Abstract
Objectives
To examine the effects of the Roy Adaptation Model-based interventions on adaptation in persons with heart failure.
Methods
A quasi-experimental study was conducted in Hangzhou, China, from March 2018 to November 2019. A convenience sample of 112 participants with heart failure from a multi-campus hospital was enrolled. Participants were allocated into an intervention group (n = 55) and a control group (n = 57) according to their hospitalized campus. A culturally-tailored care plan intervention based on the Roy Adaptation Model was performed in the intervention group. The control group received bedside patient education and a regular booklet for HF home care before discharge. Heart ultrasound, Minnesota Living with Heart Failure Questionnaire (MLHFQ), a knowledge survey, Self-care Heart failure Index (SCHFI), and Coping and Adaptation Processing Scale-Short Form (CAPS-SF) were used to measure patients’ levels of adaptation of physical function, self-concept, role function, and interdependence at baseline and six months after discharge.
Results
Ninety-one participants with complete data, 43 in the intervention group and 48 in the control group, were included in the analysis for the primary endpoints and showed adaptive improvement trends. Most patients in the intervention group completed 60% or more of the given interventions. At the sixth month after discharge, compared with the control group, the intervention group had improved adaptive behaviors showing higher scores of the MLHFQ (70.90 ± 22.45 vs. 54.78 ± 18.04), heart failure-related knowledge (13.79 ± 2.45 vs. 10.73 ± 4.28), SCHFI maintenance (57.67 ± 13.22 vs. 50.35 ± 10.88), and CAPS-SF (40.23 ± 4.36 vs. 38.27 ± 2.60) at the six-month follow-up (P < 0.05). There were no significant differences between the two groups in the scores of left ventricular ejection fraction, scores of SCHFI management and SCHFI confidence subscales (P > 0.05).
Conclusions
The findings reported evidence of positive adaptation in patients with heart failure, indicating that the Roy Adaptation Model is an effective guide for developing an implemented framework for the nursing practice of the patients. The culturally-tailored care plan intervention is helpful to improve adaptation of patients with heart failure.
Keywords: Adaptation, Heartfailure, Quality of life, Roy adaptation model, Self-care
What is known?
-
•
The Roy Adaptation Model is widely used in nursing research and real-life practice.
-
•
Personalized care planning is a promising approach for improving chronic disease management for home care patients.
-
•
Premature deaths caused by heart failure may be preventable if patients are able to recognize early escalating symptoms and seek immediate medical attention.
What is new?
-
•
This study provided empirical evidence on testing the Roy Adaptation Model in heart failure context within the Chinese culture.
-
•
Nurse-led personalized care planning is an effective intervention to activate and empower patients, thus, improves adaptation of the persons with heart failure.
-
•
Illness-related recommendations for developing behaviors within the four adaptive modes should be easily accessible to patients after discharge.
1. Introduction
Heart failure (HF) is a complex and potentially devastating chronic illness developed as a result of coronary artery disease, valvular heart disease, cardiomyopathy, or other heart diseases, affecting around 26 million people worldwide [1,2]. Recent epidemic data show that the estimated prevalence of HF is increasing among Chinese populations nationwide with an increasing prevalence of cardiometabolic disorders. Findings of the China-HF Registry indicated 4.1% in-hospital deaths among hospitalized HF patients and 32.8% readmission rate within one year after hospital discharge [3]. What’s worse, the chronicity and severity of HF complications lead to increased disability and mortality, challenging an individual’s adaptation and public health services [4,5].
Previous studies suggested that premature deaths caused by HF may be preventable if patients are able to recognize early escalating symptoms and seek immediate medical attention [6,7]. It’s acknowledged that patients require self-management strategies and self-care behaviors to help them prevent worsening and adapt to the HF [[8], [9], [10]]. Accordingly, there has been a great many studies for developing and evaluating HF management programs worldwide. The effectiveness of regular structured telephone follow-up and telemedicine monitoring for improving short-term clinical outcomes and self-care behaviors have been demonstrated among HF patients [[10], [11], [12]]. A person-centered approach, multidisciplinary strategies, and transitional care service were recommended to use in clinical settings [2,8,13]. And, researchers found that the anticipated health outcomes may be evidenced in symptom management, behavior modification, knowledge, use of health care resources, low readmission, quality of life, coping efficacy, health status, and cost of health [5,14,15].
Empirical evidence has shown that, during in-patient and immediately post-discharge, nurse-led structured personalized care planning inclusive of strategies to symptom control, lifestyle modifications, and prevention of complications for adults with chronic diseases improves health outcomes, including reduced symptom severity, a better quality of life, and lower readmission [16,17]. As reported, evidence-based individual approaches for HF in the clinical setting emphasize the need of a care plan to facilitate successful adaptation by setting specific, shared, proximal goals, developing an effective strategy for self-monitoring, scheduling a regular follow-up in-person and telephone, encouraging self-report, increasing self-efficacy, and coordinating patients’ essential relationships with the environment [8,17,18]. Any approach to the personalized nursing care of the chronically ill and their families must be implementable in individuals’ daily life and operationalized to help mitigate the diversity, complexity, and multiplicity of the symptoms that HF can cause [17,19]. While numerous individualized services exist, empirical support and practice experience of personalized care planning applied in HF management still need to be further explored to facilitate adaptation.
2. Conceptual model
Roy Adaptation Model (RAM) was used to guide this study. RAM explores the interrelationships among humanism, health, and the environment. RAM outlines theoretical concepts of stimuli, coping processes, adaptive modes such as physical, self-concept, role function, and interdependence, as well as an adaptive response including adaptive/ineffective behavior [20,21]. According to the RAM, stimuli activate coping processes. The responses and behaviors to stimuli can be observed in four interrelated adaptive modes. Physical mode refers to an individual’s physical and chemical processes involved in the function and activities of a living organism. Self-concept mode shows physical, psychological, and spiritual self at a given time, formed from internal perception and perceptions of others’ reactions. Role function mode relates to each role and its functioning unit in society. Interdependence mode represents the close relationships of people with significant others [21]. Adaptation is conceptually defined as the process and outcome whereby the thinking and feeling person uses conscious awareness and choice to create human and environmental integration [22]. Further studies on developing the operationalized concepts for practice guidance and inquiry of the adaptation process using empirical indicators in illness contexts are still needed for the RAM validation and refinement.
Stimuli are the points of interaction of the human system and environment which provoke responses [23]. In this study, focal stimuli were identified by the duration and severity of HF, while contextual stimuli were related to patients’ demographic characteristics, lifestyle behaviors, and health promotion activities. Residual stimuli operationalized as unknown factors that might be rooted in cultural beliefs and values. Previous studies showed that HF patients might experience physical symptoms, such as fatigue, edema, sleepiness, and dyspnea, and emotional symptoms, such as depression, worries, and cognitive problems [24,25]. Additionally, HF patients struggle with uncertainty, questions about the meaning of life, adjusting to the natural dynamic of the illness, and life re-adaptation [5,9,14], thereby, tend to have considerable difficulty in coping with challenging situations.
The coping progress to HF is complex and interactive for patients due to the interconnectedness of the subjectively experienced reactions to HF and various medical, sociodemographic, social, and cultural factors. Using empirical inductive and deductive strategies, the adaptive responses to HF have been delineated in previous studies [26]. These were manifested in four modes indicating physical function focused on heart function, self-concept incorporated with physical self and mental self, role function associated with individuals’ performance in illness management (disease-specific knowledge and self-care behaviors), and interdependence related to the feeling of being supported. Nurses are required to act to help patients cope with HF symptoms, arrange their personal and environmental resources, and modify lifestyle behaviors to living well with the HF. Hence, in this study, a cultural-tailored personalized care planning is assumed to bridge the gap of interactions among all these stimuli, patients’ perceptions of illness impact, and coping behaviors.
In the series of studies investigating the adaptation level to the HF, heart function, quality of life, disease-related knowledge, self-care behaviors, and the ability of coping were applied to measuring the health outcomes of living with HF. Improving knowledge and skills are beneficial for optimizing self-care behaviors [8,27]. HF self-care refers to a decision-making process that individuals and their families perform health-promoting activities to maintain health and manage illness, including self-care maintenance, self-care monitoring, and self-care management, as well as the influence of symptoms [8,28]. Moreover, quality of life, commonly used in HF management program evaluation, reflects the subjective sense of overall well-being. Extensive researches have shown that the enhancement of self-care behaviors is associated with adaptive capacity (individuals’ capacity of coping strategies), has positive impacts on reducing hospital readmissions, and leads to an improved quality of life [9,27,29]. These empirical indicators indicate the efforts a person made for physical, psychological, and social well-being, being consistent with the contents of adaptive modes. A meta-analysis by Chiou [30] also evaluated the empirical adequacy of the interrelationships between adaptive modes and supported their integrated functions for adaptation.
As noted in the RAM, individual behavior is viewed concerning the four adaptive modes and observed by coping and cognitive strategies. The concept of adaptation represents positive coping [21]. Therefore, the individual’s capacity of coping style within four modes was adopted to describe the goal of nursing as the promotion of adaptation for HF patients in this study. The readmission rate caused by HF was employed to evaluate the patients’ health outcomes in a short-term period.
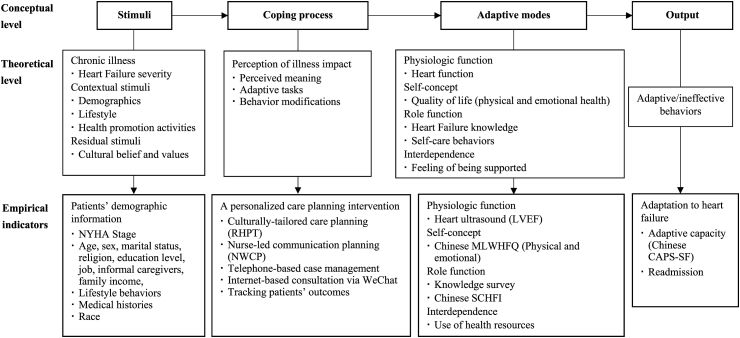
Based on the above definitions and nursing statements, a theoretically hypothesized model was proposed for guiding the development and evaluation of cultural-tailored care planning to help HF patients develop adaptive behaviors (Fig. 1). Therefore, the research objective was to evaluate the effects of the theory-guided interventions on an individual’s abilities within four adaptive modes, which were expected to be assessed by biological data, quality of life, HF knowledge, self-care behaviors, and usage of health resources. The level of adaptation to HF was measured by readmission and adaptive capacity.
Fig. 1.
Conceptual-theoretical-empirical structure of adaptation to heart failure.
NYHA = New York Heart Association. RHPT = Return to Home Planning Template. NWCP = Nurses’ Weekly Communication Planning. LVEF = Left ventricular ejection fraction. MLWHFQ = Minnesota Living with Heart Failure Questionnaire. SCHFI = Self-care Heart failure Index. CAPS-SF = Coping and Adaptation Processing Scale-Short Form.
3. Methods
3.1. Study design
This is a quasi-experimental study with a follow-up of HF participants to six months post-discharge. The study complied with the guidelines for reporting standards of nonrandomized evaluations of the behavioral intervention study, Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) Checklist [31].
3.2. Participants and setting
The entire study was conducted between March 2018 and November 2019. Using convenience sampling, patients were recruited by nurses caring for them from two cardiology departments of a single multi-campus hospital in Hangzhou, China. Inclusion criteria were: adults (18 years and older), a diagnosis with HF and defined by the New York Heart Association (NYHA) stage II-IV, HF patients after acute treatment with telephone access, fluency in Mandarin Chinese, ability to communicate verbally, and no mental disorder. Patients who were unwilling to participate were excluded. To reduce the bias caused by sample interaction, enrolled participants were allocated into the intervention and control groups according to their hospitalized campus.
3.3. Intervention
3.3.1. Control group
Participants in the control group received bedside patient education relating to symptom monitoring and self-management strategies from a nurse caring for them. A regular booklet for HF home care, including information on the treatment of HF, common health issues, development of healthy lifestyle behaviors, suggestions of intake of medication, and compliance to follow-up, would be given to patients and their caregivers before discharge. Additionally, nurses would call patients every three months to investigate their health status. Patients are encouraged to visit a cardiologist every month or in an emergency. The intervention was delivered by full-time nurses from the cardiology department.
3.3.2. Intervention group
The tailored personalized care planning was an integration of widely used and effective approaches, involving a structured tool (Return to Home Planning Template, RHPT, Appendix Table A1), nurse-led communication plan (Nurses’ Weekly Communication Planning, NWCP, Appendix Table A2), telephone follow-ups, internet-based consultation via WeChat, and tracking patients’ outcomes. Details were elaborated in Table 1, including the objectives, main tasks performed by nurses, and patients’ adaptive tasks. The period time of six months for interventions was established according to the Chinese HF guideline [32]. Intense observation after the acute treatments was also recommended for home care. Therefore, after discharge to one month, researchers gave patients phone calls once per week. In the second month, researchers reduced the frequency to one time every two weeks, then to one time per month in the following three months. Ten telephone calls were required in this study. These nursing interventions was performed by six trained researchers who had a Master degree with clinical nursing experience, as well as collaborating with the nurses caring for enrolled patients in the hospital.
Table 1.
Outline and the content of the personalized care planning intervention.
| Stage | Objectives | Main tasks performed by nurses | Patients’ adaptive tasks |
|---|---|---|---|
| Pre-discharge | |||
| Preparation for discharge | Assessing patients’ health status and making tailored recommendations |
|
|
| Post-discharge | |||
| Ten telephone calls | Facilitating patients’ adaption to HF: knowledge acquisition, skill training, behavior changing and reflecting |
|
|
| Weekly internet-based consultation | Reinforcing the interventions and creating a supportive environment by organizing WeChat forums |
|
|
| Tracing patients’ outcomes | Building a close relationship between nurses and patients |
|
|
| Clinic follow-ups (optional) | Regular follow-up and providing the support of health care professionals |
|
|
Note: HF = heart failure. RHPT = Return to Home Planning Template.
The RHPT was developed based primarily on the regular clinical booklets, evidence-based medical and nursing guidelines [10,[32], [33], [34], [35]]. Recommendations from guidelines were adapted for operationalization in the local healthcare context through informal discussions with interdisciplinary team members. RHPT served as a tool for clinicians and patients to integrate goals into daily life and to iterate personalized strategies.
The interventional study phases were presented in Table 1. In the pre-discharge phase, using RHPT, a written copy of the tailored recommendations on diet, pharmacological treatment, physical activity, self-care, symptom management, and emotional control were given to patients and their caregivers. The tailored RHPT was made by patients and their nurses after considering individuals’ health needs adequately. The post-discharge phase included the patients’ home care and follow-up visits. Patients were encouraged to continue using RHPT at home before their first follow-up visits. To trace the achievements of goals, patients were required to record their daily health behaviors, then, researchers used a teach-back approach for telephone communication. Based on patients’ or caregivers’ interpretation of the given information and reporting of problems in managing HF at home, researchers could understand the symptom experience of patients, then, provided an evaluation of patients’ accomplishments in following the RHPT, further education, advice on re-optimizing pharmacotherapy, and a summary of prioritized problems, then the data were recorded in NWCP. Furthermore, researchers discussed with patients and/or their caregivers to help them set new goals and develop appropriate adaptive behaviors. Researchers ended every telephone call with an open-ended question, for example, “Do you have any questions?” or “What else can I do for you?“, and then summarized the content of the call and set a date for the next telephone call. If needed, researchers gave patients and their caregivers suggestions for the next physician clinic visit.
To augment nursing support, WeChat (the most widely used smartphone messaging application in China, like Facebook) was used to encourage HF patients to participate in online forums that allow them and their caregivers to develop knowledge, exchange comments about self-management, and discuss health issues. The WeChat group managed by researchers consisted of patients, their caregivers, and three nursing specialists. Every Wednesday, researchers would post worthwhile information on protective lifestyle changes to improve HF symptom management. Additionally, every Friday, researchers would provide detailed recommendations for specific problems, which were collected and summarized from routine follow-up telephone contacts.
Across the whole health program, to measure progress over time, HF health status was retrieved from the electronic health record to help researchers find potential problems as adaptive tasks that patients needed to solve or report in follow-up visits to physicians. Ideally, health records enabled researchers to be familiar with patients’ health status, which leads to close relationships between researchers and patients.
3.4. Outcome measures
At enrollment and six months later, participants completed surveys that investigated demographics, adaptive behaviors, and adaptive capacity. A completion survey was investigated by a graduate nursing student. Overall, the instruments used in this study were as follows and the permissions for the use were authorized by their authors.
3.4.1. Patients’ demographic information
An investigator-developed questionnaire was used to collect patients’ demographic characteristics, lifestyle behaviors, medical histories, treatment histories, health costs, and length of stay during prior hospitalization.
3.4.2. Intervention fidelity
Each patient in the intervention group was provided with a written copy of the tailored RHPT for recording proximal goals and reminding health behaviors. To facilitate self-monitoring, participants were asked to record their HF symptoms and coping methods, then gave verbal reports in telephone follow-ups. Adherence to the intervention was assessed by their involvement in telephone call and researchers’ NWCP records during the six months. The patients’ participation of WeChat was observed by researchers, and the investigations on patients’ understandings of the given knowledge were conducted in telephone call under the teach-back method.
3.4.3. Adaptative modes
The physical adaptation was measured by heart ultrasound. To be specific, left ventricular ejection fraction (LVEF) reflecting the prognosis of HF was used for assessing heart function. The LVEF was determined by heart ultrasound.
Self-concept adaptation representing physical self and personal self was assessed by the quality of life. The Minnesota Living with Heart Failure Questionnaire (MLHFQ) including physical, emotional, and other domain was selected. The MLHFQ, a disease-specific questionnaire, is a 21-item and a 6-point response Likert scale (0–5) for investigating patients’ everyday living problems relevant to HF and their impact on physical activity, social interaction, sexual activity, and emotions [36]. The Chinese version of the MLWHF has good internal consistency with a Cronbach’s α of 0.88 [37].
Role function showing an individual’s competency in HF management was evaluated by self-care behaviors and knowledge. Further, self-care behavior was measured using the 22-item Self-care Heart Failure Index (SCHFI v.6) which included three subscales [38]. As reported, Cronbach’s α of Chinese version on self-care maintenance scale was 0.66, on self-care management scale was 0.74, and on self-care confidence scale was 0.87 [39]. Shortened from the Atlanta Heart Failure Knowledge Test (A-HFKT) [40],a 6-question and 4-point response knowledge questionnaire with a Cronbach’s α of 0.74 was used to evaluate patients’ knowledge for fluid overload prevention and HF symptom identification.
Interdependence mode relating to the relationships between patients and significant others was observed across the interventional project. Participants’ feelings of being supported would be evaluated by nurses and recorded in the NWCP. Quantitative data were collected on intervention adherence.
3.4.4. Adaptation evaluation
The 15-item Coping and Adaptation Processing Scale-Short Form (CAPS-SF), a RAM-based instrument [41], was applied to assess individuals’ adaptive capacity of coping with HF conditions. Each item is endorsed on a 4-point scale. The range of scores is from 60 to 15 with a high score indicating a more consistent use of the identified strategies of coping. The Chinese version with sound psychometric properties was translated and validated [42]. McDonald’s Omega of Chinese version was 0.82. Additionally, the readmission rate due to HF and time to the first readmission were observed according to patients’ self-report.
3.5. Data analysis
The required sample size was estimated using the G-power 3.1. According to the data from an eight-weeks personalized rehabilitation program for HF patients, an effect size of 0.5 was determined by the difference in scores of the quality of life among studied groups [43]. Considering the 20% attrition, the minimum total of 41 participants for each group would be adequate to achieve an 80% power to detect a 0.5 effect size at a 5% level of significance with the T-test method.
Patients’ demographic information was summarized using descriptive analysis. Chi-square test was used to estimate the difference in categorial data, and t-test for continuous variables (age and length of hospital stay). As per the scoring manual, raw scores from the SCHFI were tabulated into standardized 100-point scales, as well as the MLHFQ. To examine the between-group differences at baseline and completion, an independent sample t-test was adopted. Pre-intervention and post-intervention scores for each scale were compared using paired-sample t-tests. The difference in readmission rate between groups was compared using the Chi-square test. SPSS version 22.0 was defined using a 2-sided significance level of α = 0.05.
3.6. Ethic consideration
The study protocol was approved by the ethics committee of Sir Run Shaw Hospital affiliated to Zhejiang University School of Medicine (No. 20171120-14). Participants were informed about the study purpose and their rights to withdraw from the study at any time without penalty on their present or future care. Completed written consent was obtained from every participant.
4. Results
4.1. Study recruitment
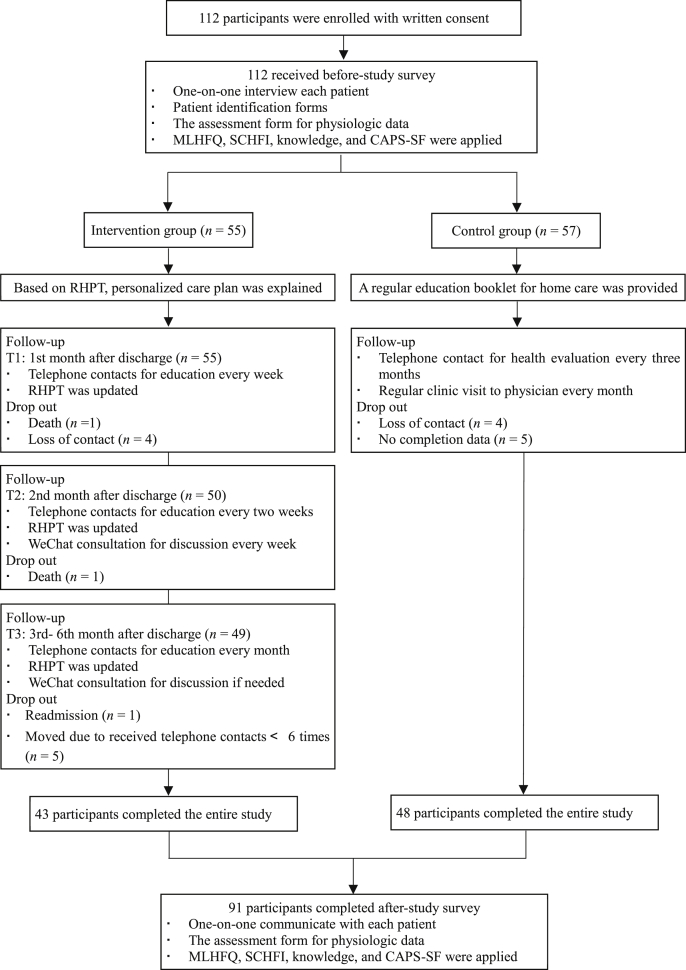
We summarized the flow of study phases (see Fig. 2). Totally 112 HF patients met inclusion criteria and agreed to participate were successfully allocated into the intervention group (n = 55) and control group (n = 57). Finally, 91 participants completed the trial and were included in data analysis. Attrition at six months was 18.8%. Two patients died of HF in the intervention group, while no death reported in the control group. Five persons in the intervention group were removed for missing more than four times telephone contacts. They were not available for accepting telephone communication for personal reasons, such as work time, terrible physical health, and feeling depressed at that time (we collected this information in the later telephone contact).
Fig. 2.
Flow of participants through the personalized care planning intervention study.
MLHFQ = Minnesota Living with Heart Failure Questionnaire. SCHFI = Self-care Heart Failure Index. CAPS-SF = Coping and Adaptation Processing Scale-Short Form. RHPT = Return to Home Planning Template.
4.2. Participants’ characteristics
Of the 112 patients at baseline, most patients were male (73.2%), Han Chinese (100%), no religions (77.7%), and had less than a high school education (75.0%). Patients were asked to take about five types of medicines during and after discharge. Most patients were on evidence-based HF therapies on admission including diuretic (85.7%, 96/112), β-blockers (76.8%, 86/112), statins (55.4%, 62/112), potassium supplement (42.0%, 47/112), and aspirin (42.0%, 47/112). Among the participants, most were dependent on caregivers for daily care. Additionally, 78.57% of patients (88/112) at baseline reported that hospitalization and health expenses caused by HF influenced their life seriously. Average hospitalization fee (53,000 CNY, 7571 USD) exceeded their family income per month if paid out-of-pocket. Participants’ characteristics were summarized. There is no statistical difference between the two groups at baseline (Table 2).
Table 2.
Patient characteristics at baseline [n (%)]
| Variables | Intervention group |
Control group |
t/χ2 |
|---|---|---|---|
| (n = 55) | (n = 57) | ||
| Age (Mean ± SD) | 65.71 ± 13.42 | 69.02 ± 12.10 | -1.372 |
| NYHA stage | 2.885 | ||
| NYHA II | 11 (20.0) | 5 (8.8) | |
| NYHA III | 26 (47.3) | 31 (54.4) | |
| NYHA IV | 18 (32.7) | 21 (36.8) | |
| Sex | 0.293 | ||
| Male | 39 (70.9) | 43 (75.4) | |
| Female | 16 (29.1) | 14 (24.6) | |
| Marital status | 1.205 | ||
| Unmarried | 2 (3.6) | 3 (5.3) | |
| Married | 50 (90.9) | 48 (84.2) | |
| Devoiced/widows | 3 (5.5) | 6 (10.5) | |
| Religion | 5.269 | ||
| No religion | 42 (76.4) | 45 (78.9) | |
| Buddhist | 11 (20.0) | 9 (15.8) | |
| Other(s) | 2 (3.6) | 3 (5.3) | |
| Education level | 1.428 | ||
| Primary school | 30 (54.5) | 35 (61.4) | |
| Middle school | 9 (16.4) | 10 (17.5) | |
| Senior high school | 10 (18.2) | 9 (15.8) | |
| University | 6 (10.9) | 3 (5.3) | |
| Job | 1.260 | ||
| Retirement | 17 (30.9) | 28 (49.1) | |
| Employed | 13 (23.6) | 3 (5.3) | |
| Unemployed | 25 (45.5) | 26 (45.6) | |
| Caregivers a | |||
| Self | 30 (54.5) | 31 (54.4) | <0.001 |
| Spouse | 34 (61.8) | 34 (59.6) | 0.055 |
| Children | 28 (50.9) | 31 (54.4) | 0.136 |
| Others | 2 (3.6) | 1 (1.8) | 0.001 |
| Family income (per month, CNY) | 0.627 | ||
| <4,000 | 21 (38.2) | 15 (26.3) | |
| 4,000–9,999 | 23 (41.8) | 17 (29.8) | |
| 10,000–15,000 | 9 (16.4) | 24 (42.1) | |
| >15,000 | 2 (3.6) | 1 (1.8) | |
| History of chronic illness a | |||
| T2DM | 22 (40.0) | 13 (22.8) | 3.817 |
| Hypertension | 26 (47.3) | 31 (54.4) | 0.562 |
| Stroke | 9 (16.4) | 13 (22.8) | 0.730 |
| Length of stay (days, Mean ± SD) | 9.54 ± 7.46 | 10.54 ± 6.71 | -0.711 |
Note: NYHA = New York Heart Association. T2DM = Type 2 diabetes mellitus. a Multiple-choice question. 1000 CNY ≈ 143 USD.
All P >0.05.
4.3. Adherence of patients
We assessed the participants’ adherence to the interventions by their acceptance of telephone calls and WeChat attendance. These data were recorded in RHPT and NWCP. Shown in Fig. 2 43 patients (78.2%, 43/55) in the intervention group provided at least six verbal reports. 49.1% of participants (27/55) were active in communicating with peers and health professionals, sharing experience and information, and discussing the health issues in WeChat forums. An analysis of patients’ online messages and feedback showed recurring responses and changing expectations to reconstruct their lives to accommodate their illnesses. Based on these, we estimated that around 70%–80% of participants completed 60% or more of the nursing interventions during the six-month follow-up period.
4.4. Effects of theory-guided intervention on behaviors within adaptive modes
The health outcomes from baseline to sixth month in the two groups were displayed in Table 3. There were no differences between the two groups at baseline. Scores of LVEF, total MLHFQ, MLHFQ physical subscale, HF knowledge, and SCHFI (management) in the control group increased with statistical significance. Patients in the intervention group got significant improvements in all the outcomes (except scores of SCHFI management and confidence subscales) and showed more significant improvement compared with the control group. No statistically significant differences for LVEF, and scores of SCHFI (management) and SCHFI (confidence) were found between two groups.
Table 3.
Health outcomes comparison between the intervention and control groups at baseline and 6th month (Mean ± SD).
| Outcomes | Intervention group (n = 43) |
Control group (n = 48) |
t1 | P1 | t2 | P2 | t3 | P3 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6th month | Baseline | 6th month | |||||||
| LVEF | 39.73 ± 13.91 | 48.75 ± 10.98 | 41.79 ± 13.42 | 44.90 ± 13.75 | 4.670 | <0.001 | 2.205 | 0.032 | 1.462 | 0.147 |
| Total MLHFQ scores | 47.11 ± 22.99 | 70.90 ± 22.45 | 45.81 ± 11.07 | 54.78 ± 18.04 | 4.956 | <0.001 | 3.367 | 0.002 | 3.792 | <0.001 |
| MLHFQ-physical subscale | 43.49 ± 25.59 | 74.59 ± 27.80 | 42.34 ± 14.23 | 56.20 ± 19.12 | 5.852 | <0.001 | 4.874 | <0.001 | 3.637 | 0.001 |
| MLHFQ-emotional subscale | 57.12 ± 27.87 | 81.40 ± 18.43 | 65.17 ± 12.99 | 62.42 ± 27.69 | 4.841 | <0.001 | −0.641 | 0.542 | 3.885 | <0.001 |
| HF knowledge | 8.30 ± 3.89 | 13.79 ± 2.45 | 8.44 ± 2.77 | 10.73 ± 4.28 | 9.485 | <0.001 | 3.742 | <0.001 | 4.245 | <0.001 |
| SCHFI-maintenance subscale | 42.82 ± 19.59 | 57.67 ± 13.22 | 48.77 ± 10.13 | 50.35 ± 10.88 | 4.575 | <0.001 | 0.938 | 0.353 | 2.898 | 0.005 |
| SCHFI-management subscale | 60.93 ± 34.85 | 71.74 ± 15.66 | 57.81 ± 10.05 | 72.29 ± 5.92 | 1.950 | 0.058 | 8.272 | <0.001 | −0.310 | 0.758 |
| SCHFI-confidence subscale | 66.98 ± 21.08 | 69.12 ± 13.35 | 65.21 ± 13.65 | 64.35 ± 13.70 | 0.633 | 0.530 | −0.318 | 0.752 | 1.678 | 0.097 |
| Total CAPS-SF scores | 36.49 ± 6.13 | 40.23 ± 4.36 | 38.50 ± 3.39 | 38.27 ± 2.60 | 4.219 | <0.001 | −0.380 | 0.705 | 2.571 | 0.012 |
Note: LVEF = Left ventricular ejection fraction. MLHFQ = Minnesota Living With Heart Failure Questionnaire. HF = Heart Failure. SCHFI = Self-care Heart failure Index. CAPS-SF = Coping and Adaptation Processing Scale-Short Form.
t1: Paired-sample t-test of the intervention group; t2: Paired-sample t-test of the control group; t3: Independent sample t-test between two groups at 6th mouth after discharge.
4.5. Effects of theory-guided intervention on adaptation
After discharge within six months, one patient in the intervention group and three in the control group readmitted. The difference in admission rate was no statistically significant (χ2 = 0.130, P = 0.718). Shown in Table 3, we found that the difference between groups in scores of CAPS-SF was statistically significant (t = 2.571, P = 0.012). There was a significant improvement in the score of CAPS-SF over time in the intervention group (t = 4.219, P<0.001), but no increase in the control group (t = −0.380, P = 0.705).
5. Discussion
The findings indicated that the RAM-based interventions aligned with patients’ treatment plans, had significant beneficial effects on the promotion of HF patients’ adaptation, and the strengths of this study should be interpreted in the context of the study limitations. As a care plan tool, RHPT emphasized the development of patients’ adaptive capacities to effectively manage HF and prepares them for their ensuing personal responsibilities.
Six months following discharge, patients’ physical data of LVEF had improved in two groups. No significant difference in LVEF between two groups was found. Considering the status of HF, the biological function of the heart is irreversible and hard to recover through nursing interventions. Previous studies revealed a similar result that physical function can improve substantially within six months with HF management service [3,44]. The finding might be explained by the usage of evidence-based medical therapies and good medication adherence, which agreed to previous studies [3,45,46]. A qualitative study reported that Chinese adults with cardiovascular diseases tend to recognize taking medicines as their responsibilities in HF self-management [9]. As the data showed, most patients were asked to take about five types of medicines for cardiac rehabilitation on admission and after discharge. Future studies on personalized care planning for promoting physical adaptation are still required, for example, personalized exercise tips aimed at physical function. Six-minute walk distance has commonly reported in other studies [47,48], showing a significant increase in physical adaptation after a 3-month and 6-month HF education.
Regarding the adaptation in self-concept mode, the total score of MLHFQ had increased in both groups. What’s more, we found that the interventional group had improved significantly more on the emotional dimension. The patient’s perceptions of the HF impact and its treatment on daily life are related to physical and personal selves [5,26]. Commonly, HF patients have feelings of weakness, fatigue, worrying, depression, and uncertainty. As well, HF patients may have difficulties in daily activities, such as walking, sleeping, and social involvement. These might result in inappropriate self-evaluation, causing the adaptive problem of low self-esteem. Melnikov et al. [49] found that the interaction between body image and depression was associated with personal well-being among patients with heart diseases. A RAM-guided study supported that patients’ quality of life can be enhanced after attending the HF rehabilitation program, and individuals’ physiologic status has a strong association with psychological and role function [48]. A systematic review revealed that personalized care planning for adults with chronic health conditions has a positive impact on psychological health, such as mitigation of depression [16]. In this way, the adoption of individuals’ feelings about themselves is needed in the nursing of patients with HF [50].
The role function mode is related to self-care activities and identity as a person with HF, showing a significant improvement. Scores of HF knowledge had increased in both groups, and the intervention group obtained a higher score than the control group. It might be attributed to the applications of the teach-back approach used in hospitalization and telephone follow-ups. As we know, teach-back is an effective strategy to reinforce education to patients in health coaching programs [51]. Lee et al. suggested that a better understanding of HF and its symptom would be more likely to have better adherence to self-care behaviors [6]. The patient-centered recommendations for exercise, dietary, weight monitoring, and medication with initial careful supervision from the RHPT were consistent with interventions set by previous studies [8,18,35], which showed that HF patients could use the given knowledge to practice a better self-care. Activation of patients in managing health is essential for adaptation to complex chronic illness such as HF [8,13,26]. Moreover, the data in the intervention group showed that self-care maintenance improved, but this was not reflected in self-care management and self-care confidence. A Home-Heart-Walk strategy for HF patients also showed that personalized exercise plans can improve self-care behaviors in short-term home care [47]. Another empirical study reported that person-centered care could promote patients’ confidence in managing illness-related symptoms [52]. Self-care confidence would mediate self-care maintenance and management, influencing the social support and HF-knowledge [18,53,54], but the approaches for supporting self-care confidence were unclear and limited. Overall, HF management programs for improving individuals’ abilities of self-care would facilitate adaptation in role function mode.
Interdependence adaptive mode is commonly manifested in nurse communication, family support, and social support. In this study, the RAM-guided interventions showed the active interactions between patients and health professionals. This provided HF patients with accessible ways to establish relationships with others and take strengths from nurses, family members, and peers. Fors et al. [52] suggested that partnerships between patients and health care professionals should be initiated and established as early as possible. The substantial effects of RHPT are likely attributed to telephone contacts and WeChat forums which promote goal-attainment, symptoms management, and behavior modifications. To increase the efficiency of nurse communication, smartphone-based interventions have been widely used as an effective alternative method for clinical programs [55]. Future use of telemedical communication could facilitate nurses ‘real-time’ support of the patient’s decision-making after discharge. Accordingly, internet-based decision support for specific problems needs to be considered and integrated into the personalized care planning in future studies. As an effective coping strategy involved in this study, social support is important to patients’ adaptation in interdependence and self-concept, whereas the uncertainty of HF and feeling of hopelessness decreases their confidence and adherence [5,56]. As the mutuality of approaches used in the personalized care planning, future studies can emphasize the development of structured interventions to maximize the impacts on the integration of a person’s inner and external resources.
Notably, there was a significant improvement in individuals’ capacity of coping styles measured by CAPS-SF in the intervention group, which is consistent with the proposal that adaptation is positively influenced by the use of coping mechanisms and self-reconstruction [29,41]. The intervention of personalized care planning that integrates patient goals, optimizes the quality of management, and cultivates coping skills, helps patients to be active communicators with health care providers and self-managers of their illness. Considering patients’ return-to-home concerns and evaluation of the significance of an HF-related event, the RHPT consisted of adaptive tasks focused on the process of reconstruction of a person’s normal life. Adaptive tasks included individuals’ coping strategies in each of the four modes, which can benefit the development of adaptive capacity [15,57]. Previous studies also demonstrated that the mitigation of symptoms, the improvement of quality of life, and the promotion of self-care behaviors are positively associated with adequate coping efficacy [7,[58], [59], [60]]. Thus, the findings suggested that RAM-guided interventions are beneficial to the development of coping skills, and further studies are required to determine the effects on adaptive behaviors.
Regarding HF readmission as an indicator of adaptation, the difference between the two groups had no statistical significance. A review illustrated that supported by specialists in hospital and follow-ups, integrating health systems between hospital and community, providing multidisciplinary service, performing personalized service, and conducting discharge planning tend to be effective interventions to reduce inappropriate readmission [61]. However, as reported, some patients would view the hospital as a safe place for easy access to health resources [5,9]. Due to the complicated reasons for readmission, the RAM-guided interventions used for reducing HF readmission require further studied.
During limited intervention time for behavioral modifications in this study, identifications of physiological, emotional, behavioral, and cognitive factors within the four modes were achieved preliminarily for patients’ adaptation to HF. Key components of HF management, including patients’ empowerment, continued education, assessment of patients’ clinical conditions, patients’ decision-making, and compliance enhancement, were implemented in this study. Recognition of HF signs, improvement in exercise capacity, and adherence to a healthy lifestyle and medical treatment may require a better integration in the RHPT to further promote coping and active patients’ engagement in HF management. The findings suggested that enhancing physical capacity, self-concept relevant to perceptions of illness impacts, the role of a self-manager, and the importance of interdependence are crucial for the operationalization of the concept of adaptation. Moreover, these behaviors are interrelated.
The current study was limited by convenience sampling from a hospital and short-term following up, which decreases the generalizability of the findings. The majority of participants was male and had a low education level, creating a highly homogeneous in this study. Therefore, future studies should recruit participants across different living environments, education levels, and social-economic status to explore the effects of RAM-guided interventions. Additionally, the contributions of informal caregivers on patients’ adaptation were unclear in this study. It is recommended that quantitative study reflecting interaction between patients and their caregivers can be conducted in the future. Also, the behaviors in interdependence mode can be studied for further development of adaptive strategies. Despite the limitations stated above, the findings of this study contribute to the knowledge of HF management and the growth of individuals’ adaptive capacities. The empirical work suggests an integration of follow-up strategies within the intervention of personalized care planning after discharge for HF patients. There are several suggestions for future researches, including employing nursing experts in case management to move the program forward, seeking structured supports from interprofessional colleagues and sharing the responsibilities with them, and integrating the interventions with current medical procedures and health service systems.
6. Conclusions
This study explored effectiveness of RAM-guided nursing interventions on promotion of HF patients’ adaptation in the context of HF within Chinese culture. The findings support that the interventions can be beneficial for individuals’ adaptation process that integrates multiple adaptive modes, providing HF patients with the coping strategies, HF-related knowledge, and self-care skills to deal with stressful challenges. A future study with a larger sample and longer-term follow-up is proposed for further evaluation of its effects and generalizability.
Funding
This work was supported by Health Commission of Zhejiang Province (grant number WKJ-ZJ-1925 and 2019ZD034).
CRediT authorship contribution statement
Xiyi Wang: Conceptualization, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Leiwen Tang: Funding acquisition, Investigation. Doris Howell: Writing - original draft, Writing - review & editing. Qi Zhang: Investigation. Ruolin Qiu: Investigation. Hui Zhang: Investigation. Zhihong Ye: Conceptualization, Supervision, Writing - review & editing.
Declaration of competing interest
None.
Acknowledgement
The authors thank all investigators and participants who participated in the study.
Footnotes
Peer review under responsibility of Chinese Nursing Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2020.09.004.
Appendices. Supplementary data
Table A1: Return to Home Planning Template (RHPT).
Table A2: Nurses’ Weekly Communication Plan Form (NWCP).
The following is the Supplementary data to this article:
References
- 1.Ponikowski P., Anker S.D., AlHabib K.F., Cowie M.R., Force T.L., Hu S. Heart failure: preventing disease and death worldwide. ESC Heart Failure. 2014;1(1):4–25. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. 2016. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y., Zhang J., Butler J., Yang X., Xie P., Guo D. Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in China: results from the China heart failure (China-HF) Registry. J Card Fail. 2017;23(12):868–875. doi: 10.1016/j.cardfail.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Lund L.H., Savarese G. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sevilla-Cazes J., Ahmad F.S., Bowles K.H., Jaskowiak A., Gallagher T., Goldberg L.R. Heart failure home management challenges and reasons for readmission: a qualitative study to understand the patient’s perspective. J Gen Intern Med. 2018;33(10):1700–1707. doi: 10.1007/s11606-018-4542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee K.S., Moser D.K., Dracup K. Relationship between self-care and comprehensive understanding of heart failure and its signs and symptoms. Eur J Cardiovasc Nurs. 2018;17(6):496–504. doi: 10.1177/1474515117745056. [DOI] [PubMed] [Google Scholar]
- 7.Heo S., Moser D.K., Lennie T.A., Fischer M., Kim J., Lee M. Changes in heart failure symptoms are associated with changes in health-related quality of life over 12 Months in patients with heart failure. J Cardiovasc Nurs. 2018;33(5):460–466. doi: 10.1097/JCN.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 8.Riegel B., Moser D.K., Buck H.G., Dickson V.V., Dunbar S.B., Lee C.S. Self-care for the prevention and management of cardiovascular disease and stroke: a scientific statement for healthcare professionals from the American heart association. Journal of the American Heart Association. 2017;6(9):1–27. doi: 10.1161/JAHA.117.006997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu R., Schick-Makaroff K., Tang L., Wang X., Zhang Q., Ye Z. Chinese hospitalized cardiovascular patients’ attitudes towards self-management: a qualitative study. Patient Prefer Adherence. 2020;14:287–300. doi: 10.2147/PPA.S236348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonkman N.H., Westland H., Groenwold R.H., Agren S., Anguita M., Blue L. What are effective program characteristics of self-management interventions in patients with heart failure? An individual patient data meta-analysis. J Card Fail. 2016;22(11):861–871. doi: 10.1016/j.cardfail.2016.06.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inglis S.C., Clark R.A., Dierckx R., Prieto-Merino D., Cleland J.G. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev. 2015;10 doi: 10.1002/14651858.CD007228.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang C.Y., Choi K.C., Ho K.M., Yu S.F. Effectiveness of nurse-led patient-centered care behavioral risk modification on secondary prevention of coronary heart disease: a systematic review. Int J Nurs Stud. 2018;84:28–39. doi: 10.1016/j.ijnurstu.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 13.American Geriatrics Society Expert Panel on Person-Centered C. Person-centered care: a definition and essential elements. J Am Geriatr Soc. 2016;64(1):15–18. doi: 10.1111/jgs.13866. [DOI] [PubMed] [Google Scholar]
- 14.Welstand J., Carson A., Rutherford P. Living with heart failure: an integrative review. Int J Nurs Stud. 2009;46(10):1374–1385. doi: 10.1016/j.ijnurstu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y., Shorey S., Seah B., Chan W.X., Tam W.W.S., Wang W. The effectiveness of psychological interventions on self-care, psychological and health outcomes in patients with chronic heart failure-A systematic review and meta-analysis. Int J Nurs Stud. 2018;78:16–25. doi: 10.1016/j.ijnurstu.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Coulter A., Entwistle V.A., Eccles A., Ryan S., Shepperd S., Perera R. Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database Syst Rev. 2015;(3):CD010523. doi: 10.1002/14651858.CD010523.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards S.T., Dorr D.A., Landon B.E. Can personalized care planning improve primary care? J Am Med Assoc. 2017;318(1):25–26. doi: 10.1001/jama.2017.6953. [DOI] [PubMed] [Google Scholar]
- 18.Lainscak M., Blue L., Clark A.L., Dahlstrom U., Dickstein K., Ekman I. Self-care management of heart failure: practical recommendations from the patient care committee of the heart failure association of the European society of cardiology. Eur J Heart Fail. 2011;13(2):115–126. doi: 10.1093/eurjhf/hfq219. [DOI] [PubMed] [Google Scholar]
- 19.Santana M.J., Manalili K., Jolley R.J., Zelinsky S., Quan H., Lu M. How to practice person-centred care: a conceptual framework. Health Expect. 2018;21(2):429–440. doi: 10.1111/hex.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy C., Andrews H.A. third ed. Pearson; New York: 2008. The Roy adaptation model. [Google Scholar]
- 21.Sister Callista Roy . third ed. ed. Pearson Education, Inc.; New Jersey: 2009. The Roy adaptation model. [Google Scholar]
- 22.Roy C. Research based on the Roy adaptation model: last 25 years. Nurs Sci Q. 2011;24(4):312–320. doi: 10.1177/0894318411419218. [DOI] [PubMed] [Google Scholar]
- 23.Wang X., Shao J., Ye Z. Understanding and measuring adaptation level among community-dwelling patients with metabolic syndrome: a cross-sectional survey. Patient Prefer Adherence. 2020;14:939–947. doi: 10.2147/ppa.s248126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moser D.K., Lee K.S., Wu J.R., Mudd-Martin G., Jaarsma T., Huang T.Y. Identification of symptom clusters among patients with heart failure: an international observational study. Int J Nurs Stud. 2014;51(10):1366–1372. doi: 10.1016/j.ijnurstu.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeVon H.A., Vuckovic K., Ryan C.J., Barnason S., Zerwic J.J., Pozehl B. Systematic review of symptom clusters in cardiovascular disease. Eur J Cardiovasc Nurs. 2017;16(1):6–17. doi: 10.1177/1474515116642594. [DOI] [PubMed] [Google Scholar]
- 26.Pollock S.E. Adaptation to chronic illness: a program of research for testing nursing theory. Nurs Sci Q. 1993;6(2):86–92. doi: 10.1177/089431849300600208. [DOI] [PubMed] [Google Scholar]
- 27.Chew H.S.J., Cheng H.Y., Chair S.Y. The suitability of motivational interviewing versus cognitive behavioural interventions on improving self-care in patients with heart failure: a literature review and discussion paper. Appl Nurs Res. 2019;45:17–22. doi: 10.1016/j.apnr.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Riegel B., Jaarsma T., Lee C.S., Stromberg A. Integrating symptoms into the middle-range theory of self-care of chronic illness. ANS Adv Nurs Sci. 2018 doi: 10.1097/ANS.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C.-C., Shun S.-C. Understanding self care coping styles in patients with chronic heart failure: a systematic review. Eur J Cardiovasc Nurs. 2015;15(1):12–19. doi: 10.1177/1474515115572046. [DOI] [PubMed] [Google Scholar]
- 30.Chiou C.P. A meta-analysis of the interrelationships between the modes in Roy’s adaptation model. Nurs Sci Q. 2000;13(3):252–258. doi: 10.1177/08943180022107663. [DOI] [PubMed] [Google Scholar]
- 31.Des Jarlais D.C., Lyles C., Crepaz N., Group T. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Publ Health. 2004;94(3):361–366. doi: 10.2105/ajph.94.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heart failure group of Chinese society of cardiology of Chinese medical A, Chinese heart failure association of Chinese medical doctor A, editorial board of Chinese journal of C. Chinese guidelines for the diagnosis and treatment of heart failure 2018. Zhonghua Xinxueguanbing Zazhi. 2018;46(10):760–789. doi: 10.3760/cma.j.issn.0253-3758.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Eckel R.H., Jakicic J.M., Ard J.D., de Jesus J.M., Miller N.H., Hubbard V.S. AHA/ACC guideline on lifestyle management to reduce cardiovascular risk. Circulation. 2013;129(25 suppl 2):S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1. 2014. [DOI] [PubMed] [Google Scholar]
- 34.Spaling M.A., Currie K., Strachan P.H., Harkness K., Clark A.M. Improving support for heart failure patients: a systematic review to understand patients’ perspectives on self-care. J Adv Nurs. 2015;71(11):2478–2489. doi: 10.1111/jan.12712. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X., Wang W., Zhao X., Li G., Jin L., Yang L. Best evidence summary for management of output and input in chronic heart failure patients Chin. J Nurs. 2020;55(3):456–461. doi: 10.3761/j.issn.0254-1769.2020.03.028. [DOI] [Google Scholar]
- 36.Riegel B., Moser D.K., Glaser D., Carlson B., Deaton C., Armola R. The Minnesota Living with Heart Failure Questionnaire: sensitivity to differences and responsiveness to intervention intensity in a clinical population. Nurs Res. 2002;51(4):209–218. doi: 10.1097/00006199-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y., Du J., Lin L., Zheqi X., Origasa H., Zheng J. The translating, editing and testing of the Minnesota living with heart failure questionnaire of Chinese version. Chin J Behav Med & Brain Sci. 2010;10(2):178–181. doi: 10.3760/cma.j.issn.1674-6554.2010.02.030. [DOI] [Google Scholar]
- 38.Riegel B., Lee C.S., Dickson V.V., Carlson B. An update on the self-care of heart failure index. J Cardiovasc Nurs. 2009;24(6):485–497. doi: 10.1097/JCN.0b013e3181b4baa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo J., Li Z., Kang X. Development and psychometric testing of Chinese version of self-care heart failure index. Chin J Nurs. 2012;47(7):653–655. doi: 10.3761/j.issn.0254-1769.2012.07.028. [DOI] [Google Scholar]
- 40.Reilly C.M., Higgins M., Smith A., Gary R.A., Robinson J., Clark P.C. Development, psychometric testing, and revision of the Atlanta heart failure knowledge test. J Cardiovasc Nurs. 2009;24(6):500–509. doi: 10.1097/JCN.0b013e3181aff0b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy C., Bakan G., Li Z., Nguyen T.H. Coping measurement: creating short form of Coping and Adaptation Processing Scale using item response theory and patients dealing with chronic and acute health conditions. Appl Nurs Res. 2016;32:73–79. doi: 10.1016/j.apnr.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Wang X., Tang L., Howell D., Shao J., Qiu R., Zhang Q. Psychometric testing of the Chinese version of the coping and adaptation processing scale-short form in adults with chronic illness. Front Psychol. 2020;11(1642):1–10. doi: 10.3389/fpsyg.2020.01642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Z., Huang S., Yuan H., Ma L., Lu X. Effects and safety of personalized rehabilitation exercise in patients with chronic heart failure. Chin J Gerontol. 2019;20(39) doi: 10.3969/j.issn.1005-9202.2019.20.005. [DOI] [Google Scholar]
- 44.Goodman H., Firouzi A., Banya W., Lau-Walker M., Cowie M.R. Illness perception, self-care behaviour and quality of life of heart failure patients: a longitudinal questionnaire survey. Int J Nurs Stud. 2013;50(7):945–953. doi: 10.1016/j.ijnurstu.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Ho P.M., Bryson C.L., Rumsfeld J.S. Medication adherence. Circulation. 2009;119(23):3028–3035. doi: 10.1161/circulationaha.108.768986. [DOI] [PubMed] [Google Scholar]
- 46.Yu M., Chair S.Y., Chan C.W., Choi K.C. A health education booklet and telephone follow-ups can improve medication adherence, health-related quality of life, and psychological status of patients with heart failure. Heart Lung. 2015;44(5):400–407. doi: 10.1016/j.hrtlng.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Du H., Newton P.J., Budhathoki C., Everett B., Salamonson Y., Macdonald P.S. The Home-Heart-Walk study, a self-administered walk test on perceived physical functioning, and self-care behaviour in people with stable chronic heart failure: a randomized controlled trial. Eur J Cardiovasc Nurs. 2017;17(3):235–245. doi: 10.1177/1474515117729779. [DOI] [PubMed] [Google Scholar]
- 48.Bakan G., Akyol A.D. Theory-guided interventions for adaptation to heart failure. J Adv Nurs. 2008;61(6):596–608. doi: 10.1111/j.1365-2648.2007.04489.x. [DOI] [PubMed] [Google Scholar]
- 49.Melnikov S., Abuhazira M., Golobov D., Yaari V., Jaarsma T., Ben Gal T. Depression and anxiety moderate the relationship between body image and personal well-being among patients with an implanted left ventricular assist device. J Cardiovasc Nurs. 2020;35(2):149–155. doi: 10.1097/JCN.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 50.Ekman I., Swedberg K., Taft C., Lindseth A., Norberg A., Brink E. Person-centered care--ready for prime time. Eur J Cardiovasc Nurs. 2011;10(4):248–251. doi: 10.1016/j.ejcnurse.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Ha Dinh TT., Bonner A., Clark R., Ramsbotham J., Hines S. The effectiveness of the teach-back method on adherence and self-management in health education for people with chronic disease: a systematic review. JBI Database System Rev Implement Rep. 2016;14(1):210–247. doi: 10.11124/jbisrir-2016-2296. [DOI] [PubMed] [Google Scholar]
- 52.Fors A., Taft C., Ulin K., Ekman I. Person-centred care improves self-efficacy to control symptoms after acute coronary syndrome: a randomized controlled trial. Eur J Cardiovasc Nurs. 2016;15(2):186–194. doi: 10.1177/1474515115623437. [DOI] [PubMed] [Google Scholar]
- 53.Massouh A., Skouri H., Cook P., Huijer H.A.S., Khoury M., Meek P. Self-care confidence mediates self-care maintenance and management in patients with heart failure. Heart Lung. 2019 doi: 10.1016/j.hrtlng.2019.07.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.Irani E., Moore S.E., Hickman R.L., Dolansky M.A., Josephson R.A., Hughes J.W. The contribution of living arrangements, social support, and self-efficacy to self-management behaviors among individuals with heart failure: a path analysis. J Cardiovasc Nurs. 2019;34(4):319–326. doi: 10.1097/JCN.0000000000000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maddison R., Rawstorn J.C., Shariful Islam S.M., Ball K., Tighe S., Gant N. mHealth interventions for exercise and risk factor modification in cardiovascular disease. Exerc Sport Sci Rev. 2019;47(2):86–90. doi: 10.1249/JES.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fivecoat H.C., Sayers S.L., Riegel B. Social support predicts self-care confidence in patients with heart failure. Eur J Cardiovasc Nurs. 2018;17(7):598–604. doi: 10.1177/1474515118762800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samson A., Siam H. Adapting to major chronic illness: a proposal for a comprehensive task-model approach. Patient Educ Counsel. 2008;70(3):426–429. doi: 10.1016/j.pec.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 58.Ditewig J.B., Blok H., Havers J., van Veenendaal H. Effectiveness of self-management interventions on mortality, hospital readmissions, chronic heart failure hospitalization rate and quality of life in patients with chronic heart failure: a systematic review. Patient Educ Counsel. 2010;78(3):297–315. doi: 10.1016/j.pec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 59.Bishop M. Quality of life and psychosocial adaptation to chronic illness and disability. Rehabil Counsel Bull. 2005;48(4):219–231. doi: 10.1177/00343552050480040301. [DOI] [Google Scholar]
- 60.Salyer J., Flattery M., Lyon D.E. Heart failure symptom clusters and quality of life. Heart Lung. 2019;48(5):366–372. doi: 10.1016/j.hrtlng.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Coffey A., Leahy-Warren P., Savage E., Hegarty J., Cornally N., Day M.R. Interventions to promote early discharge and avoid inappropriate hospital (Re)admission: a systematic review. Int J Environ Res Publ Health. 2019;16(14) doi: 10.3390/ijerph16142457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.