: 10.1186/1477-7819-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lnic Z, Inic M, Zegarac M, Inic I, Pupic G. Three cases of combined therapy in primary breast lymphoma (PBL) with successful outcomes. Clin Med Insights Oncol. 2013;7:159–163. doi: 10.4137/CMO.S12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domchek SM, Hecht JL, Fleming MD, Pinkus GS, Canellos GP. Lymphomas of the breast: primary and secondary involvement. Cancer. 2002;94(1):6–13. doi: 10.1002/cncr.10163. [DOI] [PubMed] [Google Scholar]
- 11.Wadhwa A, Senebouttarath K. Primary lymphoma of the breast: a case series. Radiol Case Rep. 2018;13(4):815–821. doi: 10.1016/j.radcr.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiseman C, Liao KT. Primary lymphoma of the breast. Cancer. 1972;29(6):1705–1712. doi: 10.1002/1097-0142(197206)29:6<1705::aid-cncr2820290640>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Ola H, Safa B, Hassan A, Eman E, Loay G, Rham A. Primary diffuse large B-cell non-Hodgkin’s lymphoma of the breast—a case report and review of the literature. Radiol Case Rep. 2019;14:22–27. doi: 10.1016/j.radcr.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anubha W, Kathleen S. Primary lymphoma of the breast: a case series. Radiol Case Rep. 2018;13:815–821. doi: 10.1016/j.radcr.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 16.Gupta V, Bhutani N, Singh S, Chhabra S, Sen R. Primary non-Hodgkin’s lymphoma of breast—a rare cause of breast lump. Hum Pathol Case Rep. 2017;7:47–50. [Google Scholar]
- 17.Aviles A, Neri N, Nambo J. The role of genotype in 104 cases of diffuse large B-cell lymphoma primary of breast. Am J Clin Oncol. 2012;35(2):126–129. doi: 10.1097/COC.0b013e318209aa12. [DOI] [PubMed] [Google Scholar]
- 18.Arkenau HT, Chong G, Cunningham D, Watkins D, Agarwal R, Sirohi B. The role of intrathecal chemotherapy prophylaxis in patients with diffuse large B-cell lymphoma. Ann Oncol. 2007;18(3):541–545. doi: 10.1093/annonc/mdl434. [DOI] [PubMed] [Google Scholar]
- 19.Boehme V, Zeynalova S, Kloess M, Loeffler M, Kaiser U, Pfreundschuh M. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma—a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL) Ann Oncol. 2007;18(1):149–157. doi: 10.1093/annonc/mdl327. [DOI] [PubMed] [Google Scholar]