Abstract
Background
As many as 40% of the 1 million children living with HIV (CLHIV) receiving antiretroviral treatment (ART) in resource limited settings have not achieved viral suppression (VS). Kenya has a large burden of pediatric HIV with nearly 140,000 CLHIV. Feasible, scalable, and cost-effective approaches to ensure VS in CLHIV are urgently needed. The goal of this study is to determine the feasibility and impact of point-of-care (POC) viral load (VL) and targeted drug resistance mutation (DRM) testing to improve VS in children on ART in Kenya.
Methods
We are conducting a randomized controlled study to evaluate the use of POC VL and targeted DRM testing among 704 children aged 1–14 years on ART at health facilities in western Kenya. Children are randomized 1:1 to intervention (higher frequency POC VL and targeted DRM testing) vs. control (standard-of-care) arms and followed for 12 months. Our primary outcome is VS (VL < 1000 copies/mL) 12 months after enrollment by study arm. Secondary outcomes include time to VS and the impact of targeted DRM testing on VS. In addition, key informant interviews with patients and providers will generate an understanding of how the POC VL intervention functions. Finally, we will model the cost-effectiveness of POC VL combined with targeted DRM testing.
Discussion
This study will provide critical information on the impact of POC VL and DRM testing on VS among CLHIV on ART in a resource-limited setting and directly address the need to find approaches that maximize VS among children on ART.
Trials registration
Keywords: HIV, Children, Antiretroviral therapy, Point-of-care (POC) testing, Viral load, Drug resistance mutations (DRM)
1. Introduction
As many as 40% of the 1 million children living with HIV (CLHIV) < 15 years of age receiving antiretroviral treatment (ART) and living in low and middle income countries (LMIC) have not achieved viral suppression (VS) [1,2]. Disturbingly, rates of VS have not improved substantially since 2000 despite the implementation of more efficacious 1st line ART regimens for children [1,3]. While the reasons for poor VS rates in children are multifactorial, drug resistance and poor access to timely viral load (VL) monitoring to facilitate early and appropriate clinical decision-making are likely major contributors. Novel approaches that are relevant to current program practices and easily scalable are urgently needed to achieve the UNAIDS 95-95-95 goal for population VS targets in CLHIV [4].
Kenya has nearly 140,000 CLHIV <15 years of age and 8000 new pediatric infections annually [5,6]. Based on national survey data, only 48% of children and adolescents on ART have achieved VS in Kenya [6]. Low VS rates contribute to pediatric HIV morbidity and mortality. Starting in 2013, the World Health Organization (WHO) recommended monitoring people on ART with routine VL testing [7]. Increasing availability of routine VL testing, implemented in Kenya starting in 2014, has highlighted the issue of treatment failure in children but has not led to higher VS rates [5,8]. Several challenges, including long turn-around times for test results from weeks to months, expense of transporting samples to centralized laboratories, and inability to monitor VL more frequently than national guidelines allow, reduce the potential benefits of laboratory-based VL testing [7,[9], [10], [11], [12], [13], [14]]. Lack of reliable supply chains for critical VL reagents, limited knowledge of VL interpretation for providers and patients, and failure to switch ART despite ongoing virologic failure further limit the potential impact of VL monitoring [[14], [15], [16], [17], [18]]. Point-of-care (POC), or even near POC, VL assessments have been shown to be feasible, accurate, and less expensive than laboratory-based VL assays [13,14,19]. Kenya has implemented a nation-wide POC testing for tuberculosis using GeneXpert® technology which can be leveraged to carry out POC VL testing as well. Furthermore, POC VL-based care models are predicted to be more cost-effective than laboratory-based VL testing [20].
Additionally, HIV drug resistance is likely to contribute substantially to virologic failure in children. Recent WHO reports warn that HIV drug resistance mutations (DRMs) will undermine the attainment of the global targets for HIV [21]. Existing data in Africa among children not achieving VS and those newly diagnosed with HIV show high rates of DRMs [[22], [23], [24], [25], [26], [27]]. Understanding current DRM patterns among children failing ART is critical to determining the optimal management of these children, and may provide evidence for revision of algorithms used to manage children with virologic failure that can be rigorously tested in the future.
This randomized controlled study will evaluate the use of higher frequency POC VL with targeted DRM testing and clinical decision support for children aged 1–14 years on ART in Kenya. We hypothesize that rapid return of results via POC VL and targeted DRM testing will facilitate earlier and more appropriate clinical decision-making resulting in improved treatment outcomes among CLHIV [28].
2. Methods
2.1. Trial design
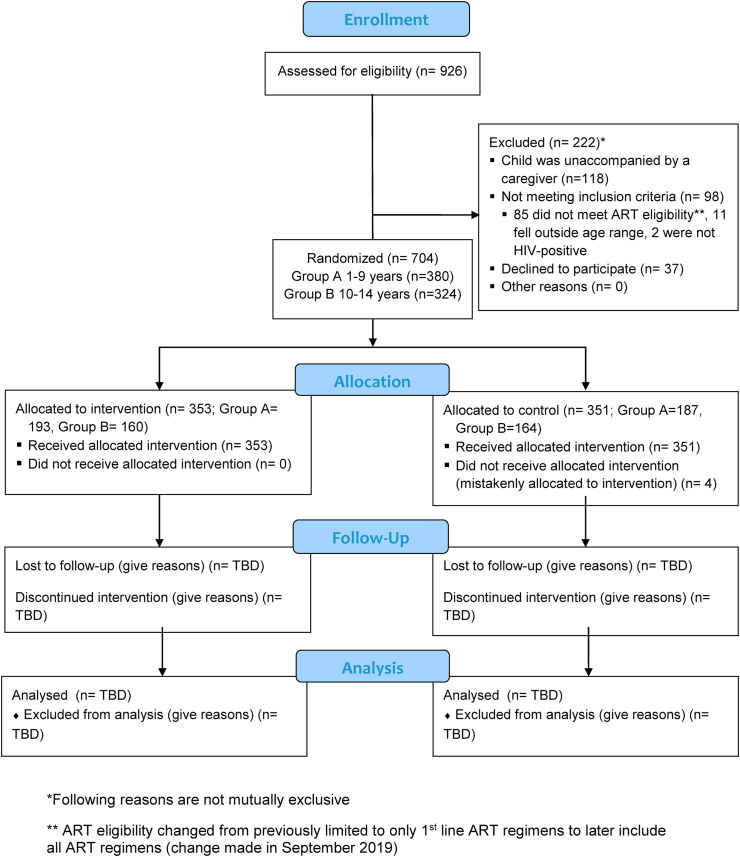
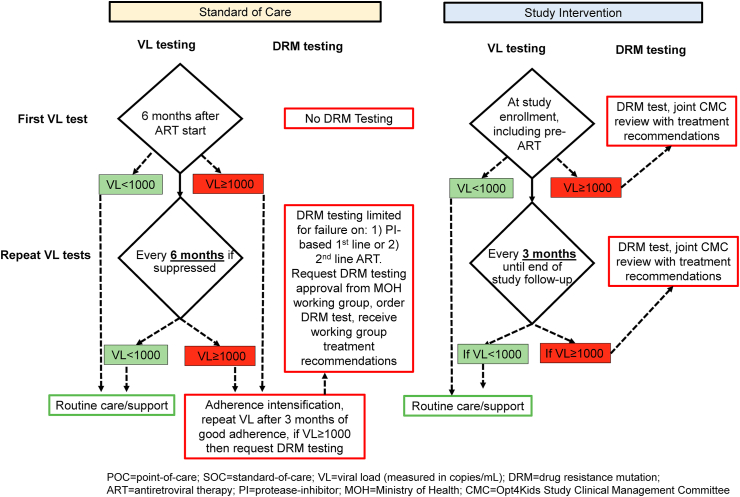
We are conducting an open-label randomized controlled trial evaluating the use of higher frequency POC VL testing with targeted DRM testing and clinical decision support among children with HIV on ART age 1–14 years over a 12-month period in high-volume HIV treatment facilities in Kisumu, Kenya (Fig. 1). We have chosen the study facilities to leverage existing POC technologies, specifically GeneXpert®. Currently, GeneXpert® technology is primarily being used for tuberculosis diagnosis in Kenya, but have been pilot tested for HIV early infant diagnosis and HIV quantitative RNA testing (e.g. VL) [[29], [30], [31]]. While validated for HIV VL monitoring, the technology remains to be tested for optimal integration into routine HIV clinical care [13,43]. At each facility, eligible children are randomized 1:1 to either receive the intervention, consisting of POC VL testing every 3 months with targeted DRM testing and clinical decision support, or standard-of-care (SOC) testing based on the existing Kenyan national guidelines (detailed later). We will follow the viral outcomes 12 months after enrollment for each child and compare VS rates, defined as VL < 1000 copies/mL by the current Kenyan national guidelines, among intervention vs. control arms (Fig. 2).
Fig. 1.
CONSORT flow diagram.
Fig. 2.
Viral load and drug resistance mutation testing algorithm for children on ART by study arm (modified from Kenya MOH ART Guidelines 2018).
2.2. Study objectives
Our leading objective is to determine the impact of POC VL by comparing the proportion of children on ART achieving VS 12 months after study enrollment (Aim 1a) and time to VS among those not suppressed or newly initiating ART (Aim 1b) between the study arms. We hypothesize that the proportion of children virally suppressed will be higher in the intervention vs. control arm.
Our secondary objectives are to determine the impact of targeted DRM testing and patterns of DRMs among children on ART without VS (Aim 2) and better understand how POC VL and targeted DRM testing influences VS and may be scaled for programmatic use, via key informant interviews (Aim 3a) and estimate programmatic costs and project incremental cost-effectiveness of the intervention (Aim 3b).
2.3. Study setting
The study is being conducted in Kisumu County, western Kenya, where adult HIV prevalence is 17.5%, 3.6 times higher than the national prevalence [6]. Kisumu County accounts for the second highest prevalence of pediatric HIV infections, with an estimated 9439 CLHIV and 369 annual HIV-related deaths in children [32]. The study is being implemented at five low-resource, high-volume, high-HIV burden primary health care facilities. These government facilities are supported by the Kenya Ministry of Health (MOH) and HIV implementing partners funded by the United States President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC).
Comprehensive pediatric HIV care and treatment services are provided in accordance with the Kenyan MOH ART guidelines. All children diagnosed with HIV are initiated on ART regardless of CD4 count per national guidelines [33]. First-line regimens in children under three years of age include lopinavir-ritonavir, lamivudine, and abacavir or zidovudine (see Table 1) [34]. For children 3–15 years of age lopinavir-ritonavir is substituted with efavirenz. Recent updates to the Kenyan guidelines recommend dolutegravir rather than efavirenz for those weighing at least 20 kg. Routine VL monitoring is offered through a laboratory network connected to a national, centralized laboratory and is recommended for children after six months of continuous ART and every six months thereafter for those with VL < 1000 copies/mL (Fig. 2). Management of children with VL ≥ 1000 copies/mL includes enhanced adherence counseling and repeat of VL testing in three months followed by possible switch of ART for those who are still not virologically suppressed. DRM testing at a national reference laboratory is restricted to children with virologic failure on a protease inhibitor (PI)-based 1st line or on 2nd or 3rd line ART who continue to have viremia after adherence optimization. This testing process requires approval by the Kenyan MOH HIV ART treatment committee, who also guides the local provider on clinical management.
Table 1.
Recommended 1st and 2nd line antiretroviral therapy (ART) regimens for children and youth in Kenya [33,34].
| Age and weight | Recommended 1st line ART at study start | Updated 1st line ART during study implementation | Recommended 2nd line ART |
|---|---|---|---|
| Birth-4 weeks | AZT + 3 TC + RAL or NVP | AZT + 3 TC + RAL or NVP | ABC + 3 TC + LPV/r |
| >4 weeks and <3 years, <20 kg | AZT (or ABC) + 3 TC + LPV/r | ABC + 3 TC + LPV/r | DRM testing-guided 2nd line |
| ≥3 years and 20–35 kg | ABC (or AZT) + 3 TC + EFV (or RAL) ABC (or AZT) + 3 TC + LPV/r | ABC + 3 TC + DTG | ABC (or AZT) + 3 TC + LPV/r DRM testing-guided 2nd line |
| ≥15 years or >35 kg | TDF (or ABC) + 3 TC + DTG (or EFV) | TDF + 3 TC + DTG | AZT (or TDF) + 3 TC + ATV/r |
ART = antiretroviral therapy; AZT = zidovidine; 3 TC = lamivudine; RAL = raltegravir; NVP = nevirapine; ABC = abacavir; LPV/r = lopinavir/boosted ritonavir; EFV = efavirenz; TDF = tenofovir; DTG = dolutegravir; DRM = drug resistance mutation; ATV/r = atazanavir/boosted ritonavir.
2.4. Study population
The study population was recruited from CLHIV ages 1–14 years newly initiating or already receiving ART at the study facilities. Infants <1 year of age were not included in the study as they frequently require more than 6 months of ART to suppress their initially high VL, and thus require specialized interpretation and management of VL results [[35], [36], [37]].
2.5. Eligibility criteria
Children eligible for inclusion were age 1–14 years living with HIV (documented HIV positive or HIV VL) and already on ART or newly initiating ART at one of the study facilities.
2.6. Randomization, allocation, and blinding
We randomized study participants 1:1 to the intervention vs. control arms using a blocked randomization scheme with varying block sizes, stratified by facility site and age groups (Group A: 1–9 or Group B: 10–14 years of age). Site coordinators ensured fidelity to the arm allocations by being the only ones able to order a POC VL. All investigators were blinded but participants, site coordinators, and the data team were not blinded to the randomization procedures.
2.7. Study procedures
Blood collection and storage: Approximately 6 mL of whole blood is collected in EDTA plasma tubes from participating children for study testing. If phlebotomy of the full amount is difficult to achieve, a minimum of 2 mL of blood is collected to perform POC VL testing and to prepare dried blood spots (DBS) for potential DRM testing if needed. The samples are processed within 6 h of collection for plasma POC VL testing by existing lab staff. The remaining plasma is placed in aliquots and transported to the KEMRI-CDC HIV Research Laboratory in coolers for potential DRM testing or stored for any future testing.
Intervention POC VL testing: Several POC VL tests have undergone analytical performance evaluations and are becoming increasingly available in resource-limited settings [[38], [39], [40], [41], [42], [43]]. Of such POC VL tests, the HIV-1 VL Assay developed by Cepheid to be run on its existing GeneXpert® systems is a leading test that has been validated and found feasible and reliable in rural African communities [43]. This assay uses reverse transcriptase polymerase chain reaction (PCR) technology to detect HIV in plasma over a range of 40 to 10 million copies/mL, with the lower limit of detection as low as 15 copies/mL, has an average run time of 90 min, and its kit is self-contained, including the necessary PCR reagents and supplies [44]. The only additional tool required is a centrifuge to separate the plasma from whole blood which is available at all the study facilities. Thus, our POC VL testing approach utilizes the existing technology and lab infrastructure already in place at the four of the five facilities for tuberculosis diagnostics. One facility does not have a GeneXpert® system on site but is within 2 km of a facility with the technology, therefore, study staff transfer the lab samples daily to this facility. The platform does not require batching tests and since the VL assay can be run simultaneously with other tests, study use of the machines does not interfere with their routine clinical use.
For participants assigned to the intervention arm, POC VL testing is done earlier than SOC, 3 vs. 6 months for those children newly initiating ART, and more frequently than SOC, 3 vs. 6 months for children already on ART (Fig. 2). For children newly initiating ART, we conduct POC VL and DRM tests pre-ART initiation, then POC VL three months post-ART initiation, and every 3 months thereafter for a total of 12 months. For children already on ART, a POC VL test is conducted at enrollment and every 3 months thereafter for a total of 12 months. Study visits are coordinated with the children's clinical visits. The study staff aim to return POC VL results to the providers and caregivers within 24 h of sample collection via text messages or phone (and paper results for providers).
Intervention HIV DRM testing: We are conducting targeted HIV DRM testing at the Kisumu branch of the KEMRI-CDC HIV Research laboratory using consensus sequencing on all plasma or DBS samples which lack VS. The HIV Research Laboratory utilizes Applied Biosystems (ABI) 3130xl Genetic Analyzers to conduct its HIV drug resistance testing on 0.5–1 mL of plasma. This optimized in-house assay detects reverse transcriptase- and PI-based mutations, and is broadly sensitive in genotyping HIV-1 subgroups detecting all mutations classified in the IAS-USA mutations list [45]. Integrase inhibitor testing is not routinely done, but may be requested. The lab conducts batch testing at periodic intervals and returns results to the study staff within 24 h of assay result. The DRM results report contain a list of the major and minor DRM genotypes as well as phenotypic interpretations, based on the scoring systems generated by the Stanford Genotypic Resistance Interpretation Algorithm [46]. Study staff then forward the DRM results to the ordering providers within 24 h of their receipt.
Intervention clinical decision support for management of children on ART with drug resistance: Overall, clinical providers are instructed to follow current Kenyan national guidelines for the management of any child with VL ≥ 1000 copies/mL which includes assessment of barriers to adherence and other potential factors related to virologic failure. For the intervention arm, a Clinical Management Committee was formed based on the existing MOH clinical and DRM case review technical working group in Kisumu County. The Committee includes the chair of the technical working group, clinical providers from the facility, study staff, MOH and other country HIV experts, and HIV implementing partner technical advisors. It meets regularly to discuss the cases and determines if a child should undergo an ART switch based on DRM testing. Facility staff aim to call back the child and caregiver within one week for counseling, adherence support, and regimen change if needed.
SOC VL testing: For those assigned to the control arm, VL testing is conducted per national guidelines (as outlined above) through laboratory networking and transport to a centralized laboratory. Children in the SOC arm who are newly initiating ART have blood collected by study staff prior to ART initiation for future VL and DRM testing to be conducted at completion of study follow up. These results will be returned to clinical providers and patients/caregivers after the study ends. Additionally, in order to have comparable outcomes, POC VL will be conducted on all SOC participants at 12 months of follow up.
SOC DRM Testing: We anticipate that very few children in the control arm will undergo SOC DRM testing as DRM testing is not currently recommended for children on 1st or 2nd line ART per national guidelines and requires centralized committee approval as described above. For those children in SOC who undergo POC VL testing, e.g. at primary endpoint of 12 months after study enrollment, if the resulting VL ≥ 1000 copies/mL, then study DRM testing is conducted with intervention clinical decision support offered.
SOC clinical decision support: Children in the control arm receive clinical management consistent with national guidelines including assessment of adherence and multi-disciplinary team review at the facility level. Providers follow their standard protocol for notification and follow up of children with high VLs.
Qualitative evaluation: We are conducting key informant interviews (KIIs) with four subgroups of key informants, including adolescent study participants, caregivers of children enrolled in our study, providers and other facility staff at our study facilities, and policy makers and other stakeholders from local to national levels. We are conducting 10–20 KIIs within each group with a total of 40–80 persons interviewed, some of whom will be interviewed serially up to four times during the study period. Our KII guide was developed from a socioecological model of pediatric VS, which includes individual, interpersonal, organizational, and structural/policy factors that influence pediatric VS, and has a distinct focus on POC VL and DRM testing. Generally, the interview guides cover the following domains: 1) barriers and facilitators to ART use, 2) VL literacy and experiences with SOC VL and DRM testing in routine care, and 3) experiences with POC VL & DRM testing via study and how best to scale up for programmatic use (see supplementary materials for interview guides). Specifically, we query particular logistical aspects of optimally operationalizing POC VL testing, e.g. how caregivers prefer to learn of the results or where facility staff see the most need for POC VL testing. These logistical aspects, such as the preferred approach to delivering results, result counseling content and methods, and provider reaction to results and additional capacity-building needs for the providers and health facilities, need to be explored further in order to optimize implementation and scale up of POC testing.
Costing and cost-effectiveness: For the costing component, we are using activity-based micro-costing, staff interviews, and time and motion studies to estimate the annual cost of HIV monitoring per child in the control and intervention arms. We use standardized cost menus to collect site costs, including start-up costs, clinic space, human resources, supplies, and VL test costs. Time and motion observation of intervention activities will be conducted to inform staff time costs and productivity assumptions and to remove research time (e.g. administering informed consent) from costs. For example, we will estimate the provider times necessary to complete the clinic visits for both arms, average number of patients seen in the clinic each day, and the time required to complete each step of the HIV care visit (VL testing and DRM, adherence counseling, etc.). Observing multiple visits by various staff members will allow estimation of the average time taken for each step; time needed for research activities will be removed from intervention time to provide an estimate of the intervention, if implemented as a government program. When data are not available from our cohort, we will utilize data from population-based studies from Kenya and sub-Saharan Africa, and we may obtain additional cost data from health facilities, published government information on labor costs, and health economics literature on an as needed basis. Analyses will follow the guidelines for costing HIV interventions [36,47,48], and will reflect the provider perspective. We will also collect data on patient out-of-pocket costs to assess if POC VL testing saves participants time and expense, reflecting the societal perspective. For the cost-effectiveness analysis, we will parameterize a model of HIV disease progression with cost data collected and VS outcomes from the trial to project HIV infections, HIV-related deaths, and disability-adjusted life years (DALYs) associated with the intervention and control scenarios.
2.7.1. Informed consent
Caregivers accompanying CLHIV age 1–14 years receiving or initiating ART were approached and invited to participate in the study during regular clinic visits. Caregivers expressing interest in participating in the study were first confirmed to be the parent or legal guardian and then taken through individual written consent in their preferred language by study staff fluent in the local languages. Caregivers receive a small transport and time reimbursement at enrollment and final study visit of approximately $5 USD. Children age 13 and older were provided information at an age appropriate level and given the opportunity to assent or decline separate from their caregiver. Consent for sample storage for future studies is documented separately from consent to participate in the study. Families who did not wish to participate continued to receive standard HIV services at the facility.
All participants in the KIIs underwent informed consent with additional assent obtained from the participating adolescents (ages 13–14 years, whose caregivers provided the consent).
2.7.2. Retention activities
The study relies on current clinic-based retention activities which include text message reminders, phone calls, home visits, and loss-to-follow-up tracing, which study staff supplement with additional phone tracing as needed. We anticipate these efforts will minimize losses to follow up and allow us to maintain sufficient participants to estimate our study outcomes well and minimize any bias due to missing outcomes.
2.8. Outcomes
Study Aim 1: Our primary outcome for Aim 1a is proportion of children virally suppressed (defined as VL < 1000 copies/mL) at 12 months after enrollment by arm. Our secondary outcome for Aim 1b is time to VS among a subset of children without VS detected at any time or those newly initiating ART by arm. We will also examine a set of process outcomes, including turn-around time for the VL testing results, retention-in-care, proportion of children switching ART, etc. by arm.
Study Aim 2: We intend to evaluate the impact of targeted HIV DRM testing on VS in the intervention arm. We will describe the proportion of children tested for DRMs with significant mutations within each class of HIV drugs, e.g. nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and PIs. We will also explore how sociodemographic, behavioral, clinical, and facility factors may be associated with the DRM patterns we observe.
Study Aim 3: In our Aim 3a, we intend to understand how our intervention influences VS by conducting KIIs which will interrogate factors which act as both facilitators and barriers to children achieving VS and focus specifically on how POC VL testing may improve VS. For Aim 3b, we will estimate costs and the incremental cost-effectiveness of POC VL combined with targeted DRM testing compared to SOC testing. We will calculate incremental cost effectiveness ratio (ICER) as the ratio of the difference in costs divided by the difference in effects across simulations for the intervention compared to SOC over a 20-year horizon. Consistent with guidelines, we will discount costs and health benefits at 3% annually, and consider ICERs below Kenya's per capita GDP to be cost-effective [60]. We will perform extensive sensitivity analyses to identify influential assumptions.
2.9. Data collection and management
Our data collection strategy includes prospective collection via REDCap systems and leveraging existing medical record systems at the study facilities. For prospective data collected through this clinical trial, we created longitudinal forms within a REDCap project, hosted by the University of Washington Institute of Translational Health Sciences, to capture the sociodemographics, behavioral, clinical, laboratory, and trial process data. Trial data collection is supplemented by capturing data from the existing paper and electronic medical record systems already running at the study facilities. When needed, for example for the pre-enrollment or SOC VL data, we obtain VL data from the National AIDS and STI Control Programme (NASCOP) online VL database and facility laboratory VL log books. For data management, we utilize existing data verification checks and quality tools within REDCap, conduct weekly quality checks, and generate quarterly reports for completeness, appropriateness, and quality of data.
The KIIs are audio recorded, last 30–60 min, occur in a room at the study facility and in the preferred language of the participants, and participants are reimbursed for their time.
For the costing studies, in addition to the staff interviews, we use standardized cost menus to collect site costs, including start-up costs, clinic space, human resources, supplies, and VL test costs and store the data in Excel files. When data are not available from our cohort, we will utilize data from population-based studies from Kenya and sub-Saharan Africa.
2.10. Sample size and power calculations
Power for the study is based on the primary outcome of proportion of VS in children in the intervention vs. control arms at 12 months after enrollment for each child (Aim 1a). We estimated requiring a sample size of 90% (630) of an estimated 700 children eligible to enroll to detect an effect size of at least 11%. Accounting for 10% loss to follow-up over 12 months, this sample size is expected to provide total of 567 children (or 284 per arm) with outcomes for analysis. These calculations are based on Fisher's exact test, two-sided α = 0.05, and initial VS rates of approximately 65% (estimated from historical facility data). We enrolled 704 children by December 2019 when further enrollment ceased.
2.11. Statistical analyses
Study Aim 1: We will provide descriptive statistics by randomization arm for study sample baseline demographics and facility characteristics, VS rates at baseline and quarterly afterwards, and process outcomes of interest. We note that VS is assessed on different schedules for the intervention arm (3-monthly) versus control arm (6-monthly unless unsuppressed and then 3-monthly until suppressed). Although testing frequency differs by arm, note that both arms include VS testing at 12 months, which is our primary outcome. In addition, both arms test quarterly in those not currently suppressed, i.e., the group for analysis for our secondary outcome (time to VS). For both outcomes, the assessment plan is similar in spite of the overall difference in testing frequency by arm.
All analyses of the intervention will be intention-to-treat (ITT), regardless of any post-randomization information, though additional secondary analyses of per-treatment may be considered.
Our primary analysis in this randomized study will compare the proportion of children with VS 12 months after enrollment for each child (primary outcome) in the intervention vs. control arms (primary predictor) using a logistic regression model, adjusting for facility and age group strata (1–9 or 10–14 years). A key secondary analysis will examine potential effect modification by age group using an interaction term between age group and intervention group in the model. While the study is not powered to test the intervention effect within age subgroups, particularly in the subgroup of 10–14 year olds, this analysis will provide data for the intervention effect within each, as well as for the difference in effect between the younger and older children.
Additional analyses including multivariate logistic regression models will estimate associations between VS and all potentially related individual-level factors (such as age, sex, duration on ART, prior VS patterns, and family demographics), as well as facility factors (such as patient volume, staff volume, urban/rural location, and tier of facility), in order to explore predictors of VS in this context. We will also do a secondary analysis separating the outlined outcomes for children on 1st vs. 2nd line therapy.
For our secondary analysis for Aim 1b, we will compare time to VS using a Cox regression model limited to individuals who are not suppressed during study follow-up or those initiating ART by study arms, with stratified baseline hazards by site and age group. A second analysis may adjust for potential confounders, such as the ones listed above, if found to be imbalanced between arms in this subpopulation.
Study Aim 2: To estimate the effect of providing timely DRM results on VS for intervention participants undergoing DRM, separately from the effect of POC VL alone, a secondary analysis will be performed with outcome of time to VS. As in Aim 1b, we will use a Cox model with primary predictor of intervention vs. control arm. To distinguish the effect of DRM results from that of POC VL testing, we will add a time-varying covariate which indicates, for each visit with a VL test, whether the clinician was notified of a positive DRM result since the child's prior VL test. If implemented as expected, all children without VS will be provided DRM testing and providers notified of the results. This model will allow us to divide the estimated effect of the intervention between the effect of POC VL testing alone (on those who do not have DRM), and the effect of POC VL testing plus DRM testing for those who do undergo DRM testing. We will perform these Cox models both unadjusted and adjusted, accounting for other factors likely to affect VS, particularly those that may vary at baseline in the intervention vs. control arms.
We will also describe the proportion of samples within the intervention arm that have any DRMs by HIV drug classes, e.g. NRTIs, NNRTIs, and PIs. We will report the proportion of samples with each type of mutation detected by drug class, and further group these mutations into major and minor ones. For example, we will examine major mutations in M184 V/I and K65R for NRTIs, K103 N, Y181C, G190A, and V106 M for NNRTIs, and V82A, I76V, 184 V, L47A, L90 M, M46I, and D30 N for PIs. With 284 participants randomized to the intervention arm, we expect approximately 35% (100) to not achieve VS at their 1st POC VL test and undergo DRM testing. With n = 100, we will be able to estimate prevalence of DRMs to within ± 5%–10% of the 95% CIs. For example, for a class of DRMs with prevalence of 80%, we anticipate generating exact 95% CIs of 71.3%–87.0%; a less common DRM at 10% will generate 95% CIs of 5.1%–17.1%. We will also use multivariate logistic regression models to identify risk factors associated with major and any DRMs.
Study Aim 3: For Aim 3a, study staff will translate and transcribe the interviews in English, with a second study staff verifying the accuracy of translation. The English transcripts are imported into a qualitative analysis software package for coding. Serial interviews for the same person are inductively coded together to maintain context for that person. We are developing a codebook documenting codes, definitions, guidelines on their use, and example quotes. 2-3 study staff independently code the transcripts, including initial double coding. The initial, primary codes are developed from the interview guides and expanded into more detailed, secondary codes during the coding process. After the initial round of coding, the study team will meet to discuss their coding process, assess intercoder agreement, and resolve discrepancies through consensus. Once the coding is complete, we will use thematic analysis to organize the data for further analysis, prioritizing longitudinal over cross-sectional analysis [49,50]. Finally, analytic memos will be written to lift the primary and secondary codes into thematic analyses that represent a full range of perspectives, both convergent and divergent. Several measures will be taken to ensure high quality data and rigorous analysis, such as principles of reflexivity [51] and rigor [[52], [53], [54]].
For Aim 3b, the micro-costing data, time and motion studies, and clinical outcomes will be used to estimate the average cost per child achieving VL suppression in the intervention compared to the control arm.
2.12. Oversight and monitoring
All serious adverse events associated with the procedures of this study are reported to the appropriate institutional review boards. The primary risk to participants in this study is social harm associated with HIV status disclosure. Field staff are trained to complete descriptions of adverse events that are communicated to the onsite study coordinator immediately, and then sent electronically to study investigators within 24 h.
Given the determination of this trial as a minimal risk study, a formal data and safety monitoring board was not assembled. Instead, the study investigators conduct periodic data and safety monitoring activities to ensure participant safety.
2.13. Trial status
This trial began participant enrollment in March 2019 and completed enrollment by December 2019. Participants will be followed for 12 months following study enrollment, which we anticipate will end in December 2020.
3. Discussion
This study will help to address the need to improve HIV treatment outcomes among children and young adolescents living with HIV in resource-limited settings. In 2018, there were an estimated 1.7 million CLHIV worldwide, with only approximately 54% accessing ART [55]. Though the number of CLHIV on ART globally has nearly doubled since 2010, VS remains suboptimal. Global estimates of VS among children on ART are limited, but range from 60 to 75% and are consistently lower than adults in similar settings [2,3,56]. Furthermore, children with virologic failure have low rates of achieving VS even with adherence interventions [57]. Achieving VS is particularly urgent for young children less than two years of age who face extremely high mortality without treatment [58,59]. Yet, VS is also important for older children whose growth and neurodevelopmental outcomes are adversely affected by poor virologic control. In adolescents, viremia not only puts youth at risk for increased morbidity, but also contributes to potential ongoing sexual transmission of HIV amongst those who are sexually active [60,61].
Several factors contribute to VS in CLHIV including access to uninterrupted, effective ART regimens, excellent adherence to medication, and regular clinical follow up. Children are often reliant on caregivers to ensure medication adherence, frequently have drug resistance prior to treatment initiation due to individual or maternal exposure to antiretrovirals, and are faced with drug formulations that are not optimized for the pediatric population often requiring complex preparation, dosing measurements, and dosing frequency more than once per day [1,62]. In addition, clinical and immunological monitoring of children's treatment success is known to be unreliable, leading to profound delays in recognizing treatment failure [63]. The introduction of routine VL monitoring presents an opportunity to reliably assess and intervene for children not reaching VS [64]. Yet, current approaches to VL monitoring which involve centralized laboratories and sample transport networks have not necessarily provided timely and regular access to VL testing [12,13,65]. POC VL technology holds substantial potential to bridge the gap addressing the need for routine VL monitoring that can impact clinical management. We hypothesize that caregivers, older children, and clinical providers will be able to use knowledge of VL monitoring to support improved adherence and clinical intervention, leading to VS, an idea supported by the principle of “cues to action” in the Health Belief Model [66,67].
Our study leverages existing POC technology, the GeneXpert® System which is already in place in Kenya for use in tuberculosis and HIV infant diagnosis, to determine if POC VL testing that is more frequent and with reduced result turn-around time to clinical providers and patients than routine VL testing will ultimately improve VS. Operationalizing such a model will foreshadow real-life implementation of such systems, where additions, such as a “hub and spoke” laboratory networking model, could be more feasible and sustainable compared to traditional, centralized laboratory models [68]. Heavy investments have already been made in GeneXpert® technologies for tuberculosis diagnostics in LMIC that should be leveraged when possible, without disrupting tuberculosis programs, in order to maximize this investment especially given the overlapping epidemics of tuberculosis and HIV in LMIC. Regardless of the results of the current study, it is arguable that POC VL technology should be utilized in LMIC in order to facilitate timely patient management and VL counseling to optimize health outcomes, particularly in high risk populations including CLHIV [64,69,70].
Furthermore, our study recognizes that VS cannot be achieved if significant drug resistance is present [71]. The most recent WHO report on HIV drug resistance shows that up to 50% of treatment-naïve infants with HIV have drug resistance to NNRTIs. At the same time, resistance among children with non-suppression on 1st line ART regimens is increasing [71]. Furthermore, re-suppression following virologic failure among children is substantially lower than in adults, suggesting enhanced adherence support may be insufficient in this population [14,57,72]. Thus, we incorporated targeted DRM testing in CLHIV with virologic failure along with supported clinical management in order to optimize clinical treatment decisions. While introduction of integrase inhibitors for younger populations, including in Kenya, is beginning, understanding current DRM prevalence may influence timing and urgency of introducing these highly potent drugs and testing for integrase resistance can be incorporated in future if needed. By determining current patterns and impact of DRM testing among children undergoing routine VL monitoring, this trial will inform the future needs of DRM testing in this population.
Lastly, the qualitative and costing components of this study will complement the findings from the primary outcomes, aiding better interpretation of results and decision-making from programmatic and policy perspectives. Already, the study has experienced concerns on the ground about use of POC VL in routine HIV care including the validity of results and their use in clinical management of patients. While the GeneXpert® systems used in this study are WHO pre-qualified and have undergone national validation in Kenya, additional local data may be required to ensure confidence in this technology [30,31]. Further, given limited options for 3rd line and salvage ART regimens in Kenya and resource-limited settings generally, stakeholders are particularly concerned about clinical management recommendations that lead to increased use of 2nd line or non-standard ART regimens. Despite substantial investment in stakeholder consultation and engagement in protocol development, ongoing barriers to uptake of POC VL technology requires further understanding which we will explore through qualitative methods.
3.1. Limitations
As designed, our study will provide important evidence regarding the impact of POC VL and DRM testing with some clinical decision support on VS in CLHIV on ART. However, there may be important study limitations to consider. First, while it would be ideal to provide same day or immediate POC VL results to participants and providers, the current clinical and laboratory systems in place at participating study facilities do not support this. Thus, the ultimate utility of a POC test may be diminished and, arguably, our intervention may be better termed “near POC” or “rapid return of results.” Nonetheless, GeneXpert® is arguably one of the more ubiquitously-available systems in resource limited settings due to the investments in tuberculosis diagnostics, and, therefore, demonstrating its use for POC VL testing may be a powerful tool for programs in many settings. Additionally, our proposed work will undoubtedly reduce the current turn-around times for centralized laboratory VL testing. Second, our current DRM testing process uses a central laboratory, which has already resulted in delays in DRM test results. We are exploring a near POC DRM testing technology in a substudy under this parent trial. Third, we chose to leverage existing technologies at the facilities to conduct POC VL testing, which meant using the GeneXpert® systems. GeneXpert® is arguably one of the more resource-intensive existing POC technologies. Other existing or in-development platforms, such as the AlereQ®, can run on battery power, are more compact, and hence more user friendly for more rural facilities. Nonetheless, the generalizability of our findings will not be limited to the GeneXpert® platform, as we are not validating this specific platform but rather testing a new approach to HIV management for children on ART which may include any POC VL testing platform. Fourth, crossovers or contamination may occur in our study if providers want to utilize POC VL testing for children in the control arm, however, such crossovers should be minimal as access to POC VL test ordering is limited to study staff only (to date our study has encountered one POC VL conducted on a child in SOC when not indicated). Fifth, because all providers are receiving additional training on management of children with virologic failure, it is possible that VS will increase for both groups. Even if this occurs, we likely still have sufficient power to detect a significant difference between study arms. Sixth, our analyses may not be able to truly account for the separate effects of the various combined intervention strategies used in the intervention, which is a limitation of all combination intervention studies. Notwithstanding these limitations, this study is poised to help optimize HIV treatment monitoring for children on ART in resource-limited settings.
4. Conclusions
Findings from this study will inform future work to combine an optimized POC VL and DRM monitoring algorithm with evidence-based socio-behavioral interventions to maximize VL suppression rates for CLHIV. The results may also support enhanced scale up of POC VL technology and access to DRM testing. Our study findings will directly address the urgent need to find interventions to maximize VS among children on ART and achieve global HIV goals.
Ethical review
Ethical approval for this study has been obtained from the African Medical and Research Foundation (AMREF) and Jaramogi Oginga Odinga Teaching and Referral Hospital (JOOTRH) Institutional Review Boards (IRBs) in Kenya, as well as the University of Washington and the University of Colorado Denver IRBs in the United States.
Acknowledgements
We recognize the study participants, caregivers, and facility staff participating in and supporting the Opt4Kids study. We acknowledge the support of the Kisumu County Health Management Team, Ministry of Health, and Family AIDS Care and Education Services. We also thank members of our Scientific Advisory Committee.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2020.100673.
Authors contributions
RP, PO, IM, KT and LA conceptualized and designed the study; and drafted the manuscript. MS has been leading the costing components of the study. BO made substantial contributions to drafting of the initial manuscript. GJS has been serving in an advisory role for the study. All authors read and approved the final manuscript.
Funding
Support for this study is provided by the National Institutes of Mental Health of the U.S. National Institutes of Health (NIH, R34MH115769). Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Washington Institute of Translational Health Sciences and supported by the National Center For Advancing Translational Sciences of the NIH (UL1 TR002319). MS received support from National Institutes of Mental Health of the NIH (K01MH115789).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Boerma R.S., Boender T.S., Bussink A.P. Suboptimal viral suppression rates among HIV-infected children in low- and middle-income countries: a meta-analysis. Clin. Infect. Dis. 2016;63(12):1645–1654. doi: 10.1093/cid/ciw645. [DOI] [PubMed] [Google Scholar]
- 2.Boender T.S., Sigaloff K.C., McMahon J.H. Long-term virological outcomes of first-line antiretroviral therapy for HIV-1 in low-and middle-income countries: a systematic review and meta-analysis. Clin. Infect. Dis. 2015;61(9):1453–1461. doi: 10.1093/cid/civ556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen S., Smit C., van Rossum A.M. Long-term response to combination antiretroviral therapy in HIV-infected children in The Netherlands registered from 1996 to 2012. AIDS. 2013;27(16):2567–2575. doi: 10.1097/01.aids.0000432451.75980.1b. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. Understanding Fast-Track: Accelerating Action to End the AIDS Epidemic by 2030.
- 5.National AIDS Control Council . Kenya Ministry of Health; 2016. Kenya AIDS Response Progress Report 2016. Nairobi, Kenya. [Google Scholar]
- 6.National AIDS and STI Control Programme (NASCOP) Kenya Ministry of Health; Nairobi, Kenya: 2020. Preliminary KENPHIA 2018 Report. [Google Scholar]
- 7.World Health Organization . World Health Organization; Geneva, Switzerland: 2013. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. [PubMed] [Google Scholar]
- 8.NASCOP . Kenya Ministry of Health; Nairobi, Kenya: 2015. National Plan for Accelerating HIV Care and Treatment 2015-2017. [Google Scholar]
- 9.Lecher S., Williams J., Fonjungo P.N. Progress with scale-up of HIV viral load monitoring—seven sub-Saharan African countries. MMWR (Morb. Mortal. Wkly. Rep.) 2016;65(47):1332–1335. doi: 10.15585/mmwr.mm6547a2. January 2015–June 2016. [DOI] [PubMed] [Google Scholar]
- 10.Lecher S., Ellenberger D., Kim A.A. Scale-up of HIV viral load monitoring—seven sub-Saharan African countries. MMWR (Morb. Mortal. Wkly. Rep.) 2015;64(46):1287–1290. doi: 10.15585/mmwr.mm6446a3. [DOI] [PubMed] [Google Scholar]
- 11.NASCOP . Kenya Ministry of Health; Nairobi, Kenya: 2016. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya. 2016. [Google Scholar]
- 12.Roberts T., Cohn J., Bonner K., Hargreaves S. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin. Infect. Dis. 15 Apr 2016;62(8):1043–1048. doi: 10.1093/cid/ciw001. Epub 2016 Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutstein S.E., Golin C.E., Wheeler S.B. On the front line of HIV virological monitoring: barriers and facilitators from a provider perspective in resource-limited settings. AIDS Care. 2016;28(1):1–10. doi: 10.1080/09540121.2015.1058896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadima J., Patterson E., Mburu M. Adoption of routine virologic testing and predictors of virologic failure among HIV-infected children on antiretroviral treatment in western Kenya. PloS One. 2018;13(11) doi: 10.1371/journal.pone.0200242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glass T.R., Motaboli L., Nsakala B. The viral load monitoring cascade in a resource-limited setting: a prospective multicentre cohort study after introduction of routine viral load monitoring in rural Lesotho. PloS One. 2019;14(8) doi: 10.1371/journal.pone.0220337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens W.S., Marshall T.M. Challenges in implenting HIV load testing in South Africa. JID (J. Infect. Dis.) 2010;201(Supplement_1):S78–S84. doi: 10.1086/650383. [DOI] [PubMed] [Google Scholar]
- 17.Hermans L.E., Moorhouse M., Carmona S. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect. Dis. 2018;18(2):188–197. doi: 10.1016/S1473-3099(17)30681-3. [DOI] [PubMed] [Google Scholar]
- 18.Etoori D., Ciglenecki I., Ndlangamandla M. Successes and challenges in optimizing the viral load cascade to improve antiretroviral therapy adherence and rationalize second‐line switches in Swaziland. J. Int. AIDS Soc. 2018;21(10) doi: 10.1002/jia2.25194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simeon K., Sharma M., Dorward J. Comparative cost analysis of point-of-care versus laboratory-based testing to initiate and monitor HIV treatment in South Africa. PloS One. 2019;14(10) doi: 10.1371/journal.pone.0223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips A., Shroufi A., Vojnov L. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature. 2015;528(7580):S68–S76. doi: 10.1038/nature16046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HIV Drug Resistance Report 2019. World Health Organization; Geneva, Switzerland: 2019. https://www.who.int/hiv/pub/drugresistance/hivdr-report-2019/en/ (WHO/CDS/HIV/19.21) (accessed April 2 2020) [Google Scholar]
- 22.Chohan B.H., Tapia K., Benki-Nugent S. Nevirapine resistance in previously nevirapine-unexposed HIV-1-Infected Kenyan infants initiating early antiretroviral therapy. AIDS Res. Hum. Retrovir. 2015;31(8):783–791. doi: 10.1089/aid.2014.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ndembi N., Hamers R.L., Sigaloff K.C. Transmitted antiretroviral drug resistance among newly HIV-1 diagnosed young individuals in Kampala. AIDS. 2011;25(7):905–910. doi: 10.1097/QAD.0b013e328346260f. [DOI] [PubMed] [Google Scholar]
- 24.Wamalwa D., Lehman D.A., Benki-Nugent S. Long-term virologic response and genotypic resistance mutations in HIV-1 infected Kenyan children on combination antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 1999;62(3):267. doi: 10.1097/QAI.0b013e31827b4ac8. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crowell C.S., Maiga A.I., Sylla M. High rates of baseline drug resistance and virologic failure among ART naïve HIV-infected children in Mali. Pediatr. Infect. Dis. J. 2017;36(11):e258. doi: 10.1097/INF.0000000000001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn L., Hunt G., Technau K.-G. Drug resistance among newly-diagnosed HIV-infected children in the era of more efficacious antiretroviral prophylaxis. AIDS (Lond.) 2014;28(11):1673. doi: 10.1097/QAD.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NASCOP . Program NASCOP. Kenya Ministry of Health; Nairobi, Kenya: 2016. Report of the 2013 cross-sectional survey of acquired HIV drug resistance among adults and children on antiretroviral therapy at sentinel sites in Kenya. [Google Scholar]
- 28.Katzenstein D.A. Oxford University Press; 2014. Editorial Commentary: HIV RNA and Genotype in Resource-Limited Settings: Can We Do Better? [DOI] [PubMed] [Google Scholar]
- 29.Sandbulte M.R., Gautney B.J., Maloba M. Infant HIV testing at birth using point-of-care and conventional HIV DNA PCR: an implementation feasibility pilot study in Kenya. Pilot and feasibility studies. 2019;5:18. doi: 10.1186/s40814-019-0402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bwana P., Ageng'o J., Mwau M. Performance and usability of Cepheid GeneXpert HIV-1 qualitative and quantitative assay in Kenya. PloS One. 2019;14(3) doi: 10.1371/journal.pone.0213865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National HIV, Refernce Lab.Standardized Protocol for Method Validation/Verification, Kenya Ministry of Health, Nairobi, Kenya.
- 32.NASCOP . Kenya Ministry of Health; Nairobi, Kenya: 2016. Kenya HIV County Profiles. [Google Scholar]
- 33.NASCOP . Kenya Ministry of Health; Nairobi, Kenya: August 2018. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya 2018 Edition. Print. [Google Scholar]
- 34.NASCOP . Ministry of Health; Nairobi, Kenya: 2019. New Guidance on ART Transition for Children and Adolescents Less than 15 Years of Age Living with HIV (CALHIV) in Kenya. [Google Scholar]
- 35.Abrams E.J., Weedon J., Steketee R.W. Association of human immunodeficiency virus (HIV) load early in life with disease progression among HIV-infected infants. JID (J. Infect. Dis.) 1998;178(1):101–108. doi: 10.1086/515596. [DOI] [PubMed] [Google Scholar]
- 36.Palumbo P.E., Kwok S., Waters S. Viral measurement by polymerase chain reaction-based assays in human immunodeficiency virus-infected infants. J. Pediatr. 1995;126(4):592–595. doi: 10.1016/s0022-3476(95)70357-8. [DOI] [PubMed] [Google Scholar]
- 37.Shearer W.T., Quinn T.C., LaRussa P. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. N. Engl. J. Med. 1997;336(19):1337–1342. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 38.UNITAID . Fourth ed. World Health Organization; Geneva, Switzerland: 2014. HIV/AIDS Diagnostics Technology Landscape 2014. [Google Scholar]
- 39.Goel N., Ritchie A.V., Mtapuri-Zinyowera S. Performance of the SAMBA I and II HIV-1 Semi-Q Tests for viral load monitoring at the point-of-care. J. Virol Methods. 2017;244:39–45. doi: 10.1016/j.jviromet.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Jani I.V., Meggi B., Mabunda N. Accurate early infant HIV diagnosis in primary health clinics using a point-of-care nucleic acid test. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2014;67(1):e1–e4. doi: 10.1097/QAI.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 41.Ritchie A.V., Ushiro-Lumb I., Edemaga D. SAMBA HIV semiquantitative test, a new point-of-care viral-load-monitoring assay for resource-limited settings. J. Clin. Microbiol. 2014;52(9):3377–3383. doi: 10.1128/JCM.00593-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott L., Gous N., Carmona S., Stevens W. Laboratory evaluation of the Liat HIV Quant (IQuum) whole-blood and plasma HIV-1 viral load assays for point-of-care testing in South Africa. J. Clin. Microbiol. 2015;53(5):1616–1621. doi: 10.1128/JCM.03325-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moyo S., Mohammed T., Wirth K.E. Point-of-care Cepheid Xpert HIV-1 viral load test in rural African communities is feasible and reliable. J. Clin. Microbiol. 2016;54(12):3050–3055. doi: 10.1128/JCM.01594-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cepheid Innovation Inc. Solna; Sweden: 2015. Xpert HIV-1 Viral Load, Package Insert. [Google Scholar]
- 45.Zhou Z., Wagar N., DeVos J.R. Optimization of a low cost and broadly sensitive genotyping assay for HIV-1 drug resistance surveillance and monitoring in resource-limited settings. PloS One. 2011;6(11) doi: 10.1371/journal.pone.0028184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang M.W., Liu T.F., Shafer R.W. The HIVdb system for HIV-1 genotypic resistance interpretation. Intervirology. 2012;55(2):98–101. doi: 10.1159/000331998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization . World Health Organization; Geneva: 2010. CHOICE: Choosing Interventions that Are Cost Effective. [Google Scholar]
- 48.Eaton J.W., Menzies N.A., Stover J. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. The Lancet Global Health. 2014;2(1):e23–e34. doi: 10.1016/S2214-109X(13)70172-4. [DOI] [PubMed] [Google Scholar]
- 49.Calman L., Brunton L., Molassiotis A. Developing longitudinal qualitative designs: lessons learned and recommendations for health services research. BMC Med. Res. Methodol. 2013;13(1):14. doi: 10.1186/1471-2288-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srivastava A., Thomson S.B. 2009. Framework Analysis: a Qualitative Methodology for Applied Policy Research. [Google Scholar]
- 51.Shaw J.A. Reflexivity and the “Acting Subject” conceptualizing the unit of analysis in qualitative health research. Qual. Health Res. 2016;26(13):1735–1744. doi: 10.1177/1049732316657813. [DOI] [PubMed] [Google Scholar]
- 52.Graneheim U.H., Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ. Today. 2004;24(2):105–112. doi: 10.1016/j.nedt.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Hammersley M. Routledge; 2013. What's Wrong with Ethnography? [Google Scholar]
- 54.Patton M.Q. Enhancing the quality and credibility of qualitative analysis. Health Serv. Res. 1999;34(5 Pt 2):1189. [PMC free article] [PubMed] [Google Scholar]
- 55.UNAIDS Global HIV & AIDS statistics — 2019 fact sheet. https://www.unaids.org/en/resources/fact-sheet
- 56.Duong T., Judd A., Collins I.J. Long-term virological outcome in children on antiretroviral therapy in the UK and Ireland. AIDS (Lond.) 2014;28(16):2395. doi: 10.1097/QAD.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ford N., Orrell C., Shubber Z., Apollo T., Vojnov L. HIV viral resuppression following an elevated viral load: a systematic review and meta-analysis. J. Int. AIDS Soc. 2019;22(11) doi: 10.1002/jia2.25415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davies M.A., Pinto J. Targeting 90-90-90--don't leave children and adolescents behind. J. Int. AIDS Soc. 2015;18(Suppl 6):20745. doi: 10.7448/IAS.18.7.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Violari A., Cotton M.F., Gibb D.M. Early antiretroviral therapy and mortality among HIV-infected infants. N. Engl. J. Med. 2008;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoare J., Heany S.J., Fouche J.P. Initiation of antiretroviral therapy after the critical neuronal developmental period of the second postnatal year affects white matter microstructure in adolescents living with HIV. J. Neurovirol. 2019;25(2):254–262. doi: 10.1007/s13365-018-0712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laughton B., Cornell M., Grove D. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS. 2012;26(13):1685–1690. doi: 10.1097/QAD.0b013e328355d0ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jenabian M.A., Costiniuk C.T., Mboumba Bouassa R.S., Chapdeleine Mekue Mouafo L., Brogan T.V., Belec L. Tackling virological failure in HIV-infected children living in Africa. Expert Rev. Anti Infect. Ther. 2015;13(10):1213–1223. doi: 10.1586/14787210.2015.1068117. [DOI] [PubMed] [Google Scholar]
- 63.Rutherford G.W., Anglemyer A., Easterbrook P.J. Predicting treatment failure in adults and children on antiretroviral therapy: a systematic review of the performance characteristics of the 2010 WHO immunologic and clinical criteria for virologic failure. AIDS. 2014;28(Suppl 2):S161–S169. doi: 10.1097/QAD.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 64.Drain P.K., Dorward J., Bender A. Point-of-Care HIV viral load testing: an essential tool for a sustainable global HIV/AIDS response. Clin. Microbiol. Rev. 2019;32 doi: 10.1128/CMR.00097-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Medicine Sans Frontiers Making viral load routine: successes and challenges in the implementation of routine HIV viral load monitoring. 2017. https://msfaccess.org/making-viral-load-routine
- 66.Janz N.K., Becker M.H. The health Belief model: a decade later. Health Educ. Q. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 67.Reddy S., Gibbs A., Spooner E. Assessment of the impact of rapid point-of-care CD4 testing in primary healthcare clinic settings: a survey study of client and provider perspectives. Diagnostics. 2020;10(2):81. doi: 10.3390/diagnostics10020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fonjungo P.N., Alemnji G.A., Kebede Y. Combatting global infectious diseases: a network effect of specimen referral systems. Clin. Infect. Dis. 2017;64(6):796–803. doi: 10.1093/cid/ciw817. [DOI] [PubMed] [Google Scholar]
- 69.Dorward J., Drain P.K., Garrett N. Point-of-care viral load testing and differentiated HIV care. The lancet HIV. 2018;5(1):e8–e9. doi: 10.1016/S2352-3018(17)30211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Setty H.G., Kumar M., Hewlett I.K. Point of care technologies for HIV. AIDS Res. Treat. 2014;2014 doi: 10.1155/2014/497046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boerma R.S., Sigaloff K.C., Akanmu A.S. Alarming increase in pretreatment HIV drug resistance in children living in sub-Saharan Africa: a systematic review and meta-analysis. J. Antimicrob. Chemother. 2017;72(2):365–371. doi: 10.1093/jac/dkw463. [DOI] [PubMed] [Google Scholar]
- 72.Nasuuna E., Kigozi J., Babirye L., Muganzi A., Sewankambo N.K., Nakanjako D. Low HIV viral suppression rates following the intensive adherence counseling (IAC) program for children and adolescents with viral failure in public health facilities in Uganda. BMC Publ. Health. 2018;18(1):1048. doi: 10.1186/s12889-018-5964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.