Highlights
-
•
Mycobacterium abscessus is a drug-resistant nontuberculous mycobacterium (NTM).
-
•
Cutaneous & subcutaneous NTM infections post-cosmetic surgery are poorly diagnosed.
-
•
Initial surgical evaluation facilitates early suspicion of M. abscessus infection.
-
•
Rapidly evolving infection & negative culture/antibiotic response are indicators.
-
•
Amikacin, imipenem, & clarithromycin combination may treat M. abscessus infection.
Keywords: Mycobacterium abscessus, Cosmetic surgery, Surgical infection, Nontuberculous mycobacteria, Clarithromycin
Abstract
Background
Mycobacterium abscessus is one of the most pathogenic and drug-resistant opportunistic microorganisms among the nontuberculous mycobacteria (NTM) involved in skin and soft tissue infections (SSTI) associated with cosmetic surgical procedures. However, NTM infection is often wrongly diagnosed initially causing prolonged suffering. Here is described the author’s experience working with patients who developed M. abscessus SSTI after cosmetic procedures.
Methods
Patients who developed NTM infection after undergoing cosmetic procedures, and who presented at the Hospital Metropolitano and Hospital Vozandes (Quito, Ecuador) between 2013–2016. A review of patient medical records was performed.
Results
Five patients with culture proven M. abscessus subspecies abscessus SSTI after cosmetic surgeries were identified. All patients were treated with aggressive surgical debridement and antibiotics.
Conclusions
A rapidly spreading wound infection presenting two or more weeks after a cosmetic procedure that fails to respond to standard antimicrobial therapy should raise suspicion for NTM infection. Samples for acid-fast bacilli smear, cultures, and PCR from infected tissue should be taken. Surgical drainage and debridement are recommended along with a long course of antibiotics. In the absence of clinical trials, a combination of amikacin, imipenem, and clarithromycin may be an adequate initial treatment for M. abscessus subspecies abscessus SSTI in immunocompetent patients.
Introduction
Non-tuberculous mycobacteria (NTM) infections of the skin and soft tissues (SSTI) have been increasing in incidence over the last few years [1]. Infection occurs by inoculation of bacteria after trauma to the skin [2]. In some cases, the sources of infection have been associated with NTM-contaminated solutions used in invasive procedures or with improperly sterilized medical equipment contaminated with NTM, most likely through tap water [3,4]. In most outbreaks, the source of the infection remains unknown [2].
M. abscessus, a rapid-growing NTM, is widely distributed in nature and can resist extreme temperatures and restricted nutrient environments. It has been reported to contaminate water sources, cleaning agents, hospital environments, reagents, and medications and can be resistant to standard disinfectants like chlorine, organomercurials, and alkaline glutaraldehyde [5].
Species in the Mycobacterium abscessus group are a major cause of SSTI infections in humans, either in isolation or in outbreaks [6]. M. abscessus comprises three subspecies: M. abscessus subsp. abscessus, M. abscessus subsp. Bolletii, and M. abscessus subsp. massiliense [7,8]. They are known for their high antimicrobial resistance arising from multiple mechanisms such as low cell envelope permeability, drug export systems, and expression of drug-target-modifying enzymes [9]. Therefore, antimicrobial treatment for infections caused by the M. abscessus group complex becomes difficult, due to both natural and acquired resistance to most of the currently available antibiotics [2,9].
SSTI caused by the M. abscessus group has been reported in cases of medical tourism after cosmetic or surgical procedures, from countries such as Switzerland, Spain, and USA [10,11]. Outbreaks in Ecuador have not been described to date.
Here we report five cases of immunocompetent patients with no comorbidities pursuing medical attention in Quito-Ecuador, who were diagnosed with M. abscessus subspecies abscessus soft-tissue infection after a cosmetic or surgical procedure was performed in Guayaquil-Ecuador.
Materials and methods
Isolation and identification of M. abscessus subspecies abscessus
Tissue samples for cultures were collected in the operating room during drainage and debridement of the infected site. Acid-fast stain (AFS) and Gram stain were performed for direct examination. The samples were placed in culture media, including blood agar, chocolate agar, MacConkey agar, Lowenstein-Jensen, and Middlebrook broth, using the BACTECMGIT 320 system (Becton Dickinson, USA) [12].
Antimicrobial susceptibility
The standard broth dilution method with cation-supplemented Mueller-Hinton broth (Becton Dickinson, USA) recommended by the CLSI was used. Microtiter plates with antibiotics were purchased from Trek Diagnostics (RapidMyco Sensititre, Thermo Scientific, USA) and were provided together with demineralized water and Mueller-Hinton broth (Becton Dickinson, USA). Susceptibility testing using the broth microdilution method was performed according to CLSI guidelines described in document M24-A [13].
Molecular identification
The High Pure PCR Template Preparation Kit (Roche Diagnostics, Germany) was used for DNA extraction, following the manufacturer's specifications. Subsequently, partial sequencing of the 65-kDa heat shock protein gene (hsp65) conserved in Mycobacterium was carried out through conventional PCR using primers TB11 and TB12 according to a previously described protocol [12]. The PCR products were digested with the restriction enzymes, BstEII and HaeIII, and the resulting fragments were separated by electrophoresis on a 4% agarose gel using a 50 bp molecular marker. Interpretation of the fragments obtained was performed following an identification algorithm introduced by Chimara et al. [14].
Species and subspecies were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDITOF) [15] and confirmed by sequencing the rpoB gene [16]; then, the sequences were analyzed using BLAST.
Case 1
A 22-year-old woman received several silicone injections in both thighs four weeks prior to clinical presentation. She consulted because she noticed erythema and painful papules around the injection sites on her right thigh, one with an abscess-forming tendency. In our first encounter, she presented with multiple tender erythematous papules with regular borders and serosanguineous discharge compromising around 20 × 10 cm2 of the distal third of the right thigh. Laboratory analysis showed mild neutrophilic leukocytosis, C-reactive protein (124 mg/dL), and blood chemistry panel without alterations. Biopsy of the lesion showed multiple fragments of adipose panniculus with fibrosis, scarce chronic inflammatory infiltrate, and large empty vacuoles, evidencing factitious panniculitis triggered by silicone injection. AFS was not performed. The patient received clindamycin and oxacillin for 7 days, but returned complaining of worsening pain, with many papules forming plaques of varying size, the largest being 1.5 × 1.0 cm2 and some of them ulcerated. Extensive surgical drainage was performed, and the lesions were sampled; acid-fast stain (AFS) was positive and cultures for atypical mycobacteria and PCR-RFLP analysis (PRA) were performed. Amikacin and imipenem were administered for 3 months plus oral clarithromycin for 7 months.
Case 2
A 42-year-old woman underwent abdominoplasty, bilateral mastopexy, liposuction of the arms and neck, and had multiple plasma injections at surgical sites. Two weeks after surgical intervention she noticed a yellowish, malodorous discharge from the abdominal incision. She did superficial cleaning for two weeks with normal saline solution with no improvement. She subsequently noticed a similar discharge from the breast wounds and decided to seek medical attention. The other surgical wounds and injection sites healed properly. Five weeks after surgery, the patient presented to our clinic complaining of generalized weakness, fever, diffuse burning abdominal pain, and foul-smelling serosanguineous discharge through abdominal and breast wounds. Incisional borders were erythematous but showed no other superficial skin lesions. Hemogram, chemistry panel, and CRP were within normal limits. Extensive incision and drainage were performed. AFB was positive, cultures and PRA were positive for M. abscessus, and ESBL-Escherichia coli was isolated. The patient received antibiotic therapy with clarithromycin, amikacin, and imipenem; a month and a half later, she presented positive to Clostridioides difficile toxin. She received 500 mg of metronidazole TID for 14 days (oral vancomycin and fidaxomicin were not available in Ecuador). On completion of colitis treatment, clarithromycin was resumed for seven months. Amikacin and imipenem were discontinued.
Case 3
A 37-year-old woman had undergone an abdominoplasty, bilateral mastopexy, and botulinum toxin injections bilaterally in the malar region. Two weeks after intervention, she complained of abundant purulent discharge in the surgical incisions. She was diagnosed with wound dehiscence and her surgeon prescribed ciprofloxacin and clindamycin for one month. Despite the antibiotic treatment, the purulent discharge continued. The patient came to the hospital six weeks after surgery presenting multiple painful, warm, erythematous, indurated skin nodules around the abdominal incision, the right malar region, the suprapatellar region (bilaterally), the inner left and right thighs, and the legs; the abdominal and breast wounds showed purulent discharge. Extensive surgical debridement was performed. AFS, cultures, and PRA positive for M. abscessus were obtained. The patient received amikacin and imipenem for 3 months and clarithromycin for 7 months; however, three months after treatment was completed, she had a recurrence around the abdominal scar. Thus, debridement of the area was performed, and a second round of antibiotics was given. New cultures and PRA were positive for M. abscessus. The second antibiotic course consisted of amikacin and imipenem for one month plus clarithromycin for 7 months. The infection resolved, and no recurrence was observed during the one-year follow-up. She has recovered well with healed suture lines and no additional wound drainage.
Case 4
A 43-year-old woman had undergone bilateral augmentation mammoplasty. Three weeks after the procedure, she complained of fever, pain, and surgical wound secretion. Her surgeon prescribed ciprofloxacin and clindamycin for 7 days. Staphylococcus epidermidis grew in culture secretions and the patient took the oral antibiotic with no improvement. We evaluated her two months after the surgical intervention and noticed wound dehiscence around the nipple-areolar complex bilaterally, accompanied with a whitish secretion, and perilesional edema and erythema. A CT scan was performed, showing multiple pre-pectoral collections bilaterally. We removed foreign bodies and did extensive debridement, and drainage; 80 % of the pectoralis major fascia was absent. AFS, cultures, and PRA were positive for M. abscessus. Amikacin and imipenem were administered for 3 months and clarithromycin for 7 months. She has since been followed in the office with no signs of infection and her lesions have healed well.
Case 5
A 27-year-old woman who underwent abdominoplasty and bilateral mastopexy presented three months after surgery due to multiple abdominal and bilateral breast masses that appeared around 10 weeks after the procedure. Her infectious disease doctor diagnosed a surgical wound infection. Multiple microorganisms, including Mycobacterium, were cultivated, and PRA for M. abscessus was run with negative results. Despite these results, she received antibiotic therapy with IV vancomycin for one month, amikacin and imipenem for 3 months, and oral clarithromycin for 7 months, which resolved the nodules and breast wound infection. However, fluid oozed out of the abdominal wound after few weeks, and an additional three-month course of oral clarithromycin and moxifloxacin was prescribed, but with no success.
Two years after her plastic surgery, she presented at our clinic complaining of persistent abdominal wound discharge. We observed serosanguineous drainage through the abdominal and right breast wounds and multiple painful nodules in the right arm. The persistent abdominal wound drainage prompted an MRI that identified multiple collections in the anterior abdominal wall around the umbilicus and two collections in the surgical wound. A surgical intervention with extensive debridement and scar excision was performed, and multiple collections of dense white fluid surrounded by fibrous tissue were found, the largest of which was a 10 × 6 × 2 cm3 periumbilical collection. Cultures and PRA confirmed M. abscessus, but AFS was negative. Finally, amikacin and imipenem were administered for three months and oral clarithromycin, for seven months.
Discussion
Increasing reports of NTM SSTI in recent years have attracted much attention in the medical community. Initially, it was considered a reflection of the increase in the immunosuppressed population, however numerous reports document this type of infection in healthy individuals. In fact, all the patients described in the present study were immunocompetent. The exact incidence of NTM SSTI infections has not yet been determined, as it is not a notifiable infection. The largest population-based study on the incidence of NTM showed an incidence of 2.0 per 100,000 person-years, and a nearly threefold increase in the incidence of NTM skin infections over a 30-year period in the USA [17]. Most data on NTM refer to isolates from respiratory tract infections. Few data on skin and soft tissues caused by NTM are found in Latin America [18] and in Ecuador [19,20].
Generally, the NTM most frequently causing SSTIs are: M. chelonae, M. fortuitum, and M. abscessus subsp. abscessus. After M. fortuitum, M abscessus is the second most common NTM species isolated from clinical samples, and the most pathogenic of the three mentioned. M. abscessus has been associated with postsurgical wound infections, post-injection infections, localized community-acquired wound infections, and disseminated skin infections. In ∼25 % of the cases resulting in localized infections, the disease developed after a skin surface break and subsequent direct contact with contaminated water or soil [2,21,22].
In the last few decades, a growing amount of cases of M. abscessus infections caused by non-sterile techniques or contaminated materials after Mohs surgery, liposuction, soft tissue augmentation, mesotherapy, and acupuncture have been reported [[23], [24], [25], [26]].
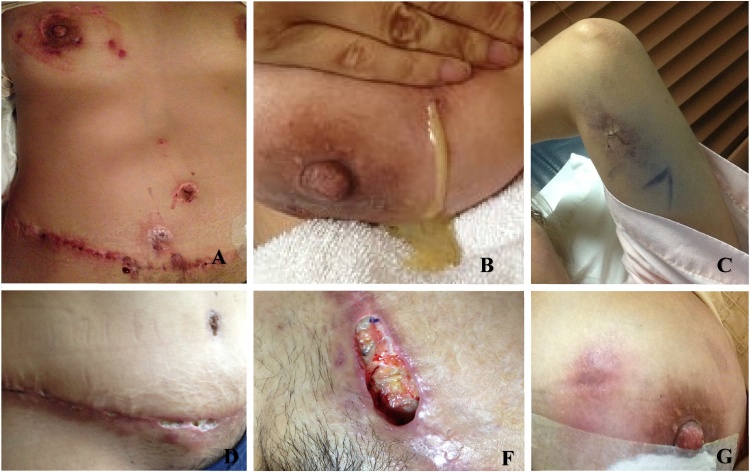
The clinical presentation of the NTM SSTIs is nonspecific and includes abscesses, cellulitis, nodules, sporotrichoid nodules, ulcers, panniculitis, sinus drainage, folliculitis, papules, and plaques. Patients often have multiple red to violet subcutaneous nodules, and lymphadenopathy, and sometimes can present systemic symptoms [2,[26], [27], [28]]. Fig. 1 shows skin lesions in our patients.
Fig. 1.
Different manifestations in skin and soft tissues caused by M. abscessus after cosmetic surgery. M. abcessus infection usually follows penetrating trauma in immunocompetent individuals. Initial presentation includes the formation of a tender, fluctuating subcutaneous abscess at the site of inoculation (G). Other presentations include ulcerations, sinus drainage, or nodules (A, C, D). Abscesses that drain large amounts of pus (B) or chronic ulcers (F). The primary lesion is often followed by a sporotrichoid aspect of ascending lymphadenitis.
In our patients, the clinical presentation was characterized by an indolent course of initial wound healing and subsequent sinus drainage with deeper abscesses along the abdominal wall. Clinical cases 1–3 had umbilical wound openings with serous fluid-like yellow drainage. All three patients presented with a healing abdominoplasty incision that later developed blisters or open wounds and drained yellow fluid.
A high index of suspicion is necessary for NTM SSTI diagnoses. Factors that raise suspicion for M. abscessus SSTI are concomitant presentation of wound dehiscence or unsuccessful wound healing, poor response to antimicrobial agents against common bacterial invaders, absence of bacterial growth in standard cultures that may be due to inadequate sampling, and rapid progression of the infection involving the skin and subcutaneous tissue [2,29,30].
The reported incidence of surgical site infection after plastic surgery is around 0.08 %, varying with the type of procedure, and the most common organism isolated is S. aureus [31]. In general, antibiotics against skin organisms, such as streptococci or staphylococci, are administered in cases of wound infection after surgical procedures. However, when facing postoperative infections with negative cultures or lack of response to typical antimicrobial treatments, a mycobacterial infection should be considered. This is especially important when treating patients who underwent surgery in areas endemic with atypical mycobacteria [29].
Detection of NTM can be difficult since Gram stains and cultures are frequently negative. Although, M. abscessus performs well on Chocolate and MacConkey agar, failure to maintain cultures for more than 72 h can lead to the loss of this bacteria, which typically requires 5- to 7-day cultivation to obtain visible colonies on conventional agar [32,33].
Based on our experience at our institution, multiple cultures and histopathological samples for identifying resistant acid alcohol bacilli should be obtained from fluid and tissue contents to maximize culture yield. Swabs from lesions should be avoided. Intraoperative cultures are good samples for cultivating mycobacteria on media, such as Lowenstein Jensen medium and Middlebrook broth, that become positive for M. abscessus between 6 days and 2 weeks [19,20].
Currently, there are no standard guidelines for treating M. abscessus skin infection. M. abscessus is generally considered to be the most pathogenic and drug resistant fast-growing NTM, and it is characterized by in vitro resistance to standard tuberculosis therapy. It also has limited susceptibility to antibiotics, including imipenem, doxycycline, and trimethoprim-sulfamethoxazole. M. abscessus is often susceptible to clarithromycin and amikacin [34], but can show inducible resistance to clarithromycin and other macrolide antibiotics when it carries the erm gene [35].
While clarithromycin is a drug of choice in the treatment of M. abscessus infections, it is often administered in combination with one or two antibiotics [36]. Parenteral medications, such as amikacin, cefoxitin, tigecycline, or imipenem may be initially required [2].
Treatment should be based primarily on the in vitro sensitivities of the isolated microorganism, but given the complexity of antimicrobial resistance in M. abscessus isolates, empirical therapy generally must be deferred until the laboratory confirms the organism’s growth in cultures [2,36]. M. abscessus responds slowly to antibiotics and the duration of therapy typically lasts around 4–6 months, but it largely depends on each patient's clinical course (Table 1).
Table 1.
Summary of the presentation, treatment and outcomes of the cases reported.
| Case | Age (years) | Time from procedure to symptoms onset (weeks) | Clinical features | Procedure | Antibiotic treatmenta | Outcome |
|---|---|---|---|---|---|---|
| 1 | 22 | 4 | Erythematous papules, serosanguineous discharge compromising around 20 × 10 cm2 of the distal third of the right thigh. No lesions were found on the left thigh. | Several silicone injections in both thighs | IV amikacin and imipenem for 3 months plus oral clarithromycin for 7 months | Recovered |
| 2 | 42 | 2 | Subjective fever diffuses burning abdominal pain, and foul-smelling serosanguineous discharge through abdominal and breast wounds | Abdominoplasty, bilateral mastopexy, liposuction of the arms and neck, and multiple plasma injections | IV amikacin and imipenem for 1.5 months plus oral clarithromycin for 7 months | Recovered |
| 3 | 37 | 2 | Multiple painful, warm, erythematous, indurated skin nodules around the abdominal incision, the right malar region, the suprapatellar region bilaterally, the inner left and right thighs, and the legs; the abdominal and breast wounds showed purulent discharge. | Abdominoplasty, bilateral mastopexy, and botulinum toxin injections bilaterally in the malar region | IV amikacin and imipenem for 3 months plus oral clarithromycin for 7 months. Same regimen was repeated after I&D | Recovered after recurrence |
| 4 | 43 | 3 | Dehiscence around the nipple-areolar complex bilaterally, accompanied by a whitish secretion, perilesional edema, and erythema. | Bilateral breast augmentation | IV amikacin and imipenem for 3 months plus oral clarithromycin for 7 months | Recovered |
| 5 | 27 | 10 | Multiple abdominal and bilateral breast masses | Abdominoplasty and bilateral mastopexy | First regimen (failed): Vancomycin 1 month; amikacin IV and imipenem for 3 months; clarithromycin for 7 months. Second regimen (after first recurrence) failed: clarithromycin and moxifloxacin for 3 months. Third regimen started after extensive I&D: IV amikacin and imipenem for 1 month plus Clarithromycin 7 months | Recovered after recurrence |
All the patients were females.
All the patients underwent incision and drainage in addition to the antibiotic treatment.
In all the cases reported here, amikacin and imipenem were administered through an implantable catheter, and clarithromycin was given orally 500 mg bid. We use intravenous Imipenem 1 gm every 12 h and Amikacin every day for 15 days at 15 mg/kg/day and rest for 15 days to avoid renal or hearing impairment; Amikacin and imipenem were administered for 3 months and clarithromycin for 7 months. None of the patients have side effects except one who had a Clostridioid infection. Table 2 shows the sensitivity patterns of M. abscessus isolated from our patients. Except for case 2, which showed in vitro multidrug resistance, the other cases were sensitive to clarithromycin and amikacin. However, in vitro resistance to imipenem was observed in all the cases. The treatment was initially guided by clinical judgement (since all the patients showed improvement by the time the sensitivities were available) and subsequently was maintained considering the reported discordance between antimicrobial in vitro sensitivity and in vivo efficacy in NTM infections [2,37]. The patients described in this study were considered clinically cured when the lesions disappeared completely and there was no evidence of relapse 12 months after treatment completion.
Table 2.
Antibiotic MIC for M. abscessus subsp. abscessus of the reported cases.
-TIG = tigecyclina, AMK = amikacin, MOX = moxifloxacin, LNZ = linezolid, CIP = ciprofloxacin, IMI = imipenem, SXT cotrimoxazol, MIN = minocycline, DOX = doxycycline CLA = claritromycin.
-In gray, the resistant breakpoint.
In general terms, meticulous surgical drainage is needed when the area compromised has a thick subcutaneous layer, as in the abdomen and thighs, since failed or delayed resolution may result in a prolonged course of antibiotics. Further, the removal of foreign bodies, as in the case of mastopexy, is essential for recovery [38].
In the case of NTM infections after abdominoplasty with strong clinical suspicion and/or radiographic evidence of multiple subcutaneous fluid collections, complete removal of infectious foci with elevation of the entire abdominoplasty flap, excision of the scar, and shipment of fluid and debridement contents for routine cultures and AFB, are required. All areas of tissue granulation and plication sutures should be removed, followed by abundant pulse saline irrigation. Drains are placed and removed when production is less than 30 cc per day for consecutive days and signs of infection resolve [25]. A case by case evaluation by an infectious disease expert along with the surgeon is recommended to treat NTM SSTI.
Conclusion
A rapid diagnosis of atypical mycobacterial infections following cosmetic surgery, such as prosthesis placement, liposuction, botox injections, or mastopexy, requires a high suspicion index based on clinical presentation and empirical antibiotic treatment failure. AFB smears and cultures with sensitivities to guide treatment are recommended for patients suspected of having atypical mycobacterial infections. We believe that NTM infections should be considered in all patients who develop skin infections after surgical or cosmetic procedures not responding to empirical antimicrobial therapy and presenting repetitive conventional negative cultures. In the absence of clinical trials, a combination of amikacin, imipenem, and clarithromycin seems to be an adequate treatment for soft-tissue M. abscessus subspecies abscessus infections along with surgical evaluation. Close follow-up after antimicrobial cessation is recommended since recurrences are possible.
Funding
The authors declare that they have no sponsors to influence the work reported in this paper.
Consent
Written informed consent was obtained from the patient for publication of this case series and accompanying images.
Author contribution
Each author in this paper contributed with the data collections, data analysis and writing in this paper. Mean author also did the study design.
Ethical
Written informed consent was obtained from the patient for publication of this case report and accompanying images
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Sander M.A., Isaac-Renton J.L., Tyrrell G.J. Cutaneous nontuberculous mycobacterial infections in Alberta, Canada: an epidemiologic study and review. J Cutan Med Surg. 2018;22(5):479–483. doi: 10.1177/1203475418776945. [DOI] [PubMed] [Google Scholar]
- 2.Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 3.Le Dantec C., Duguet J.P., Montiel A., Dumoutier N., Dubrou S., Vincent V. Occurrence of mycobacteria in water treatment lines and in water distribution systems. Appl Environ Microbiol. 2002;68(11):5318–5325. doi: 10.1128/AEM.68.11.5318-5325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Ingen J., Blaak H., De Beer J., De Roda Husman A.M., Van Soolingen D. Rapidly growing nontuberculous mycobacteria cultured from home tap and shower water. Appl Environ Microbiol. 2010;76(17):6017–6019. doi: 10.1128/AEM.00843-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falkinham J.O., III Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol. 2009;107(2):356–367. doi: 10.1111/j.1365-2672.2009.04161.x. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 6.Wallace R.J., Brown B.A., Griffith D.E. Nosocomial outbreaks/pseudo outbreaks caused by nontuberculous mycobacteria. Annu Rev Microbiol. 2002;52(1):453–490. doi: 10.1146/annurev.micro.52.1.453. [DOI] [PubMed] [Google Scholar]
- 7.Leao S.C., Tortoli E., Paul Euzé J., Garcia M.J. Proposal that Mycobacterium massiliense and Mycobacterium bolletii be united and reclassified as Mycobacterium abscessus subsp. bolletii comb. nov., designation of Mycobacterium abscessus subsp. abscessus subsp. nov. and emended description of Mycobacteri. Int J Syst Evol Microbiol. 2011;61(9):2311–2313. doi: 10.1099/ijs.0.023770-0. [DOI] [PubMed] [Google Scholar]
- 8.Lee Meng-Rui, Sheng W.-H., Hung Chien-Ching, Chong-Jen Y.u, Lee Li-Na, Hsueh Po-Ren. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis J. 2015;21(9):1638–1646. doi: 10.3201/2109.141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luthra S., Rominski A., Sander P. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobacterium abscessus drug resistance. Front Microbiol. 2018;9(September):1–13. doi: 10.3389/fmicb.2018.02179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurer F.P., Castelberg C., Braun Von A., Wolfensberger A., Bloemberg G.V., Böttger E.C. Postsurgical wound infections due to rapidly growing mycobacteria in Swiss medical tourists following cosmetic surgery in Latin America between 2012 and 2014. Eurosurveillance. 2014;19(37):1–4. doi: 10.2807/1560-7917.es2014.19.37.20905. [DOI] [PubMed] [Google Scholar]
- 11.Llenas-García J., Vicente J., Ruiz-García M.M., Valencia-Ramírez I., Masiá M. A “lipo-tourist” with chronic cutaneous lesions after surgery in Ecuador: a diagnostic challenge. Travel Med Infect Dis. 2018;25:77–78. doi: 10.1016/j.tmaid.2018.07.013. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 12.Telenti A., Marchesi F., Balz M., Bally F., Bottger E., Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31(2):175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CLSI . Clinical and Laboratory Standards Institute; Wayne, PA: 2018. Susceptibility testing of mycobacteria N, and other aerobic actinomycetes. 3rd ed. CLSI document M24; p. 2018. 2018. [PubMed] [Google Scholar]
- 14.Chimara E., Ferrazoli L., Ueky S.Y.M., Martins M.C., Durham A.M., Arbeit R.D. Reliable identification of mycobacterial species by PCR-restriction enzyme analysis (PRA)-hsp65 in a reference laboratory and elaboration of a sequence-based extended algorithm of PRA-hsp65 patterns. BMC Microbiol. 2008;8:1–12. doi: 10.1186/1471-2180-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng S.H., Chen C.M., Lee M.R., Lee T.F., Chien K.Y., Teng L.J. Matrix-assisted laser desorption ionization-time of flight mass spectrometry can accurately differentiate between Mycobacterium masilliense (M. abscessus subspecies bolletti) and M. abscessus (Sensu Stricto) J Clin Microbiol. 2013;51(9):3113–3116. doi: 10.1128/JCM.01239-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adékambi T., Colson P., Drancourt M. rpoB-based identication of nonpigmented and late-pigmenting rapidly growing mycobacteria. J Clin Microbiol. 2003;41(12):5699–5708. doi: 10.1128/JCM.41.12.5699-5708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wentworth A., Drage L.A., Wengenack N.L., Wilson J.W., Lohse C.M. Increased incidence of cutaneous nontuberculous mycobacteral infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88(1):38–45. doi: 10.1016/j.mayocp.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira R.M.C., Saad M.H.F., Da Silva M.G., De Souza Fonseca L. Non-tuberculous mycobacteria I: one year clinical isolates identification in Tertiary Hospital Aids Reference Center, Rio de Janeiro, Brazil, in pre highly active antiretroviral therapy era. Mem Inst Oswaldo Cruz. 2002;97(5):725–729. doi: 10.1590/s0074-02762002000500024. [DOI] [PubMed] [Google Scholar]
- 19.Zurita J., Ortega-Paredes D., Mora M., Espinel N., Parra H., Febres L. Characterization of the first report of Mycobacterium timonense infecting an HIV patient in an Ecuadorian hospital. Clin Microbiol Infect. 2014;20(12):O1113–O1116. doi: 10.1111/1469-0691.12675. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 20.Romero J.J., Herrera P., Cartelle M., Barba P., Tello S., Zurita J. Panniculitis caused by Mycobacterium monacense mimicking erythema induratum: a case in Ecuador. New Microbes New Infect. 2016;10:112–115. doi: 10.1016/j.nmni.2016.01.006. [Internet]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace R.J., Swenson J.M., Silcox V.A., Good R.C., Tschen J.A. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis. 1983;5(4) doi: 10.1093/clinids/5.4.657. [DOI] [PubMed] [Google Scholar]
- 22.Uslan D.Z., Kowalski T.J., Wengenack N.L., Virk A., Wilson J.W. Skin and soft tissue infections due to rapidly growing mycobacteria. Infect Chemother. 2015;142 doi: 10.1001/archderm.142.10.1287. [DOI] [PubMed] [Google Scholar]
- 23.Fisher E.J., Gloster H.M. Infection with Mycobacterium abscessus after Mohs micrographic surgery in an immunocompetent patient. Dermatol Surg. 2005;31(7 Part I):790. doi: 10.1097/00042728-200507000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Wongkitisophon P., Rattanakaemakorn P., Tanrattanakorn S., Vachiramon V. Cutaneous mycobacterium abscessus infection associated with mesotherapy injection. Case Rep Dermatol. 2011;3(1):37–41. doi: 10.1159/000324766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engdahl R., Cohen L., Pouch S., Rohde C. Management of Mycobacterium abscessus post abdominoplasty. Aesthetic Plast Surg. 2014;38(6):1138–1142. doi: 10.1007/s00266-014-0410-7. [DOI] [PubMed] [Google Scholar]
- 26.Cusumano L.R., Tran V., Tlamsa A., Chung P., Grossberg R., Weston G. Rapidly growing Mycobacterium infections after cosmetic surgery in medical tourists: the Bronx experience and a review of the literature. Int J Infect Dis. 2017;63:1–6. doi: 10.1016/j.ijid.2017.07.022. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 27.Liu R., To K.K.W., Teng J.L.L., Choi G.K.Y., Mok K.Y., Law K.I. Mycobacterium abscessus bacteremia after receipt of intravenous infusate of cytokine-induced killer cell therapy for body beautification and health boosting. Clin Infect Dis. 2013;57(7):981–991. doi: 10.1093/cid/cit443. [DOI] [PubMed] [Google Scholar]
- 28.Su S.H., Chen Y.H., Tsai T.Y., Huang S.C., Lin C.Y., Chen T.C. Catheter-related Mycobacterium abscessus bacteremia manifested with skin nodules, pneumonia, and mediastinal lymphadenopathy. Kaohsiung J Med Sci. 2013;29(1):50–54. doi: 10.1016/j.kjms.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furuya E.Y., Paez A., Srinivasan A., Cooksey R., Augenbraun M., Baron M. Outbreak of Mycobacterium abscessus wound infections among “Lipotourists” from the United States who underwent abdominoplasty in the Dominican Republic. Clin Infect Dis. 2008;46(8):1181–1188. doi: 10.1086/529191. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Santiago T.M., Drage L.A. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33(3):563–577. doi: 10.1016/j.det.2015.03.017. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 31.Nazarian Mobin S.S., Keyes G.R., Singer R., Yates J., Thompson D. Infections in outpatient surgery. Clin Plast Surg. 2013;40(3):439–446. doi: 10.1016/j.cps.2013.04.009. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 32.Paulose R.M., Joseph J., Narayanan R., Sharma S. Clinical and microbiological profile of non-tuberculous mycobacterial endophthalmitis—experience in a tertiary eye care centre in Southern India. J Ophthalmic Inflamm Infect. 2016;6(1) doi: 10.1186/s12348-016-0096-x. [Internet]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.England P.H. 2018. UK standards for microbiology investigations: investigation of specimens for Mycobacterium species.https://www.gov.uk/government/publications/smi-b-40-investigation-of-specimens-for-mycobacterium-species [Internet]. SMI B40. 2018 [cited 2020 Jun 9]. Available from: [Google Scholar]
- 34.Namkoong H., Morimoto K., Nishimura T., Tanaka H., Sugiura H., Yamada Y. Clinical efficacy and safety of multidrug therapy including thrice weekly intravenous amikacin administration for Mycobacterium abscessus pulmonary disease in outpatient settings: a case series. BMC Infect Dis. 2016;16(1) doi: 10.1186/s12879-016-1689-6. [Internet]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown-Elliott B.A., Nash K.A., Wallace R.J. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev. 2012;25(3):545–582. doi: 10.1128/CMR.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esteban J., Ortiz-Perez A. Current treatment of atypical mycobacteriosis. Expert Opin Pharmacother. 2009;10(17):2787–2799. doi: 10.1517/14656560903369363. [DOI] [PubMed] [Google Scholar]
- 37.Greendyke R., Byrd T.F. Differential antibiotic susceptibility of Mycobacterium abscessus variants in biofilms and macrophages compared to that of planktonic bacteria. Antimicrob Agents Chemother. 2008;52(6):2019–2026. doi: 10.1128/AAC.00986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett J., Dolin R., Douglas R. 7th ed. Elsevier; Philadelphia: 2009. E. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. [Google Scholar]