Abstract
Objectives:
We aimed to assess the in-hospital outcomes in patients with mitral regurgitation treated with percutaneous mitral valve repair (PMVR) among patients with chronic obstructive pulmonary disease (COPD).
Background:
There is lack of data on the outcomes of PMVR for mitral regurgitation in patients with COPD.
Methods:
We analyzed the national inpatient sample (NIS) database from January 2012 to December 2016.
Results:
A total of 9125 patients underwent PMVR in the period between January 2012 and December 2016, of whom 2,495 (27.3%) patients had concomitant COPD. Comparing COPD patients to non-COPD patients, COPD patients had higher proportion of females (48.3% vs. 46.6%, p = .16), were younger (75.8 ± 10.0 years vs. 76.4 ± 12.2 years; p = .04), had higher prevalence of peripheral vascular disease (17.4% vs. 13.5%; p < .01) and renal failure (39.3% vs. 37%; p < .01). After propensity matching, there was no significant difference in mortality among the COPD group versus non-COPD patients (2.6% vs. 2.9%; p = .6). Patients with COPD had higher proportion of in-hospital morbidities including St-segment elevation myocardial infarction (1.8% vs. 1.0%; p = .02), cardiogenic shock (1.4% vs. 0.4%; p < .01), vascular complications (2% vs. 0.8; p < .01), pneumothorax (1% vs. 0.4%; p < .01), and septic shock (1.2% vs. 0.4%; p < .01). Moreover, surrogates of severe disability (mechanical intubation and non-home discharges), cost of hospitalization, and length of stay were higher in the COPD group.
Conclusions:
There was no difference in mortality between the COPD and non-COPD patients after PMVR. Moreover, we observed higher rates of in-hospital morbidities, surrogates of severe disability, and higher resources utilization by the COPD group.
Keywords: chronic obstructive pulmonary disease, mitral clip, mitral regurgitation, national in-patient sample database, percutaneous mitral valve repair
1 |. INTRODUCTION
Mitral regurgitation (MR) is the most common valvular heart disease in the United States with more than 2 million patients with moderate to severe MR.1While surgical options were the only available strategies for treatment, recently percutaneous mitral valve repair (PMVR) using the commercially available (Mitraclip system; Abbot Vascular, Inc) has revolutionized the field and emerged as an effective therapeutic option for patients with primary or secondary MR who are deemed to be at high risk for surgery.2,3
Chronic obstructive pulmonary disease (COPD) affects around 25% of the patients who undergo surgical mitral valve interventions and its presence as comorbidity has been associated with the decision not to operate in patients with MR.4,5 Moreover, recent data from the tanscatheter aortic valve replacement (TAVR) for patients with COPD and severe aortic stenosis suggested that the presence of COPD is associated with higher mortality after TAVR.6 There is currently lack of similar data on the interaction between COPD and outcomes after treatment with PMVR. To bridge the gap, we sought to assess the nationwide trends, characteristics, and in-hospital outcomes in patients with MR treated with PMVR among patients with or without COPD within the national inpatient sample (NIS) database.
2 |. MATERIALS AND METHODS
The data, analytic methods, and study materials will be made available upon request to other researchers for purposes of reproducing the results. The NIS database is part of healthcare cost and utilization project (HCUP) databases and is sponsored by the agency for healthcare research and quality (AHRQ). The NIS is the largest publicly available all payer administrative claims based database and contains information about patient discharges from 1,000 hospitals in 45 states. It contains clinical and resource utilization information on 5–8 million discharges annually, with safeguards to protect the privacy of individual patients, physicians, and hospitals. These data are stratified to represent 20% of U.S. inpatient hospitalizations across different hospital and geographic regions (random sample). National estimates (NE) of the entire U.S. hospitalized population were calculated using the AHRQ sampling and weighting method.7 Institutional review board approval and informed consents were not required for this study given the de-identified nature of the NIS database and public availability.
We analyzed NIS data from January 2012 to December 2016 using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes and International Classification of Diseases, 10th Revision, Clinical Modification ICD-10-CM codes (ICD-10-CM). All patients undergoing PMVR who are 18 years and older were identified using ICD-9-CM code of 35.97 and ICD-10-CM code of 02UG3JZ. The study population was then divided into two groups (COPD vs. no-COPD). To account for potential confounding factors and selection bias, a propensity score-matching model was applied using logistic regression to derive two matched groups for comparative outcomes analysis. To avoid data loss, missing values were calculated for non-outcome variables using Markov chain Monte Carlo (MCMC) method. A nearest neighbor 1:1 variable ratio, parallel, balanced propensity-matching model was made using a caliper width of 0.2.
The primary endpoint of the study was in-hospital mortality. Secondary endpoints were (a) in-hospital morbidities, (b) surrogates of severe disability (non-home discharges and mechanical ventilation), and (c) cost of hospitalization and length of stay.
Associated procedures and hospital morbidities were identified using ICD-9-CM and ICD-10-CM codes (Online S1). Flow chart of our selection of patients is shown in Figure 1.
FIGURE 1.

Flow sheet of our study [Color figure can be viewed at wileyonlinelibrary.com]
Descriptive statistics were presented as frequencies with percentages for categorical variables and as means with standard deviations for continuous variables. Baseline characteristics were compared using a Pearson χ2 test and Fisher’s exact test for categorical variables and independent samples t test for continuous variables.
Logistic regression was performed to estimate odds ratios (ORs) with 95% confidence intervals (CIs) to determine predictors for mortality in patients undergoing PMVR. Binomial multiple logistic regression model was used to identify variables from demographic data. A type I error rate of <0.05 was considered statistically significant. All statistical analyses were performed using statistical package for social science (SPSS) version 26 (IBM Corp) and R, version 3.5 for propensity matching.
3 |. RESULTS
A total of 9,125 patients underwent PMVR in the period between January 2012 and December 2016, of whom 2,495 (27.3%) patients had concomitant COPD. Out of the total population, 4,295 (47.1%) were females, mean age was 76.2 ± 11.6, 6,635 (78.7%) were Caucasian, 625 (7.4%) were African American, and 565 (6.7%) were Hispanics. The majority of cases were elective 6,765 (74.3%) with fewer elective cases in patients with COPD compared to non-COPD (71.1% vs. 75.6%, p < .01). Comparing COPD patients to non-COPD patients, COPD patients had higher proportion of females (48.3% vs. 46.6, p = .16), were younger (75.8 ± 10.0 years vs. 76.4 ± 12.2 years; p = .04), had higher prevalence of peripheral vascular disease (17.4% vs. 13.5%; p < .01), diabetes mellitus (21.6% vs. 17.9%; p < .01), and renal failure (39.3% vs 37%; p < .01). The baseline characteristics of the two groups are shown in Tables 1 and 2.
TABLE 1.
Baseline characteristics of the study population
| Variables | No COPD (n = 6,630) | COPD (n = 2,495) | All PMVR (n = 9,125) | P value |
|---|---|---|---|---|
| Age (mean [SD]) years | 76.4 (12.2) | 75.8 (10.0) | 76.2 (11.6) | .04 |
| Gender | .16 | |||
| Female | 3,090 (46.6%) | 1,205 (48.3%) | 4,295 (47.1%) | |
| Male | 3,535 (53.4%) | 1,290 (51.7%) | 4,825 (52.9%) | |
| Race | ||||
| Caucasian | 4,760 (77.8%) | 1,875 (81.0%) | 6,635 (78.7%) | <.01 |
| African American | 395 (6.5%) | 230 (9.9%) | 625 (7.4%) | |
| Hispanics | 480 (7.8%) | 85 (3.7%) | 565 (6.7%) | |
| Asian or Pacific islander | 185 (3.0%) | 70 (3.0%) | 255 (3.0%) | |
| Native American | 30 (0.5%) | 15 (0.6%) | 45 (0.5%) | |
| Elective admission | 4,995 (75.6%) | 1,770 (71.1%) | 6,765 (74.3%) | <.01 |
| AHRQ medical comorbidity | ||||
| Anemia | 65 (1.0%) | 35 (1.4%) | 100 (1.1%) | .08 |
| Collagen vascular diseases | 270 (4.1%) | 145 (5.8%) | 415 (4.5%) | <.01 |
| Congestive heart failure | 85 (1.3%) | 45 (1.8%) | 130 (1.4%) | .06 |
| Coagulopathy | 875 (13.2%) | 330 (13.2%) | 1,205 (13.2%) | .9 |
| Diabetes | 1,190 (17.9%) | 540 (21.6%) | 1,730 (19.0%) | |
| Diabetes with complications | 440 (6.6%) | 195 (7.8%) | 635 (7.0%) | .05 |
| Hypertension | 4,455 (67.2%) | 1,710 (68.5%) | 6,165 (67.6%) | .22 |
| Chronic liver disease | 190 (2.90%) | 45 (1.80%) | 235 (2.60%) | |
| Fluid and electrolyte disorders | 1,370 (20.7%) | 555 (22.2%) | 1,925 (21.1%) | .09 |
| Neurological disorders | 280 (4.2%) | 135 (5.4%) | 415 (4.5%) | .01 |
| Obesity | 615 (9.3%) | 270 (10.8%) | 885 (9.7%) | .02 |
| Peripheral vascular disorders | 800 (12.10%) | 435 (17.40%) | 1,235 (13.50%) | <.01 |
| Pulmonary circulation disorders | 50 (0.80%) | 25 (1.00%) | 75 (0.80%) | .24 |
| Renal failure | 2,400 (36.2%) | 980 (39.3%) | 3,380 (37.0%) | <.01 |
| Weight loss | 275 (4.1%) | 165 (6.6%) | 440 (4.8%) | <.01 |
| Hospital location | ||||
| Rural | 10 (0.2%) | 10 (0.4%) | 20 (0.2%) | <.01 |
| Urban nonteaching | 660 (10.0%) | 225 (9.0%) | 885 (9.7%) | |
| Urban teaching | 5,960 (89.9%) | 2,260 (90.6%) | 8,220 (90.1%) | |
| Bed size of the hospital | ||||
| Small | 410 (6.2%) | 90 (3.6%) | 500 (5.5%) | <.01 |
| Medium | 1,075 (16.2%) | 405 (16.2%) | 1,480 (16.2%) | |
| Large | 5,145 (77.6%) | 2,000 (80.2%) | 7,145 (78.3%) | |
| Region | ||||
| New England | 170 (2.6%) | 75 (3.0%) | 245 (2.7%) | <.01 |
| Mid-Atlantic | 920 (13.9%) | 355 (14.2%) | 1,275 (14.0%) | |
| East north central | 810 (12.2%) | 380 (15.2%) | 1,190 (13.0%) | |
| West north central | 710 (10.7%) | 240 (9.6%) | 950 (10.4%) | |
| South Atlantic | 1,345 (20.3%) | 555 (22.2%) | 1,900 (20.8%) | |
| East south central | 335 (5.1%) | 145 (5.8%) | 480 (5.3%) | |
| West south central | 490 (7.4%) | 170 (6.8%) | 660 (7.2%) | |
| Mountain | 570 (8.6%) | 215 (8.6%) | 785 (8.6%) | |
| Pacific | 1,280 (19.3%) | 360 (14.4%) | 1,640 (18.0%) | |
| Median household income percentile | ||||
| 0–25th | 1,450 (22.2%) | 625 (25.7%) | 2,075 (23.2%) | <.01 |
| 26–50th | 1,425 (21.8%) | 645 (26.5%) | 2,070 (23.1%) | |
| 51–75th | 1,880 (28.8%) | 635 (26.1%) | 2,515 (28.1%) | |
| 76–100th | 1,770 (27.1%) | 530 (21.8%) | 2,300 (25.7%) | |
Abbreviations: AHRQ, agency for healthcare research and quality; COPD, chronic obstructive pulmonary disease; PMVR, percutaneous mitral valve clip.
TABLE 2.
Baseline characteristics after propensity matching
| Variable | No COPD (n = 2,455) | COPD (n = 2,495) | P value |
|---|---|---|---|
| Age (mean [SD]) years | 76.2 (12.0) | 75.8 (10.1) | .58 |
| Gender | .65 | ||
| Female | 1,150 (46.8%) | 1,205 (48.3%) | |
| Male | 1,305 (53.2%) | 1,290 (51.7%) | |
| Race | |||
| Caucasian | 1980 (80.7%) | 2020 (81.0%) | .86 |
| African American | 200 (8.1%) | 245 (9.8%) | |
| Hispanics | 120 (4.9%) | 100 (4.0%) | |
| Asian or Pacific Islander | 80 (3.3%) | 70 (2.8%) | |
| Native American | 20 (0.8%) | 20 (0.8%) | |
| AHRQ medical comorbidities | |||
| Anemia | 15 (0.6%) | 35 (1.4%) | .21 |
| Collagen vascular diseases | 24 (4.9%) | 29 (5.8%) | .52 |
| Congestive heart failure | 35 (1.4%) | 45 (1.8%) | .63 |
| Coagulopathy | 260 (10.6%) | 330 (13.2%) | .20 |
| Diabetes | 455 (18.5%) | 540 (21.6%) | .22 |
| Diabetes with complications | 160 (6.5%) | 195 (7.8%) | .43 |
| Hypertension | 1,700 (69.2%) | 1,710 (68.5%) | .81 |
| Chronic liver disease | 25 (1.0%) | 45 (1.8%) | .29 |
| Fluid and electrolyte disorders | 470 (19.1%) | 555 (22.2%) | .23 |
| Neurological disorders | 90 (3.7%) | 135 (5.4%) | .20 |
| Obesity | 275 (11.2%) | 270 (10.8%) | .85 |
| Peripheral vascular disorders | 430 (17.5%) | 435 (17.4%) | |
| Pulmonary circulation disorders | 20 (0.8%) | 25 (1.0%) | .76 |
| Renal failure | 935 (38.1%) | 980 (39.3%) | .74 |
| Hospital location Rural | |||
| Rural | 10 (0.4%) | 10 (0.4%) | .98 |
| Urban nonteaching | 230 (9.4%) | 225 (9.0%) | |
| Urban teaching | 2,215 (90.2%) | 2,260 (90.6%) | |
| Bed size of the hospital | |||
| Small | 115 (4.7%) | 90 (3.6%) | .58 |
| Medium | 360 (14.7%) | 405 (16.2%) | |
| Large | 1,980 (80.7%) | 2,000 (80.2%) | |
| Region | |||
| New England | 15 (3.1%) | 15 (3.%) | .80 |
| Mid-Atlantic | 74 (15.1%) | 71 (14.2%) | |
| East north central | 69 (14.1%) | 76 (15.2%) | |
| West north central | 59 (12.%) | 48 (9.6%) | |
| South Atlantic | 104 (21.2%) | 111 (22.2%) | |
| East south central | 24 (4.9%) | 29 (5.8%) | |
| West south central | 39 (7.9%) | 34 (6.8%) | |
| Mountain | 31 (6.3%) | 43 (8.6%) | |
| Pacific | 76 (15.5%) | 72 (14.4%) | |
| Median income | |||
| 0–25th | 470 (19.1%) | 635 (25.5%) | .06 |
| 26–50th | 630 (25.7%) | 675 (27.1%) | |
| 51–75th | 710 (28.9%) | 645 (25.9%) | |
| 76–100th | 645 (26.3%) | 540 (21.6%) | |
Abbreviations: AHRQ, agency for healthcare research and quality; COPD, chronic obstructive pulmonary disease.
Crude in-hospital mortality rate of the overall population was 2.5%. There was no difference between the COPD and non-COPD groups in term of in-hospital mortality (2.6% vs. 2.4%; p = .6). Patients with COPD had higher proportion of in-hospital morbidities including st-segment elevation myocardial infarction (STEMI) (1.8% vs. 0.8%; p < .01), non-st-segment elevation myocardial infarction (NSTEMI) (1.6% vs. 1.3%; p < .01), respiratory complications (1.6% vs. 1.1%; p = .03), pneumothorax (1% vs. 0.5%; p < .0), stroke (0.6% vs. 0.4%; p = .04), vasopressor use (2.2% vs. 1.6%, p < .01), and vascular complications (2.0% vs. 1.4%; p = .03). On the other hand, patients with COPD had fewer crude rates of cardiogenic shock (2.8% vs. 4.5%; p < .01) (Table 3). Moreover, surrogates of severe disability (mechanical intubation and non-home discharges) were more frequent in the COPD group, who also had longer hospitalization and higher cost of care (Table 3).
TABLE 3.
Hospital outcomes in unmatched and matched cohorts
| Unmatched cohorts |
Matched cohorts |
|||||
|---|---|---|---|---|---|---|
| Variables no. (%) | No COPD (n = 6,630) | COPD (n = 2,495) | P value | No COPD (n = 2,455) | COPD (n = 2,495) | P value |
| In-hospital mortality | 160 (2.4%) | 65 (2.6%) | .60 | 70 (2.9%) | 65 (2.6%) | .60 |
| In-hospital morbidities | ||||||
| STEMI | 50 (0.8%) | 45 (1.8%) | <.01 | 25 (1.0%) | 45 (1.8%) | .02 |
| NSTEMI | 85 (1.3%) | 40 (1.6%) | <.01 | 25 (1.0%) | 40 (1.6%) | .07 |
| Tamponade | 30 (0.5%) | 10 (0.4%) | .74 | 20 (0.8%) | 10 (0.4%) | .06 |
| Iatrogenic cardiac complications | 170 (2.6%) | 80 (3.2%) | .09 | 80 (3.3%) | 80 (3.2%) | .90 |
| Cardiogenic shock | 300 (4.50%) | 70 (2.80%) | <.01 | 10 (0.4%) | 30 (1.2%) | <.01 |
| Postprocedure respiratory complication | 70 (1.1%) | 40 (1.6%) | .03 | 45 (1.8%) | 40 (1.6%) | .54 |
| Pneumothorax | 20 (0.30%) | 25 (1.00%) | <.01 | 10 (0.4%) | 25 (1.0%) | .01 |
| Vascular complications | 90 (1.40%) | 50 (2.00%) | .03 | 20 (0.8%) | 50 (2.0%) | <.01 |
| Transfusion during the procedure | 605 (9.10%) | 245 (9.80%) | .31 | 220 (9.0%) | 245 (9.8%) | .30 |
| Acute kidney injury | 525 (7.9%) | 165 (6.6%) | .04 | 190 (7.7%) | 165 (6.6%) | .13 |
| Septic shock | 55 (0.8%) | 30 (1.2%) | .09 | 10 (0.4%) | 30 (1.2%) | <.01 |
| Vasopressors use | 90 (1.40%) | 55 (2.20%) | <.01 | 30 (1.2%) | 55 (2.2%) | .73 |
| Stroke (after procedure) | 20 (0.30%) | 15 (0.60%) | .04 | 15 (0.6%) | 15 (0.6%) | .96 |
| Severe disability surrogates | ||||||
| Non-home discharge | 855 (13.2%) | 400 (16.4%) | <.01 | 250 (10.5) | 400 (16.4) | <.01 |
| Mechanical ventilation | 395 (6.0%) | 195 (7.8%) | <.01 | 125 (5.1%) | 195 (7.8%) | <.01 |
| Resources utilization | ||||||
| Length of stay, mean (SD), days | 5.7 (9.2) | 6.6 (8.3) | <.01 | 5.1 (7.4) | 6.6 (8.3) | .02 |
| Cost of hospitalization-mean (SD), $ | 204,446 (173,932) | 217,970 (150,018) | <.01 | 194,939 (167,692) | 217,225 (149,564) | .03 |
Abbreviations: COPD; chronic obstructive pulmonary disease, STEMI; ST-segment elevation myocardial infarction; NSTEMI; non-St-segment elevation myocardial infarction.
After vigorous propensity matching and adjusting for baseline demographics, clinical co-morbidities, region and hospital characteristics, we were able to match 2,495 COPD patients to 2,455 non-COPD patients, the two groups were well matched(Table 2).
After propensity matching, there continue to be no significant difference in mortality among the COPD group versus non-COPD patients (2.6% vs. 2.9%; p = .6). Patients with COPD had higher proportion of in-hospital morbidities including STEMI (1.8% vs. 1.0%; p = .02), cardiogenic shock (1.4% vs. 0.4%; p < .01), vascular complications (2% vs. 0.8, p < .01), pneumothorax (1% vs. 0.4%; p < .01) and septic shock (1.2% vs. 0.4%; p < .01). Moreover, surrogates of severe disability (mechanical intubation and non-home discharges) were more frequent in the COPD group. The mean cost of hospitalization (217,225$ vs. 194,939$; p < .01) and length of stay (6.6 days vs. 5.1 days; p < .01) were higher in COPD group as compared to non-COPD group (Table 3).
Mortality has decreased over the years in both groups, though this is has shown more reduction in non-COPD group (Figure 2). There has been decrease in the mean length of stay over the year for both groups with more reduction in non-COPD group (Figure 3).
FIGURE 2.

Yearly trends in mortality in percutaneous mitral valve replacement [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
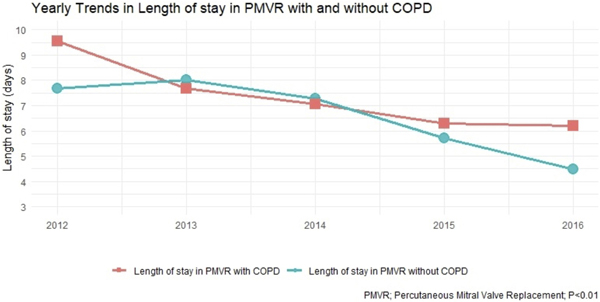
Yearly trends in length of hospital stay for patients undergoing percutaneous mitral valve replacement [Color figure can be viewed at wileyonlinelibrary.com]
Predictors of mortality for patients undergoing PMVR are shown in Figure 4. COPD was not associated with higher odds of mortality (OR, 1.84[95% CI, 0.72–4.78, p = .20]). Congestive heart failure (OR, 8.61 [95% CI, 4.18–17.71, p < .01), chronic renal failure (OR, 1.61[95% CI, 1.13–2.30, p < .01]), chronic liver disease (OR, 4.09 [95% CI, 2.14–7.84, p < .01]), diabetes mellitus (OR, 1.61[95% CI, 1.08–2.55, p < .01]), and coagulopathy (OR, 2.7[95% CI, 1.82–3.92, p < .01]) were associated with higher mortality. The overall mortality has improved over the years (OR, 0.79 [95% CI, 0.69–092, p < .01).
FIGURE 4.

Predictors of mortality in percutaneous mitral valve replacement [Color figure can be viewed at wileyonlinelibrary.com]
4 |. DISCUSSION
In the current analysis from the NIS database comparing outcomes of PMVR in patients with COPD versus no-COPD, we observed several findings. First, the overall in-hospital mortality is low and improved over years, with no difference in mortality between the two groups. Second, we observed higher rates of in-hospital morbidities, surrogates of severe disability and higher resources utilization by the COPD group. Third, the presence of COPD was not predictor for mortality.
COPD is a common comorbidity in patients with chronic cardiac diseases and contributes to poor outcomes including increased mortality following cardiac procedures.6–11 Its role in mitral valve periprocedural complications has been seldom studied. One small, prospective observational study showed COPD to be an independent predictor of composite mortality, cardiac re-hospitalization, re-intervention, and major cerebro-vascular and cardiac events.11 A meta-analysis of mitral valve interventions found an increased rate of mitral annular calcification in chronic lung disease patients, which increased risk of mortality in PMVR.12 Our study showed no increased in-hospital mortality risk for COPD patients compared to their propensity-matched cohorts for PMVR but did show increased risk in other clinically relevant endpoints. These findings are consistent with other observational data looking specifically at pulmonary comorbidities for this procedure.13,14 Additionally, we found that in-hospital mortality risk decreased from 2012 to 2016 which speaks to improvements in patient selection and operator skill levels.
Although we saw no significant differences in in-hospital mortality, the sample with COPD had higher rates of other in-hospital morbidities including acute coronary syndrome, cardiogenic shock, respiratory failure requiring mechanical ventilation, and septic shock. They also had longer and higher cost of hospitalizations; as well as, required more post-hospital rehabilitation and skilled nursing home placement. These findings should not come as a surprise. COPD in patients undergoing cardiothoracic surgery is a known risk factor for similar poor outcomes and may lead to respiratory failure in up to 22% of patients.15–17 Risk stratification tools, such as the Society of Thoracic Surgeons risk score, for other mitral valve interventions account for COPD. Still, the major clinical trials for PMVR did not even report subgroup analysis data for COPD patients.3,18,19
Our study highlights the importance of COPD management and planning in the setting of PMVR utilization trends. Following the positive results of the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) trial, the usage of PMVR in heart failure patients will continue to increase.3,20 Monitoring for COPD in this population will be clinically imperative. COPD is an independent predictor of mortality and increased hospitalizations in heart failure patients. Furthermore, spirometry is not considered as accurate in these patients, which leads to underdiagnosis.21–23 Recognizing recent treatment of COPD may also be important. Our study found an increased risk of infection causing septic shock, which may be due to periodic oral and inhaled steroid use. One study found these patients to be at a significantly increased septic risk up to 5 months following oral steroid therapy.24
Our study has several limitations that need to be addressed. NIS is an administrative claim-based database that uses ICD-9-CM and ICD-10-CM codes for diagnosis, although we have used procedure codes that are less prone to error, it may be subject to error. NIS collects data on in-patient discharges and each admission is registered as an independent event. NIS samples are not designed to follow patients longitudinally, so long-term outcomes could not be assessed from the present dataset. This limitation may be considerable for post procedural outcomes as follow-up data from transcutaneous aortic valve replacement patients showed pulmonary disease to be an significant predictor of mortality at 1 year, a trend that was not seen at 30 days.6,10 Although a similar trend may be present in PMVR patients, our study was not able to assess for it due to the use of this particular registry. Lastly, our retrospective database study demonstrates only associations and cannot conclusively show causation. Although the negative implications of COPD in patients undergoing PMVR have physiological reason, our data are not as strong as controlled trials.
5 |. CONCLUSIONS
In the current analysis from the NIS database comparing outcomes of PMVR in patients with COPD versus no-COPD, there was no difference in mortality between the two groups. Moreover, we observed higher rates of in-hospital morbidities, surrogates of severe disability and higher resources utilization by the COPD group.
Supplementary Material
Abbreviations:
- AHRQ
agency for healthcare research and quality
- COPD
chronic obstructive pulmonary disease
- HCUP
healthcare cost and utilization project
- ICD-10-CM
international classification of diseases, 10th revision, clinical modification
- ICD-9-CM
international classification of diseases, 9th revision, clinical modification
- MR
mitral regurgitation
- NSTEMI
non-st-segment elevation myocardial infarction
- PMVR
percutaneous mitral valve repair
- STEMI
st-segment elevation myocardial infarction
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. [DOI] [PubMed] [Google Scholar]
- 2.Kar S, Feldman T, Qasim A, et al. Five-year outcomes of transcatheter reduction of significant mitral regurgitation in high-surgical-risk patients. Heart. 2019;105(21):1622–1628. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379 (24):2307–2318. [DOI] [PubMed] [Google Scholar]
- 4.Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28(11):1358–1365. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien SM, Shahian DM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg. 2009;88(1 Suppl):S23–S42. [DOI] [PubMed] [Google Scholar]
- 6.Crestanello JA, Popma JJ, Adams DH, et al. Long-term health benefit of Transcatheter aortic valve replacement in patients with chronic lung disease. JACC Cardiovasc Interv. 2017;10(22):2283–2293. [DOI] [PubMed] [Google Scholar]
- 7.Ponomarev D, Kamenskaya O, Klinkova A, et al. Chronic lung disease and mortality after cardiac surgery: a prospective cohort study. J Cardiothorac Vasc Anesth. 2018;32(5):2241–2245. [DOI] [PubMed] [Google Scholar]
- 8.Roversi S, Fabbri LM, Sin DD, Hawkins NM, Agusti A. Chronic obstructive pulmonary disease and cardiac diseases. An urgent need for integrated care. Am J Respir Crit Care Med. 2016;194(11):1319–1336. [DOI] [PubMed] [Google Scholar]
- 9.Bundhun PK, Gupta C, Xu GM. Major adverse cardiac events and mortality in chronic obstructive pulmonary disease following percutaneous coronary intervention: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2017;17(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes DR Jr, Brennan JM, Rumsfeld JS, et al. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015; 313(10):1019–1028. [DOI] [PubMed] [Google Scholar]
- 11.Paranskaya L, D’Ancona G, Bozdag-Turan I, et al. Residual mitral valve regurgitation after percutaneous mitral valve repair with the MitraClip(R) system is a risk factor for adverse one-year outcome. Catheter Cardiovasc Interv. 2013;81(4):609–617. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro RVP, Yanagawa B, Legare JF, et al. Clinical outcomes of mitral valve intervention in patients with mitral annular calcification: a systematic review and meta-analysis. J Card Surg. 2020;35(1):66–74. [DOI] [PubMed] [Google Scholar]
- 13.Zuern CS, Bauer A, Lubos E, et al. Influence of non-cardiac comorbidities on outcome after percutaneous mitral valve repair: results from the German transcatheter mitral valve interventions (TRAMI) registry. Clin Res Cardiol. 2015;104(12):1044–1053. [DOI] [PubMed] [Google Scholar]
- 14.Tigges E, Blankenberg S, von Bardeleben RS, et al. Implication of pulmonary hypertension in patients undergoing MitraClip therapy: results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail. 2018;20(3):585–594. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov A, Yossef J, Tailon J, et al. Do pulmonary function tests improve risk stratification before cardiothoracic surgery? J Thorac Cardiovasc Surg. 2016;151(4):1183–1189. e3. [DOI] [PubMed] [Google Scholar]
- 16.Saleh HZ, Mohan K, Shaw M, et al. Impact of chronic obstructive pulmonary disease severity on surgical outcomes in patients undergoing non-emergent coronary artery bypass grafting. Eur J Cardiothorac Surg. 2012;42(1):108–113. discussion 13. [DOI] [PubMed] [Google Scholar]
- 17.Trouillet JL, Combes A, Vaissier E, et al. Prolonged mechanical ventilation after cardiac surgery: outcome and predictors. J Thorac Cardiovasc Surg. 2009;138(4):948–953. [DOI] [PubMed] [Google Scholar]
- 18.Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364(15):1395–1406. [DOI] [PubMed] [Google Scholar]
- 19.Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379(24):2297–2306. [DOI] [PubMed] [Google Scholar]
- 20.Panaich SS, Arora S, Badheka A, et al. Procedural trends, outcomes, and readmission rates pre-and post-FDA approval for MitraClip from the National Readmission Database (2013–14). Catheter Cardiovasc Interv. 2018;91(6):1171–1181. [DOI] [PubMed] [Google Scholar]
- 21.Kohli P, Staziaki PV, Janjua SA, et al. The effect of emphysema on readmission and survival among smokers with heart failure. PLoS One. 2018;13(7):e0201376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, McMurray JJ. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11 (2):130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Light RW, George RB. Serial pulmonary function in patients with acute heart failure. Arch Intern Med. 1983;143(3):429–433. [PubMed] [Google Scholar]
- 24.Ernst P, Coulombe J, Brassard P, Suissa S. The risk of sepsis with inhaled and Oral corticosteroids in patients with COPD. COPD. 2017; 14(2):137–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


