Abstract
Objective:
To quantitatively examine the association between gestational weight gain (GWG) and risk of autism spectrum disorder (ASD) in offspring.
Methods:
Electronic databases were searched for studies of excessive or inadequate GWG, as compared to recommended GWG, in relation to the risk of ASD in offspring. Measures of the association from primary studies were pooled using a meta-analytic approach and expressed as weighted odds ratios (ORs) with 95% confidence intervals (95% CIs).
Results:
Nine studies were identified, including 323,253 participants with 4,135 cases of ASD from five cohort studies, and 1,462 cases and 3,265 controls from four case-control studies. Evidence from cohort studies indicates that both excessive and inadequate GWG were significantly associated with a higher risk of ASD in offspring. The pooled OR of ASD was 1.10 (95% CI: 1.02–1.18) for excessive GWG and 1.13 (95% CI: 1.04–1.24) for inadequate GWG using recommended GWG as the reference. Evidence from case-control studies suggests that excessive GWG (1.38 [95% CI: 1.19–1.62]), but not inadequate GWG (0.87 [95% CI: 0.72–1.04]) was significantly associated with a higher risk of ASD.
Conclusions:
The accumulated evidence supports that gaining weight outside the recommended GWG is associated with a higher risk of ASD in offspring.
Keywords: gestational weight gain, pregnancy, weight gain
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder commenced from early childhood that is manifested by persistent social interaction deficits in parallel with repetitive and stereotypic activities (1). ASD is a life-long disorder. Most of the children with ASD may not work full-time or even live independently after they reach adulthood, which may bring substantial economic burden to their families, healthcare system, and society (2). Since ASD is estimated to affect approximately 1% of people globally (3), identifying modifiable risk factors for ASD is of great public health significance.
Gestational weight gain (GWG) is one of the few modifiable factors related to maternal and neonatal health outcomes (4). However, many women neither received consistent advice on GWG from healthcare providers nor realized the risks of inappropriate GWG to maternal and fetal health (5, 6). According to a recent review of more than 1 million women worldwide, approximately 47% of pregnant women gained excessive weight, and 23% of them gained inadequate weight during pregnancy (4). Both excessive and inadequate GWG are indicators of suboptimal nutritional and metabolic status, and can lead to an unfavorable intrauterine environment for the development of the fetus (7). It has been suggested that gaining gestational weight outside the current recommended guidelines may play a critical role in triggering the manifestations of ASD phenotypes in predisposing individuals during the prenatal period. However, existing studies have provided inconsistent evidence. Some reported a moderate association between excessive GWG and ASD risk in offspring, but some others found no association (8–16). Findings for inadequate GWG are even more controversial (8–12, 14–16).
A recent meta-analysis summarized data from two cohort studies and three case-control studies on this topic (17), however, this meta-analysis missed three large cohort studies and one case-control study. Of note, the authors pooled results from cohort and case-control studies together due to the limited number of primary studies, which is not an ideal analytic strategy and may lead to biased results. In addition, the data from one cohort study (9) used a different reference group for excessive and inadequate weight gain, which might bias the pooled analysis. Therefore, we conducted this meta-analysis that includes de novo results from the primary studies to provide the most comprehensive data to the literature.
Methods
Study selection
This meta-analysis was conducted based on the criteria of Preferred Reporting Items for Systematic Reviews and Meta Analyses statement (Supporting Information Table S1). Studies that examined the association between GWG and ASD risk in offspring and published before March 19, 2020, were identified in PubMed and Embase databases. The literature search was based on the following terms: (maternal OR gestational OR prenatal OR pregnant OR pregnancy) AND (weight gain OR weight growth OR weight change) AND (autistic OR autism OR autism spectrum disorder OR ASD OR Asperger). Bibliographies of relevant studies and Google Scholar were searched for additional eligible studies. Published studies with English or Chinese languages were reviewed.
Two independent investigators (L.S. and C.C.) reviewed the titles, abstracts, and full-texts of potentially eligible studies. Disagreements were resolved by group discussion. Studies were considered eligible if they met the following inclusion criteria: 1) cohort, case-control, or cross-sectional studies that investigated excessive GWG or inadequate GWG in relation to risk of ASD in offspring, compared with recommended GWG; and 2) the relative risk (RR), hazard ratio (HR), or odds ratio (OR) with corresponding 95% confidence intervals were reported, or such information can be derived from published results.
The detailed study selection process is shown in Figure 1. From 206 non-duplicate articles identified from the databases, 93 irrelevant studies, 53 animal studies, and 23 non-original studies (reviews or letter-to-editors) were excluded. After reviewing the full-texts, 29 articles were further excluded because the association of interest was not reported (n=26), or the available data could not be combined with other studies (n=3). In order to appropriately combine data from the primary studies, we requested for de novo results from three studies (12, 18, 19), and obtained the new results from Xiang et al.(12). Therefore, 9 studies (5 cohort and 4 case-control studies) met the inclusion criteria and were included in the present meta-analysis.
Figure 1.

Flow diagram of the study selection process
Quality assessment
A modified Newcastle-Ottawa Scale (NOS) was used to assess the quality of the included studies (20). All primary studies scored equal to or above 5 out of 7. The mean score of the five cohort studies was 5.7 out of 7, while the mean score of the four case-control studies was 5.9 out of 7. Thus, the identified studies were considered to have medium to high quality and thus were included in the current meta-analysis (Supporting Information Table S2–5).
Data extraction
Information on the primary studies was extracted by L.S. and verified by C.C. independently. By using a standardized data extraction form, the following information was retrieved: the first author’s last name and published year, the region of study, number of cases and participants (or number of cases and controls in case-control studies), offspring age, study period (for cohort studies), GWG assessment method, ASD confirmation, guidelines of GWG, risk estimates compared with the recommended GWG, and adjusted covariates.
Eight included studies (8, 10–16) categorized GWG based on the 2009 Institute of Medicine (IOM) guidelines, which were endorsed by the American College of Obstetricians and Gynecologists (21). These studies reported the multivariable-adjusted ORs or HRs that compared excessive or inadequate GWG to the recommended GWG. In Dodds et al. (9), the authors used the 1990 IOM guidelines and calculated the multivariable-adjusted RR of excessive GWG (GWG ≥18 kilograms) by comparing them to the rest participants including both inadequate GWG (GWG < 7 kilograms) and recommended GWG, and vice versa for the multivariable-adjusted RR of inadequate GWG. Since the results presented in Dodds et al. (9) cannot be directly combined with that of other primary studies, we calculated the crude ORs of excessive and inadequate GWG as compared to the recommended GWG based on the available data presented in the study.
Statistical analysis
HR was considered equivalent to OR in this meta-analysis. OR and HR were transformed into their natural logarithms (ln) and the corresponding 95% CIs were used to calculate their standard errors. The association between inadequate or excessive GWG, compared to recommended GWG, and risk of ASD in offspring were expressed as weighted ORs in cohort and case-control studies. Fixed-effect models were used in all analyses since heterogeneities across studies were found to be low-to-moderate when evaluated using I2 statistics along with Cochran’s Q test. Random-effects models were used in a sensitivity analysis. In addition, the influence of each study on the overall association was assessed by excluding one study each time. Publication bias was assessed by using Begger’s regression asymmetry test. A two-sided P value ≤0.05 was considered statistically significant. All analyses were conducted with STATA software (Version 16.0, STATA Corporation LP, College Station, TX).
Results
Study characteristics
Nine studies were included in this meta-analysis: 323,253 participants with 4,135 ASD cases in five cohort studies (8–12), and 1,462 cases and 3,265 controls in four case-control studies (13–16). One study was conducted in Europe (10), four in North America (8, 9, 12, 16), and the rest four in Asia (11, 13–15). Other characteristics of the study populations are shown in Table 1 and 2.
Table 1.
Characteristics of the cohort studies
| Author (year) | Region | Cases/participants | Offspring age | Study period | GWG assessment | ASD confirmation | GWG guidelines | Risk estimates compared with recommended GWG (referent) | Adjusted covariates | |
|---|---|---|---|---|---|---|---|---|---|---|
| Excessive GWG | Inadequate GWG | |||||||||
| Brumbaugh (2020) | USA | 266/7,876 | Median, 6.6 yearsa (Interquartile range, 4.3–9.2 years) | 1994–2000 | Birth certificate data | Research-identified ASD cases based on DSM-IV | Modified 2009 IOM guidelines: reference GWG is 11–41 lbs for singleton gestation; 25–55 lbs for multiple gestation | HR (95% CI): 0.94 (0.63–1.42) | HR (95% CI): 1.50 (0.86–2.63) | History of maternal psychiatric disorder, sex of infant, gestational age, size for gestational age, Apgar at 5 min, maternal age, maternal unmarried status, maternal education level, tobacco use during pregnancy, parity, mode of delivery |
| Sun (2016) | China | 290/3,663 | 51.5±5.58 Months | 2008–2010 | Self-report | SAT ascertained by CABS | 2009 IOM guidelines | OR (95% CI): 0.95 (0.70–1.28) | OR (95% CI): 1.54 (1.10–2.18) | Maternal education level and vomiting in the first trimester |
| Gardner (2015) | Sweden | 1,947/113,469 | 4–27 years | 1984–2007 | Medical records | ASD ascertained by ICD-9, ICD-10, DSM-IV | 2009 IOM guidelines | OR (95% CI): 1.12 (1.10–1.25) | OR (95% CI): 1.17 (1.04–1.31) | Maternal BMI, gestational age at birth, gender of child, birth year of the child, maternal age, parity, paternal age, maternal country of birth, socioeconomic status factors, and parental history of psychiatric treatment |
| Xiang (2015)b | USA | 708/68,512 | Median, 4.0 years (Interquartile range, 3.2–5.0 years) | 1995–2009 | Medical records | ASD ascertained by ICD-9 | 2009 IOM guidelines | HR (95% CI): 0.95 (0.80–1.13) | HR (95% CI): 0.87 (0.71–1.07) | Pre-pregnancy BMI, birth year, maternal age, parity, education, household income, race/ethnicity, history of comorbidity other than diabetes, smoking during pregnancy, preeclampsia/eclampsia, diabetes status, sex of child |
| Dodds (2011)c | Canada | 924/129,733 | 1–17 years | 1990–2002 | Medical records | ASD ascertained by ICD-9, ICD-10 | Modified 1990 IOM guidelines: referent GWG is 7–18 kg | OR (95% CI): 1.26 (1.08–1.48) | OR (95% CI): 1.16 (0.90–1.49) | None |
Abbreviations: 95% CI, 95% confidence interval; ASD, Autism spectrum disorder; BMI, body mass index; CABS, Clancy Autism Behavior Scale; DSM, Diagnostic and Statistical Manual of Mental Disorders; GWG, gestational weight gain; ICD, International Classification of Diseases; IOM, Institute of Medicine; OR, odds ratio; RR, relative risk; SAT, sub-threshold autism trait.
Median age of ASD-R cases are reported.
De nova results offered by Xiang et al.
Calculated crude results from data in published paper.
Table 2.
Characteristics of the case-control studies
| Author (year) | Region | Cases/controls | Offspring agea, y | GWG assessment | ASD confirmation | GWG guidelines | Risk estimates compared with recommended GWG (referent) | Adjusted covariates | |
|---|---|---|---|---|---|---|---|---|---|
| Excessive GWG | Inadequate GWG | ||||||||
| Qiu (2018) | China | 36/72 | 1.5–5 | Self-report | ASD ascertained by DSM-IV | 2009 IOM guidelines | OR (95% CI): 1.11 (0.36–3.27) | OR (95% CI): 0.73 (0.29–1.80) | Parents with overweight/obesity, maternal age, birth weight, gestational age, and folic acid supplementation |
| Shen (2018) | China | 705/2,236 | 2–9 | Self-report | ASD ascertained by DSM-IV | Modified 2009 IOM guidelines for Chinese population | OR (95% CI): 1.33 (1.02–1.73) | OR (95% CI): 0.86 (0.69–1.06) | Gender of child, age of child, parental age, and family annual income |
| Windham (2018) | USA | 540/776 | 2–5 | Self-report | ASD identified by previous diagnosis; with a SCQ score of 11 or higher; ascertained by clinician during inperson assessment | 2009 IOM guidelines | OR (95% CI): 1.29 (1.00–1.66) | OR (95% CI): 0.92 (0.63–1.34) | Maternal age, parity, race/ethnicity, and smoking |
| Ling (2015) | China | 181/181 | 1–5 | Self-report confirmed by hospital data monitor system or prenatal health handbook | ASD ascertained by DSM-IV | 2009 IOM guidelines | OR (95% CI): 1.64 (1.21–2.21) | Not reported | Maternal education level, maternal age, number of births, threatened miscarriage, prenatal nutritional status, history of diseases, and drug use. |
Abbreviations: 95% CI, 95% confidence interval; ASD, Autism spectrum disorder; BMI, body mass index; CABS, Clancy Autism Behavior Scale; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders; GWG, gestational weight gain; ICD, International Classification of Diseases; IOM, Institute of Medicine OR, odds ratio; SCQ, Social communication questionnaire.
Range of age was reported.
Excessive GWG with ASD risk
Excessive GWG was significantly associated with a higher risk of ASD in offspring (Figure 2). Compared with the children whose mothers’ weight gain during pregnancy within the recommended range, the pooled OR of ASD for children whose mothers had excessive GWG was 1.10 (95% CI: 1.02–1.18) in cohort studies and 1.38 (95% CI: 1.19–1.62) in case-control studies. The heterogeneity was moderate (I2=44.5%, p=0.13) in cohort studies and low (I2=0.0%, p=0.62) in case-control studies. Sensitivity analysis indicated that excluding the study of Gardner et al. (10) (pooled OR: 1.08, 95% CI: 0.97–1.19) or the study of Dodds et al. (9) (pooled OR: 1.05, 95% CI: 0.97–1.15) slightly attenuated the overall association in cohort studies. Using the random-effects model, the combined association (pooled OR: 1.08, 95% CI: 0.96–1.21) in cohort studies was also attenuated. The association in case-control studies did not appreciably change by using the random-effects model (pooled OR: 1.38, 95% CI: 1.19–1.62). In addition, evidence on publication bias was not found in either cohort studies (p=0.62) or case-control studies (p=0.50).
Figure 2. Meta-analysis of excessive gestational weight gain in relation to the risk of autism spectrum disorder in offspring.

Abbreviations: GWG, gestational weight gain; IOM, Institute of Medicine; OR, odds ratio; 95% CI, 95% confidence interval.
Inadequate GWG with ASD risk
In comparison with the children whose mothers had recommended GWG, the pooled OR of ASD for children whose mothers had inadequate GWG was 1.13 (95% CI: 1.04–1.24) in cohort studies (Figure 3). Of note, this association was not seen when combining case-control studies (pooled OR: 0.87, 95% CI: 0.72–1.04). The heterogeneity was medium in cohort (I2=62.8%, p=0.03) and low in case-control studies (I2=0.0%, p=0.89). Sensitivity analysis indicated that excluding the study of Gardner et al. (10) attenuated the association (pooled OR: 1.08, 95% CI: 0.94–1.24) in cohort studies. Using the random-effects model, the pooled point estimated in cohort studies (pooled OR: 1.16, 95% CI: 0.97–1.38) was higher, but 95% confidence interval was wider. The pooled OR was not appreciably changed in case-control studies (pooled OR: 0.87, 95% CI: 0.72–1.04) using the random-effects model. Evidence of publication bias was not observed in either cohort (p=0.33) or case-control studies (p=0.60).
Figure 3. Meta-analysis of inadequate gestational weight gain in relation to the risk of autism spectrum disorder in offspring.
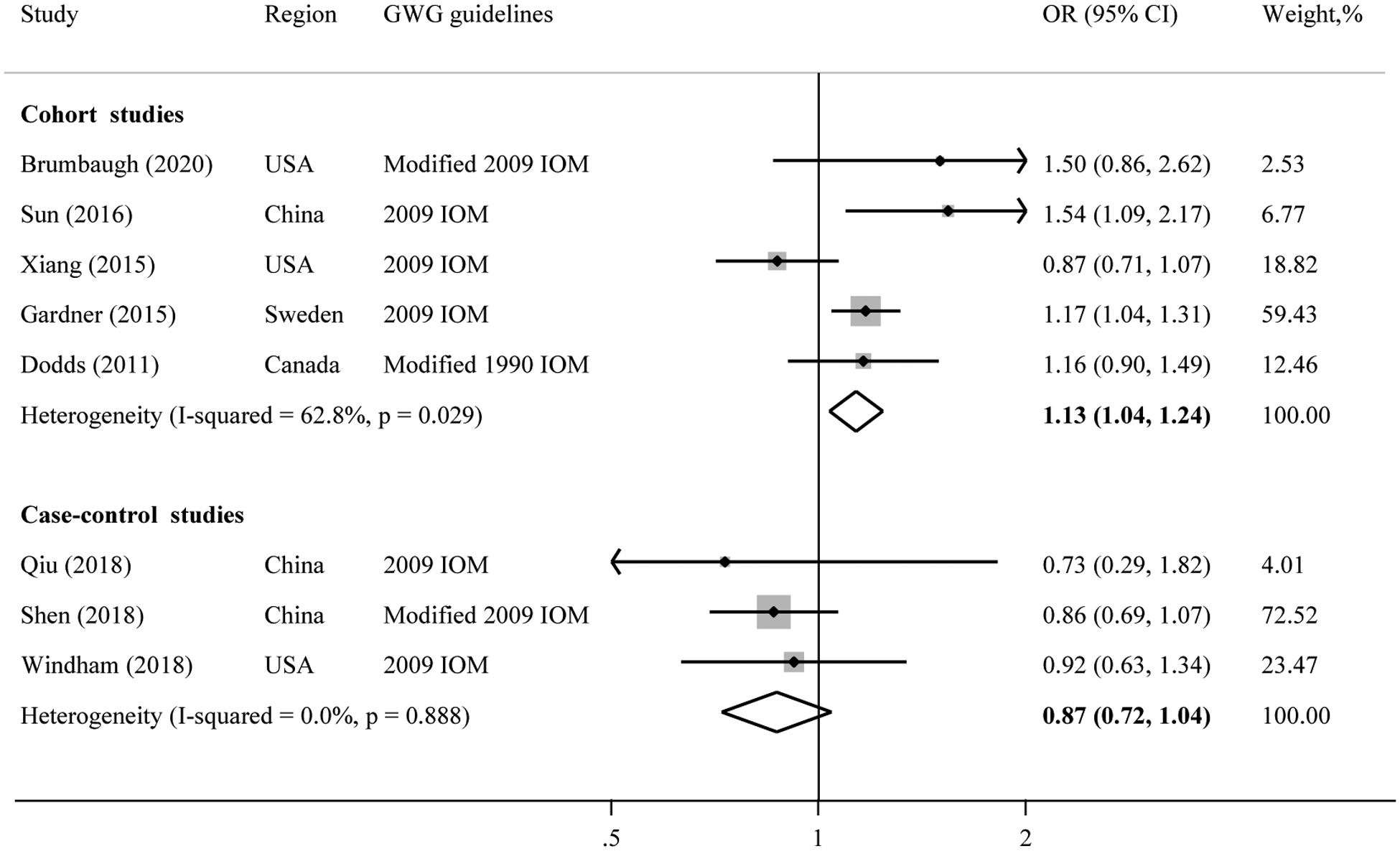
Abbreviations: GWG, gestational weight gain; IOM, Institute of Medicine; OR, odds ratio; 95% CI, 95% confidence interval.
Discussion
This meta-analysis indicates a higher risk of ASD in offspring if pregnant women’s GWG are outside the recommended ranges. The association for inadequate GWG is not consistently seen in cohort and case-control studies.
Maternal weight status may influence the neurodevelopment of offspring. A growing number of studies have investigated the relationship between maternal obesity and ASD in offspring (22). However, previous studies mainly focused on maternal BMI at the first antenatal visit as a proxy of pre-pregnancy maternal weight status (23, 24). Recently, GWG was suggested to be a more important risk factor for ASD compared to pre-pregnancy obesity (10, 15, 18). In a large cohort study with 17,860 mothers and 333,057 children, Gardner et al. (10) reported that both pre-pregnancy obesity and GWG were significantly associated with increased risk of ASD in offspring. However, in their matched-siblings analysis, which is less likely to be affected by confounding from genetic susceptibility and living environment, the association with pre-pregnancy obesity was attenuated to non-significance, while the association with GWG remained. Similarly, two case-control studies found a modest association between excessive GWG and ASD risk in offspring, but no association with pre-pregnancy BMI (15, 18). These studies suggest that, compared to pre-pregnancy obesity, GWG may play more critical roles in the development of ASD in offspring or serve as a reliable indicator for this disorder.
Although the underlying mechanisms by which GWG affects neurodevelopment of offspring are still unclear, it has been suggested that excessive GWG may induce disturbed blood leptin signaling in offspring, which may consequently lead to adverse neurobiological conditions (25). Leptin is a proinflammatory cytokine produced by adipose tissue and placenta, and its receptors are widely expressed in brain regions that are related to behavior regulation (26). A proper level of leptin has neurotrophic effects and stimulates neuronal differentiation during fetal neurodevelopment (27). Previously, a positive correlation between GWG and blood leptin levels in pregnant women has been observed (28, 29). Neonates whose mothers gained excessive GWG had significantly elevated leptin levels in their cord blood (30). In addition, a few cohort and case-control studies found that leptin levels were associated with a higher risk of ASD (31–33). Therefore, the observed association between excessive GWG and ASD risk may be partially explained by the dysregulation of leptin signaling during the fetal neurodevelopment period.
How inadequate GWG may affect the neurodevelopment of children is rarely studied. Inadequate GWG may be caused by pregnancy complications such as anorexia nervosa, hyperemesis gravidarum, and malabsorption. It may be considered as a marker of suboptimal nutritional status of the fetus, and nutrient deficiencies have been linked to poor neurodevelopment and ASD risk in children (34, 35). In animal models, maternal protein malnutrition in rats can induce autism-like symptoms in their offspring (36). Although a smaller proportion of pregnant women has inadequate GWG compared to excessive GWG, it has been suggested that women with insufficient GWG benefit more from interventions during pregnancy (37).
Of note, the 2009 IOM guidelines for GWG was designed for the American population (21), and thus may not be suitable for Asian women, similar to the definition of metabolic syndrome and obesity (38, 39). Misclassification of GWG is possible in Asian populations using IOM guidelines, and may lead to biased results towards the null in the primary studies. We performed a stratified analysis by study regions (Supporting Information Figure S1, S2). The association of excessive GWG with ASD was generally consistent when comparing Western to Asian studies. However, the association of inadequate GWG with ASD persisted in Western, but was not seen in Asian studies. A modified recommendation of GWG for Asian populations merits further investigation.
Some limitations need to be acknowledged. First, a strong association between inadequate GWG and the risk of ASD in offspring was not consistently seen when we examined cohort studies and case-control studies separately. The inherent limitations of case-control study design, e.g., potential selection and/or recall bias may be one explanation for this inconsistency. Second, the association found in this meta-analysis was slighted attenuated in our sensitivity analyses using the “leave-one-out” approach or the random-effects models. The attenuated association in “leave-one-out” sensitivity analysis may be due to the fact that some studies have substantially larger sample sizes than others, and excluding them may change the overall results. Because of the low-to-moderate heterogeneities tested across primary studies, we chose to use the random-effects models only in the sensitivity analysis. There is also little evidence that the underlying mechanism between GWG and ASD in offspring vary across different populations. The point estimates of ORs in random-effects models were similar or slightly higher than the fixed-effect models, but the confidence intervals were wider, suggesting a lower statistical power possibly due to the limited number of primary studies. Third, GWG was self-reported in the majority of the primary studies. GWG is better directly measured and collected by trained study personnel. Self-reported GWG is prone to measurement error, though it is commonly used in the previous studies (40). However, one study that examined the correlation between self-reported and clinically measured GWG found that the proportions of women that gained excessive, recommended, and inadequate GWG were essentially identical using two approaches (41). Fourth, the definitions of ASD cases were not completely identical across the primary studies, which might cause misclassification in ASD. Clinically diagnosed ASD was examined in seven studies with different diagnostic assessments including ICD-9, ICD-10, and DSM-IV (9, 10, 12–16). One study investigated research-identified ASD (ASD-R) by the epidemiological approach (8). Another study determined sub-threshold autism traits (SAT), which is the broader phenotype of autism that not yet meets the clinical diagnosis of ASD (11). However, when we excluded these two studies in a sensitivity analysis, the pooled association remained. Fifth, covariates’ adjustments were different across primary studies. Important confounders, such as genetic susceptibility indicated by maternal psychiatric disorders, were considered in only two studies (8, 10). Thus, the possibility of confounding from unmeasured or unknown factors in primary studies cannot be completely ruled out. Finally, the association between GWG and ASD may differ depending on weight status. For example, Shen et al. (15) found that women with overweight or obesity who gain excessive GWG had a higher risk of ASD in offspring than normal-weighted women with excessive GWG. However, most of the primary studies did not report BMI-stratified results. We were not able to explore the potential effect modification by pre-pregnancy weight status due to limited data. Thus, future prospective studies with large sample sizes, using clear ASD diagnostic criteria and reporting the association between GWG and ASD risk in offspring stratified by BMI categories are needed.
Conclusion
This meta-analysis combines the most comprehensive literature and suggests that gaining gestational weight outside the current recommended guidelines is associated with a higher risk of ASD in offspring. Women are generally highly motivated to improve diet and lifestyle during pregnancy for the sake of babies. Thus, promoting optimal GWG could be a cost-effective preventive strategy for neurodevelopment disorders such as ASD.
Supplementary Material
Study importance questions.
What is already known about this subject?
More than half of pregnant women gained weight outside the recommended guidelines worldwide.
Existing studies provided inconsistent evidence for the association between suboptimal gestational weight gain and autism spectrum disorder.
What are the new findings in your manuscript?
This is an updated meta-analysis regarding the association between gestational weight gain and risk of autism spectrum disorder in offspring.
Gaining gestational weight outside of the current recommended guidelines, including both excessive and inadequate gestational weight gain, is associated with a higher risk of autism spectrum disorder in offspring.
Funding:
This study is partially supported by the National Institutes of Health (NIH) grants (R01DK116603 and R01AG056111).
Footnotes
Disclosure: The authors declared no conflict of interest.
Reference
- 1.Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. The Lancet. 2018;392(10146):508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavelle TA, Weinstein MC, Newhouse JP, Munir K, Kuhlthau KA, Prosser LA. Economic burden of childhood autism spectrum disorders. Pediatrics. 2014;133(3):e520–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychological medicine. 2015;45(3):601–13. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. Jama. 2017;317(21):2207–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanstone M, Kandasamy S, Giacomini M, DeJean D, McDonald SD. Pregnant women’s perceptions of gestational weight gain: A systematic review and meta synthesis of qualitative research. Maternal & child nutrition. 2017;13(4):e12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deputy NP, Sharma AJ, Kim SY, Olson CK. Achieving appropriate gestational weight gain: the role of healthcare provider advice. Journal of Women’s Health. 2018;27(5):552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kominiarek MA, Peaceman AM. Gestational weight gain. American journal of obstetrics and gynecology. 2017;217(6):642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brumbaugh JE, Weaver AL, Myers SM, Voigt RG, Katusic SK. Gestational Age, Perinatal Characteristics, and Autism Spectrum Disorder: A Birth Cohort Study. The Journal of Pediatrics. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. Journal of autism and developmental disorders. 2011;41(7):891–902. [DOI] [PubMed] [Google Scholar]
- 10.Gardner RM, Lee BK, Magnusson C, Rai D, Frisell T, Karlsson H, et al. Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: Results from a Swedish total population and discordant sibling study. International journal of epidemiology. 2015;44(3):870–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y. Progestational and Gestational Risk Factors of Sub-threshold Autism Trait in Pre-school Children:A Birth Cohort Study [Master’s thesis]: Anhui Medical University; 2016. [Google Scholar]
- 12.Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, et al. Association of maternal diabetes with autism in offspring. Jama. 2015;313(14):1425–34. [DOI] [PubMed] [Google Scholar]
- 13.Ling Z, Wang J, Li X, Zhong Y, Qin Y, Xie S, et al. Association between mothers’ body mass index before pregnancy or weight gain during pregnancy and autism in children. Zhonghua liu xing bing xue za zhi= Zhonghua liuxingbingxue zazhi. 2015;36(9):949–52. [PubMed] [Google Scholar]
- 14.Qiu T, Guo BB, Wang LZ, Zhang H, Xu Y, Jiang XY. Association between overweight/obesity in parents and autism spectrum disorders in offspring. Zhongguo dang dai er ke za zhi= Chinese journal of contemporary pediatrics. 2018;20(5):383–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Y, Dong H, Lu X, Lian N, Xun G, Shi L, et al. Associations among maternal pre-pregnancy body mass index, gestational weight gain and risk of autism in the Han Chinese population. BMC psychiatry. 2018;18(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Windham GC, Anderson M, Lyall K, Daniels JL, Kral TVE, Croen LA, et al. Maternal Pre-pregnancy Body Mass Index and Gestational Weight Gain in Relation to Autism Spectrum Disorder and other Developmental Disorders in Offspring. Autism Research. 2019;12(2):316–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian Z-X, Wan M, Gao Y-L, Wu B-F, Xie Y, Liu J, et al. Gestational weight gain and risk of autism spectrum disorders in offspring: a systematic review and meta-analysis. Journal of Obstetrics and Gynaecology. 2019:1–8. [DOI] [PubMed] [Google Scholar]
- 18.Bilder DA, Bakian AV, Viskochil J, Clark EAS, Botts EL, Smith KR, et al. Maternal prenatal weight gain and autism spectrum disorders. Pediatrics. 2013;132(5):e1276–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can. 2010;30(4):125–34. [PubMed] [Google Scholar]
- 20.Stang A Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 21.National Research C Weight gain during pregnancy: reexamining the guidelines: National Academies Press; 2010. [PubMed] [Google Scholar]
- 22.Li Y-M, Ou J-J, Liu L, Zhang D, Zhao J-P, Tang S-Y. Association between maternal obesity and autism spectrum disorder in offspring: a meta-analysis. Journal of autism and developmental disorders. 2016;46(1):95–102. [DOI] [PubMed] [Google Scholar]
- 23.Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129(5):e1121–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyall K, Pauls DL, Santangelo S, Spiegelman D, Ascherio A. Maternal early life factors associated with hormone levels and the risk of having a child with an autism spectrum disorder in the nurses health study II. Journal of autism and developmental disorders. 2011;41(5):618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tessier DR, Ferraro ZM, Gruslin A. Role of leptin in pregnancy: consequences of maternal obesity. Placenta. 2013;34(3):205–11. [DOI] [PubMed] [Google Scholar]
- 26.Valleau JC, Sullivan EL. The impact of leptin on perinatal development and psychopathology. Journal of chemical neuroanatomy. 2014;61:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desai M, Li T, Ross MG. Fetal hypothalamic neuroprogenitor cell culture: preferential differentiation paths induced by leptin and insulin. Endocrinology. 2011;152(8):3192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perichart-Perera O, Muñoz-Manrique C, Reyes-López A, Tolentino-Dolores M, y Sosa SE, Ramírez-González MC. Metabolic markers during pregnancy and their association with maternal and newborn weight status. PLoS One. 2017;12(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacroix M, Battista M-C, Doyon M, Moreau J, Patenaude J, Guillemette L, et al. Higher maternal leptin levels at second trimester are associated with subsequent greater gestational weight gain in late pregnancy. BMC pregnancy and childbirth. 2016;16(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logan CA, Bornemann R, Koenig W, Reister F, Walter V, Fantuzzi G, et al. Gestational Weight Gain and Fetal-Maternal Adiponectin, Leptin, and CRP: results of two birth cohorts studies. Scientific reports. 2017;7(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashwood P, Kwong C, Hansen R, Hertz-Picciotto I, Croen L, Krakowiak P, et al. Brief report: plasma leptin levels are elevated in autism: association with early onset phenotype? Journal of autism and developmental disorders. 2008;38(1):169–75. [DOI] [PubMed] [Google Scholar]
- 32.Blardi P, de Lalla A, Ceccatelli L, Vanessa G, Auteri A, Hayek J. Variations of plasma leptin and adiponectin levels in autistic patients. Neuroscience letters. 2010;479(1):54–7. [DOI] [PubMed] [Google Scholar]
- 33.Raghavan R, Zuckerman B, Hong X, Wang G, Ji Y, Paige D, et al. Fetal and infancy growth pattern, cord and early childhood plasma leptin, and development of autism spectrum disorder in the Boston birth cohort. Autism Research. 2018;11(10):1416–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeVilbiss EA, Gardner RM, Newschaffer CJ, Lee BK. Maternal folate status as a risk factor for autism spectrum disorders: a review of existing evidence. British Journal of Nutrition. 2015;114(5):663–72. [DOI] [PubMed] [Google Scholar]
- 35.Madore C, Leyrolle Q, Lacabanne C, Benmamar-Badel A, Joffre C, Nadjar A, et al. Neuroinflammation in autism: plausible role of maternal inflammation, dietary omega 3, and microbiota. Neural plasticity. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batista TH, Giusti-Paiva A, Vilela FC. Maternal protein malnutrition induces autism-like symptoms in rat offspring. Nutritional neuroscience. 2019;22(9):655–63. [DOI] [PubMed] [Google Scholar]
- 37.Brawarsky P, Stotland NE, Jackson RA, Fuentes Afflick E, Escobar GJ, Rubashkin N, et al. Pre-pregnancy and pregnancy-related factors and the risk of excessive or inadequate gestational weight gain. International Journal of Gynecology & Obstetrics. 2005;91(2):125–31. [DOI] [PubMed] [Google Scholar]
- 38.Tan C-E, Ma S, Wai D, Chew S-K, Tai ES. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes care. 2004;27(5):1182–6. [DOI] [PubMed] [Google Scholar]
- 39.Who EC. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England). 2004;363(9403):157. [DOI] [PubMed] [Google Scholar]
- 40.Huber LRB. Validity of self-reported height and weight in women of reproductive age. Maternal and child health journal. 2007;11(2):137–44. [DOI] [PubMed] [Google Scholar]
- 41.Johnson J, Clifton RG, Roberts JM, Myatt L, Hauth JC, Spong CY, et al. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine guidelines. Obstetrics and gynecology. 2013;121(5):969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


