Summary
A major feature of amyotrophic lateral sclerosis (ALS) pathology is the accumulation of ubiquitin (Ub) into intracellular inclusions. This sequestration of Ub may reduce the availability of free Ub, disrupting Ub homeostasis and ultimately compromising cellular function and survival. We previously reported significant disturbance of Ub homeostasis in neuronal-like cells expressing mutant SOD1. Here, we show that Ub homeostasis is also perturbed in neuronal-like cells expressing either TDP-43 or FUS. The expression of mutant TDP-43 and mutant FUS led to UPS dysfunction, which was associated with a redistribution of Ub and depletion of the free Ub pool. Redistribution of Ub is also a feature of sporadic ALS, with an increase in Ub signal associated with inclusions and no compensatory increase in Ub expression. Together, these findings suggest that alterations to Ub homeostasis caused by the misfolding and aggregation of ALS-associated proteins play an important role in the pathogenesis of ALS.
Subject Areas: Molecular Biology, Neuroscience, Protein Folding
Graphical Abstract

Highlights
-
•
The expression of TDP-43M337V and FUSR495X causes UPS dysfunction in NSC-34 cells
-
•
The aggregation of TDP-43M337V and FUSR495X depletes the free Ub pool in cells
-
•
Ub homeostasis is altered in spinal cord tissue from patients with sALS
-
•
Perturbed Ub homeostasis is a common feature of ALS
Molecular Biology; Neuroscience; Protein Folding
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease characterized by the progressive loss of motor neurons in the spinal cord and motor cortex, resulting in paralysis and eventually death typically by respiratory failure (Hardiman et al., 2017). The cause(s) of most cases of ALS remains largely unknown (sporadic ALS; sALS), with only approximately 10% of all cases having a clear inherited genetic cause (familial ALS; fALS). There are now over 25 genes known to be associated with ALS (Nguyen et al., 2018), which can be broadly classified into three functional groups including RNA metabolism, trafficking, and protein degradation that have each been proposed to perturb proteome homeostasis (Yerbury et al., 2020). Mutations in RNA-binding proteins TAR DNA-binding protein 43 (TDP-43) and fused-in-sarcoma (FUS) account for a small proportion of fALS cases (∼5%) (Chen et al., 2013). However, the wild-type forms of both TDP-43 and FUS are both strongly associated with the etiology of sALS and frontotemporal dementia (FTD) (Neumann et al., 2006, 2009; Arai et al., 2006; Blair et al., 2010; Lai et al., 2011), suggesting these genes play a pivotal role in disease pathology. A growing list of genes encoding components or regulators of the ubiquitin-proteasome system (UPS) and autophagy implicate defective protein degradation in ALS. Mutations in VCP (Johnson et al., 2010), SQSTM1 (Fecto et al., 2011), UBQLN2 (Deng et al., 2011), OPTN (Maruyama et al., 2010), TBK1 (Cirulli et al., 2015), CCNF (Williams et al., 2016), and DNAJC7 (Farhan et al., 2020) have all been associated with ALS and are all components of cellular protein degradation machinery.
A hallmark of many neurodegenerative diseases, including FTD and ALS, is the abnormal accumulation of proteins into insoluble aggregates or inclusions (Yerbury et al., 2016). It remains to be determined whether these aggregates are a cause or consequence of disease. Evidence suggests that a correlation exists between aggregate load and motor neuron loss in ALS (Ticozzi et al., 2010; Brettschneider et al., 2014; Giordana et al., 2010), and our previous work has shown that mutant superoxide dismutase 1 (SOD1) aggregation propensity correlates with toxicity in the neuronal-like NSC-34 cells (McAlary et al., 2016). Protein inclusions are heterogeneous in their protein composition and contain not just pathological proteins but also molecular chaperones (Sherman and Goldberg, 2001; Yerbury and Kumita, 2010), UPS components (Huang and Figueiredo-Pereira, 2010), and other proteins susceptible to aggregation (Ciryam et al., 2013, 2015). In ALS, the composition of inclusions varies depending on the ALS subtype (sALS versus fALS) and even the underlying mutated gene itself. For example, a majority of sALS and fALS cases have inclusions positive for TDP-43 (Turner et al., 2013) but not SOD1 or FUS; however, SOD1- or FUS-associated fALS cases show exclusive deposition of SOD1 and FUS respectively, with no TDP-43 immunoreactivity. In addition to the variation in composition, there is evidence that these different inclusions form by distinct processes in cells (Farrawell et al., 2015). The deposition of specific pathological proteins in different cases suggests that the cellular toxicity may be a result of the sequestration of vital proteins into the inclusions affecting their normal function. Accordingly, we have shown that the sub-proteome that co-aggregates with SOD1, TDP-43, and FUS is composed of supersaturated proteins (Ciryam et al., 2017), with cellular concentrations that exceed their predicted solubility (Ciryam et al., 2013, 2015). These results are consistent with a collapse in the proteostasis capacity of motor neurons in ALS, which is not surprising given that motor neurons have been shown to be particularly susceptible to UPS stress (Bax et al., 2019). Moreover, motor neurons also have a reduced UPS capacity (Brockington et al., 2013) and a more metastable proteome (Yerbury et al., 2019) when compared with ALS-resistant oculomotor neurons, making them particularly vulnerable.
The sequestration of Ub into inclusions is common to all forms of ALS, regardless of the underlying genetics. Ub is best known for its role in targeting proteins for degradation via the proteasome, but it also plays essential roles in a variety of cellular processes including signal transduction, endocytosis, transcription, and DNA repair (Hershko and Ciechanover, 1998; Chen and Sun, 2009). Ub is covalently linked to target proteins through a highly ordered, three-step enzymatic cascade, with differences in Ub chain length and topology determining the fate of the target protein (Pickart, 2001). Inside cells, Ub exists in a dynamic equilibrium between free unconjugated Ub and Ub conjugated in chains (Dantuma et al., 2006; Groothuis et al., 2006). The pool of free Ub is limited and is maintained through controlling Ub expression and the rates of Ub degradation and by recycling Ub from its target substrates. The availability of Ub is particularly important in neurons as it has been shown to regulate differentiation and many aspects of synaptic function including neurogenesis, neuronal excitability, neurotransmission, and synapse formation and elimination (Bax et al., 2019; Mabb and Ehlers, 2010; Kawabe and Brose, 2011). The sequestration of Ub into inclusions may also reduce the availability of free Ub, which is essential for cellular function and survival (Groothuis et al., 2006; Ben Yehuda et al., 2017).
Recently, we showed altered Ub homeostasis in a mutant SOD1 cell model of ALS (Farrawell et al., 2018). The aggregation of pathogenic SOD1A4V in NSC-34 cells led to alterations in UPS activity and redistribution of Ub, disrupting Ub homeostasis and causing mitochondrial dysfunction. It is not currently known if perturbed Ub homeostasis is common to all forms of ALS. Here, we show that Ub homeostasis is disrupted in multiple cell models of ALS. The expression of the mutant forms of ALS-associated proteins, TDP-43 and FUS, caused UPS dysfunction in cells, which was associated with a redistribution of Ub and decreased levels of free monomeric Ub. Increased levels of Ub were also found to be associated with inclusions in postmortem tissue from patients with sALS, confirming that redistribution of Ub is also a feature of sALS. Importantly, this work highlights that misfolded proteins and aggregates associated with ALS contribute to UPS dysfunction and that Ub homeostasis is a key target for monitoring pathological changes in ALS.
Results
TDP-43 and FUS Aggregates Contain K48 and K63-Linked Ubiquitin Chains
K48- and K63-linked polyubiquitin chains are the two most abundant chain types known to target proteins for degradation by the UPS or direct them for removal by autophagy, respectively. We have previously shown that SOD1A4V aggregates contain both UbK48 and UbK63 chains and that Ub was present from the earliest stages of aggregation (Farrawell et al., 2018), whereas TDP-43 and FUS aggregates are ubiquitinated relatively late by comparison (Farrawell et al., 2015). Here, we show that both TDP-43M337V aggregates (Figure 1A) and FUSR495X aggregates (Figure 1B) contain both UbK48 and UbK63 polymers. In fact, there was a large amount of overlap between TDP-43M337V aggregates and both UbK48 and UbK63, with 60% of the TDP-43M337V aggregates identified within transfected cells colocalized with UbK48 and 73% of TDP-43M337V aggregates colocalized with UbK63. Furthermore, the intensity of UbK48 and UbK63 signal associated with TDP-43M337V aggregates was significantly higher than the signal observed to be associated with soluble TDP-43 M337V (Figure 1C). In cells containing FUSR495X aggregates, ∼ 55% of FUSR495X aggregates were positive for UbK48 or UbK63 and the intensity of UbK48 and UbK63 staining was significantly greater in these aggregates when compared with areas containing soluble FUS (Figure 1D).
Figure 1.
TDP-43 and FUS Aggregates Contain K48 and K63-Linked Ubiquitin Chains
NSC-34 cells transfected with mutant TDP-43-GFP (A) or mutant FUS-GFP (B) were fixed, permeabilized, and stained for UbK48 and UbK63 polymers 48 h post transfection. The mean fluorescence intensity of UbK48 and UbK63 associated with soluble and insoluble TDP-43M337V (C) or FUSR495X (D) was quantified using ImageJ. Data shown are mean ± SD (n = 17 TDP-43/UbK48, n = 25 TDP-43/UbK63, n = 19 FUS/UbK48, n = 25 FUS/UbK63) and statistical significance between groups was determined using an unpaired Student's t test (∗∗∗∗p < 0.0001). Scale bars represent 10 μm.
Mutations in TDP-43 and FUS Cause UPS Dysfunction
We have previously shown that cells containing SOD1A4V aggregates have a dysfunctional UPS, as evidenced by the accumulation of significantly higher amounts of the fluorescent UPS reporter tdTomatoCL1 compared with cells expressing SOD1WT (Farrawell et al., 2018). To investigate whether cells expressing ALS-associated mutant TDP-43 or FUS have a dysfunctional UPS, we co-transfected NSC-34 cells with TDP-43-GFP, FUS-GFP, or tGFP and tdTomatoCL1 and measured tdTomatoCL1 accumulation in the presence of increasing concentrations of MG132 by flow cytometry. Cells expressing TDP-43WT and TDP-43M337V showed a dose-dependent increase in tdTomatoCL1 signal with MG132 treatment, which was significantly higher than the signal observed in cells expressing the tGFP control at all the concentrations tested (Figure 2A). Modest increases in tdTomatoCL1 signal were observed with increasing MG132 concentrations in cells expressing the tGFP control (Figure S1), suggesting that UPS function was not completely impaired at the concentrations tested. Although cells expressing TDP-43M337V exhibited higher levels of tdTomatoCL1 fluorescence compared with cells expressing TDP-43WT, this increase was only significant at the highest concentration of MG132 tested. In contrast, cells expressing FUSR495X exhibited significantly higher levels of tdTomatoCL1 signal than cells expressing FUSWT at all the concentrations tested (Figure 2B). Together, these results suggest that the overexpression of ALS-associated TDP-43 and FUS causes UPS dysfunction.
Figure 2.
ALS Mutant TDP-43 and ALS Mutant FUS Alter UPS Activity in Living Cells
Dose-dependent response of UPS activity (tdTomatoCL1 fluorescence) in NSC-34 cells co-transfected with TDP-43-GFP (A) or FUS-GFP (B) after overnight treatment (18 h) with the proteasome inhibitor, MG132. Data represent mean tdTomatoCL1 fluorescence ±SEM (n = 3) ∗p < 0.05, ∗∗∗p < 0.001 indicate significant difference to WT (two-way ANOVA with Bonferroni post-test). See also Figure S1.
Ubiquitin Pools Are Disturbed in Cells Expressing ALS Mutants of TDP-43 and FUS
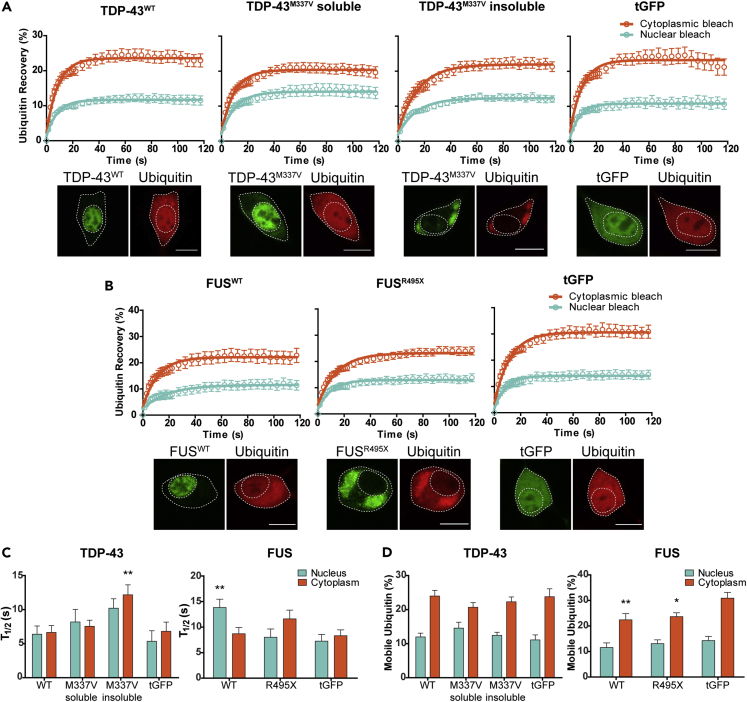
To determine whether the UPS dysfunction observed in cells expressing mutant TDP-43 and mutant FUS was associated with altered Ub homeostasis, we assessed the mobility and distribution of Ub in cells using fluorescence recovery after photo bleaching (FRAP) (Axelrod et al., 1976; Dantuma et al., 2006). By bleaching regions of interest in the nucleus and cytoplasm of cells co-expressing mCherry-Ub and TDP-43-GFP or FUS-GFP (Figure S2), we could measure the recovery of mCherry-Ub into these regions to determine the rate of nucleocytoplasmic Ub diffusion and cellular availability of mobile Ub. In the case of cells expressing mutant TDP-43M337V-GFP, we examined two subpopulations of cells, those expressing soluble TDP-43M337V-GFP and those containing insoluble TDP-43M337V-GFP aggregates (defined as bright fluorescent puncta > 2 μm). This distinction between subpopulations was not made in cells expressing FUSR495X-GFP, as the majority of FUSR495X-GFP mislocalizes to the cytoplasm where it forms small foci and larger aggregates. For this set of experiments, cells containing large FUSR495X-GFP aggregates were selected for FRAP.
Patterns of Ub recovery for cells expressing both TDP-43-GFP (Figure 3A) and FUS-GFP (Figure 3B) were similar to those observed previously with NSC-34 cells expressing SOD1-GFP (Farrawell et al., 2018), in that levels of cytoplasmic recovery were higher than levels of recovery in the nucleus, indicating increased Ub mobility. However, cells expressing soluble TDP-43M337V-GFP appeared to have slightly lower levels of cytoplasmic recovery when compared with other cell populations expressing TDP-43-GFP and the tGFP control (Figure 3A). Cells expressing either FUSWT-GFP or FUSR495X-GFP both had slightly lower levels of cytoplasmic recovery in comparison with the tGFP control (Figure 3B), suggesting differences in the levels of mobile Ub. After calculating the mean half-life of recovery (T1/2), significant increases were observed in the cytoplasm of cells containing insoluble TDP-43M337V-GFP and in the nucleus of cells expressing FUSWT-GFP when compared with the relevant tGFP control (Figure 3C), suggesting that the kinetics of Ub diffusion is altered in these cells (presumably as Ub is being incorporated into larger complexes). When the amount (%) of mobile Ub available to cells was quantified, no significant differences were observed between populations expressing TDP-43-GFP. However, cells expressing both FUSWT-GFP and FUSR495X-GFP had significantly lower levels of mobile Ub in the cytoplasm in comparison with the tGFP control (Figure 3D).
Figure 3.
Ubiquitin Distribution Is Altered in Cells Expressing ALS Mutants of TDP-43 and FUS
(A−C) NSC-34 cells co-transfected with TDP-43-GFP (A) or FUS-GFP (B) and mCherry-Ub were photobleached in either the nucleus or cytoplasm and recovery of Ub fluorescence was monitored for 120 s. Data shown are means ± SEM (n ≥ 9, combined from three independent experiments). Scale bars represent 10 μm. (C) The mean half-life of mCherry-Ub recovery (T1/2) was measured in both the nucleus and cytoplasm of NSC-34 cells co-transfected with either TDP-43-GFP or FUS-GFP. Data shown are means ± SEM combined from three independent experiments (n ≥ 9). Two-way ANOVA with Bonferroni post-test was used to compare differences to the tGFP control (∗∗p ˂ 0.01).
(D) Quantification of the proportion of mobile Ub in the nucleus and cytoplasm of cells expressing either TDP-43-GFP or FUS-GFP. Data shown are means ± SEM combined from three independent experiments (n ≥ 9). ∗p ˂ 0.05, ∗∗p ˂ 0.01 indicate significant difference to tGFP control (two-way ANOVA with Bonferroni post-test).
See also Figure S2.
Free Ubiquitin Availability Is Lowered in Cells Expressing ALS Mutants of TDP-43 and FUS
To test whether the expression of TDP-43-GFP and FUS-GFP altered the amount of free monomeric Ub in cells, we fractionated cell lysates of NSC-34 cells expressing either wild-type or mutant TDP-43-GFP and FUS-GFP and analyzed the relative amount of monomeric Ub by western blotting (Figure S3). Similar to our previous findings in SOD1-GFP-transfected cells (Farrawell et al., 2018), we were unable to detect significant differences in free Ub levels using this method, most likely as this analysis represents a measurement of both transfected and non-transfected cells in culture. Therefore, we examined the monomeric Ub pool in cells expressing TDP-43-GFP and FUS-GFP by fluorescence recovery after nuclear photobleaching (FRANP) (Farrawell et al., 2018). Using the nuclear pore as a molecular sieve, we bleached the entire nucleus of cells co-expressing mCherry-Ub and TDP-43-GFP (Figures 4A and S4A) or FUS-GFP (Figures 4B and S4B) and monitored the diffusion of monomeric mCherry-Ub back into the nucleus. We observed a significant decrease in the amount of monomeric Ub available to cells containing insoluble TDP-43M337V-GFP aggregates, but there were no significant differences in the levels of monomeric Ub between cells expressing TDP-43WT-GFP, soluble TDP-43M337V–GFP, and the tGFP control (Figure 4C). A significant decrease in monomeric Ub levels was also observed in cells expressing FUSR495X-GFP in comparison with cells expressing FUSWT-GFP and tGFP (Figure 4C).
Figure 4.
Reduced Levels of Free Monomeric Ubiquitin in NSC-34 Cells Expressing Mutant TDP-43 and Mutant FUS
(A and B) The entire nucleus of NSC-34 cells co-expressing TDP-43-GFP (A) or FUS-GFP (B) and mCherry-Ub was photobleached, and the recovery of nuclear Ub was monitored as a proportion of cytoplasmic fluorescence for 120 s. Data represent mean ± SEM (n ≥ 10 TDP-43, n ≥ 12 FUS, combined from three independent experiments). Scale bars, 10 μm.
(C) The percentage of mobile Ub in the nucleus at the final read was quantified as a proportion of cytoplasmic fluorescence. Data represent mean ± SEM (n ≥ 10 TDP-43, n ≥ 12 FUS, combined from three independent experiments). One-way ANOVA with a Tukey's multiple comparison post-test was used to determine statistical significance compared with tGFP control (∗∗p ˂ 0.01).
See also Figure S4.
To determine whether TDP-43-GFP and FUS-GFP expression also alters the endogenous monomeric Ub pool, we used the probe tUI-HA, which is designed to bind strongly and specifically to free Ub (Choi et al., 2019). Using confocal microscopy, we confirmed the specificity and sensitivity of this probe for free Ub by measuring tUI-HA levels in NSC-34 cells treated with the E1/UBA1 inhibitor TAK-243 (Hyer et al., 2018). We observed a time-dependent increase in tUI-HA fluorescence in cells incubated with the E1 inhibitor compared with cells treated with the DMSO control (Figure S5), consistent with previous reports (Choi et al., 2019) and confirming the specificity of the tUI-HA probe for free Ub. We then measured tUI-HA fluorescence in NSC-34 cells transfected with TDP-43-GFP or FUS-GFP (Figure 5A) and observed a significant reduction in tUI-HA fluorescence in cells expressing TDP-43M337V-GFP or FUSR495X-GFP when compared with cells expressing TDP-43WT-GFP or FUSWT-GFP (Figure 5B). To test whether the aggregation of TDP-43M337V-GFP would also deplete the levels of monomeric Ub available to cells, we manually segregated cells expressing TDP-43M337V-GFP into insoluble and soluble populations before quantifying tUI-HA fluorescence. Cells containing insoluble aggregates of TDP-43M337V-GFP were found to have significantly lower levels of tUI-HA fluorescence in comparison with cells expressing soluble TDP43M337V-GFP or TDP-43WT-GFP (Figure 5C). These data indicate that the expression and aggregation of mutant TDP-43 and mutant FUS decrease the cellular availability of endogenous free monomeric Ub and are consistent with observations using mCherry labeled Ub reporter (above).
Figure 5.
Free Ubiquitin Staining in NSC-34 Cells Expressing TDP-43 and FUS
(A) NSC-34 cells transfected with TDP-43-GFP or FUS-GFP were fixed, permeabilized, and stained for free Ub using the free Ub sensor tUI-HA 48 h post-transfection. Scale bars, 20 μm.
(B) Violin plots of tUI-HA fluorescence in cells expressing either TDP-43-GFP or FUS-GFP calculated from high-throughput image analysis using CellProfiler. Data shown are median, 25th and 75th quartiles with the width of the plot indicating frequency (n = 139 TDP-43WT, n = 253 TDP-43M337V, n = 273 FUSWT, n = 335 FUSR495X). Statistical significance between populations was determined using a Mann-Whitney U test.
(C) Cells expressing TDP-43M337V-GFP were further divided into soluble and insoluble populations via manual segregation, and mean tUI-HA fluorescence was measured in ImageJ. Data are shown as a violin plot with median, 25th-75th quartile and overall data range (n = 250 TDP-43WT, n = 221 TDP-43M337Vsoluble, n = 27 TDP-43M337V, insoluble). Statistical significance was determined using Kruskal-Wallis test (∗p < 0.05, ∗∗p < 0.01).
See also Figure S5.
Ubiquitin Homeostasis in ALS Spinal Cord
Inclusions consisting primarily of ubiquitinated and aggregated TDP-43 are a pathological hallmark of sALS. Having shown that the aggregation of TDP-43 in neuronal-like cells resulted in substantial disruption to Ub homeostasis via sequestration of cellular Ub and significant depletion of free monomeric Ub (above), we next sought to interrogate spinal cord motor neurons from sALS postmortem tissue for evidence of perturbed Ub homeostasis. We first probed for both TDP-43 and Ub and confirmed that the distribution of Ub was dispersed evenly throughout spinal cord motor neurons in the absence of TDP-43 inclusions (Figure 6A). In contrast, we observed a significant increase in Ub fluorescence associated with TDP-43 aggregates in cells containing inclusions (Figures 6A, 6B, and S6), consistent with the results of our cell culture models (above). To investigate potential increases in Ub gene expression to compensate for the depletion of free Ub following the accumulation of Ub into inclusions, we analyzed microdissected lumbar spinal cord ventral horn cells from 11 patients with sALS and controls (D'Erchia et al., 2017). Of the four genes encoding Ub (UBB, UBC, UBA52, and RPS27), only UBB was significantly reduced in sALS (Figure 6C), but when the expression of all four Ub genes are considered together, there is no overall difference in Ub expression between sALS and controls.
Figure 6.
Ubiquitin Homeostasis in sALS
(A) Human ALS postmortem tissue was stained for both TDP-43 and Ub. Inclusions were imaged across two cases of sALS. Representative images of motor neurons with the absence or presence of large skeins that colocalize to Ub staining are shown. Scale bar, 10 μm.
(B) The mean fluorescence intensity of Ub associated with soluble and insoluble TDP-43 was quantified using ImageJ. Data shown are mean ± SD (n = 3) and statistical significance was determined using an unpaired Student's t test (∗∗∗∗p < 0.0001).
(C) Relative levels of Ub gene expression that were detected in microdissected ventral horn ALS spinal tissue (from D'Erchia et al., 2017).
(D) Waterfall plot of relative expression or fold change of genes in the UPS and autophagy KEGG pathways. Representative genes from three different regions are displayed.
(E) Key processes contributing to free Ub homeostasis and the difference in these processes in ALS compared with control.
A broader analysis of genes involved in the maintenance of Ub homeostasis (predominantly genes controlling UPS and autophagy) showed significant differences in the relative expression of a number of genes encoding Ub ligases, de-ubiquitinase enzymes (DUBs), and proteases between ALS and control neurons. We observed a pattern of generally decreased expression of UPS genes in ALS (Figure 6D, Tables S1 and S2). We hypothesize that the decrease in expression across the Ub ligases and DUBs, along with the accumulation of Ub chains on soluble and insoluble proteins, would act in concert to reduce the pool of free monomeric Ub in sALS (Figure 6E).
Discussion
Ub is integral to neuronal health and function as it regulates essential cellular processes including protein quality control, protein trafficking, cell cycle regulation, and DNA repair (Hershko and Ciechanover, 1998; Chen and Sun, 2009). The accumulation of Ub-positive inclusions is a hallmark of ALS pathology (Leigh et al., 1991), and the associated sequestration of Ub into inclusions likely disrupts Ub homeostasis by depleting the free Ub pool to levels where cellular functions are severely compromised and ultimately result in cell death. We recently showed that SOD1A4V aggregation caused UPS dysfunction through depletion of the free Ub pool and subsequent disruption of Ub homeostasis (Farrawell et al., 2018). To determine if regulation of Ub homeostasis is perturbed in other models of ALS, we examined the distribution of Ub in cell models expressing ALS-associated proteins TDP-43 and FUS and in spinal cord motor neurons from sALS postmortem tissue. Our results confirm that expression of both mutant TDP-43 and mutant FUS cause UPS dysfunction and alterations to Ub homeostasis. Furthermore, gene expression data from the ventral horn of male patients with ALS suggests that these processes play an important role in the pathogenesis of ALS (D'Erchia et al., 2017).
Ub-mediated protein degradation plays a vital role in maintaining proteostasis (Yerbury et al., 2016), and the UPS has been shown to regulate the levels of ALS-associated proteins in cells (Scotter et al., 2014; Miyazaki et al., 2004), with proteasome inhibition resulting in the accumulation and aggregation of ubiquitinated TDP-43 (Nonaka et al., 2009; van Eersel et al., 2011). Our previous work in NSC-34 cells established that TDP-43 and FUS aggregates accumulate Ub late in comparison with SOD1 aggregates (Farrawell et al., 2015), which contain Ub at the earliest stage of aggregation (Farrawell et al., 2018). Furthermore, we confirmed that SOD1 aggregates contain both K48- and K63-linked polyubiquitin chains, targeting them for degradation by the UPS and/or directing them for removal by autophagy, respectively (Farrawell et al., 2018). In line with these results, we now show that TDP-43 and FUS aggregates contain both K48- and K63-linked polyubiquitin chains. These results are consistent with previous work in human SH-SY5Y cells, which demonstrated that both the UPS and autophagy pathway play a role in the clearance of TDP-43 (Scotter et al., 2014). The presence of both UbK48 and UbK63 linkages suggests that K63 polyubiquitin labeling may be directing aggregates to the Ub-aggresome route when the UPS is inhibited (Yamamoto and Simonsen, 2011). Interestingly, cells expressing mutant FUS have increased levels of UbK48 in comparison with cells expressing FUSWT (Kamelgarn et al., 2018), suggesting a dysfunctional UPS. These results highlight the dependence on tightly regulated Ub homeostasis in neurons and are consistent with the model that mutant ALS-associated proteins compromise UPS function and disrupt Ub homeostasis.
The UPS regulates cellular processes that are fundamental to the structure and function of the nervous system (Mabb and Ehlers, 2010; Chen et al., 2011), and a dysfunctional UPS is thought to contribute to the development of neurodegenerative disorders including ALS. Previously, using the proteasome reporter tdTomatoCL1, we have shown that the aggregation of SOD1A4V results in UPS dysfunction in NSC-34 cells. Here, we show that this dysfunction is not specific to SOD1, as an accumulation of tdTomatoCL1 was observed in cells expressing both TDP-43M337V and FUSR495X. In addition, we found that the levels of UPS dysfunction in cells expressing TDP-43M337V were only significantly different to cells expressing TDP-43WT at the highest concentration of MG132 tested. These results may also be partly explained by the fact that overexpression of TDP-43WT is toxic to cells (Watanabe et al., 2013; Yamashita et al., 2014; Park et al., 2017; Baskaran et al., 2018) and causes motor dysfunction and death in mice (Becker et al., 2017). Furthermore, UPS inhibition causes the aggregation of both TDP-43WT and mutant TDP-43 (Scotter et al., 2014), and the aggregation of TDP-43 leads to the accumulation of the UPS reporter GFP-CL1 in SH-SY5Y cells (Nonaka et al., 2013). The UPS reporter UbG76V-GFP has also been observed to accumulate in the motor neurons of SOD1G93A mice (Cheroni et al., 2009), highlighting that UPS dysfunction is a common feature of ALS. In fact, UPS dysfunction alone is sufficient to drive ALS pathology, with the motor neuron-specific knockout of the proteasome subunit Rpt3 inducing the aggregation of ALS-associated proteins and motor neuron degeneration in mice (Tashiro et al., 2012).
The aggregation of ALS-associated proteins may exacerbate UPS dysfunction and alter Ub homeostasis in cells by depleting the free Ub pool. We have previously shown that the UPS dysfunction observed in NSC-34 cells containing SOD1A4V aggregates was associated with a redistribution of Ub and decreased levels of free Ub (Farrawell et al., 2018). In this study, we show that the aggregation of mutant TDP-43M337V and mutant FUSR495X also results in changes to Ub homeostasis through the redistribution of Ub and depletion of the free Ub pool. Cellular Ub exists in dynamic pools where free Ub is maintained at an adequate level in order to be able to respond to different cellular conditions (Park and Ryu, 2014). Stress to the proteome causes dramatic changes to the Ub equilibrium by increasing the amount of poly-ubiquitinated protein aggregates and decreasing the free Ub levels in cells (Salomons et al., 2009). Inhibition of the UPS induces similar changes, with MG132 treatment resulting in the accumulation of poly-ubiquitinated proteins and decreased rates of Ub diffusion (Dantuma et al., 2006). Consistent with this, the aggregation of TDP-43M337V and FUSR495X led to changes in the mobility and kinetics of Ub diffusion. Significant changes in Ub mobility were also observed in cells expressing FUSWT, but this is consistent with the fact that the overexpression of FUSWT has been shown to alter the nuclear function of endogenous FUSWT (Sephton et al., 2014) and lead to the accumulation of Ub-positive deposits in mice (Mitchell et al., 2013).
The accumulation of Ub in inclusions is a potential mechanism for Ub depletion, and previous studies have suggested the effects of protein aggregation on Ub homeostasis will be similar regardless of which protein is aggregated (Ben Yehuda et al., 2017). TDP-43 and FUS are both large components of ubiquitinated inclusions in ALS tissue (Arai et al., 2006; Neumann et al., 2006), and here, we confirm through multiple investigations that the aggregation of mutant TDP-43 and mutant FUS deplete the free Ub pool. The consequences of Ub depletion in neurons can be dire, causing defects in neuronal outgrowth and impaired synaptic development (Ryu et al., 2014), which, if prolonged, can lead to cell death. Reduction in cellular Ub levels through disruption to the UBB poly-Ub gene gives rise to a progressive neurodegenerative condition in mice (Ryu et al., 2008). Subsequent studies in these UBB-deficient mice revealed that compensatory expression from the UBC gene is significantly upregulated in an attempt to maintain free Ub levels and protect against neuronal dysfunction (Park et al., 2012). Interestingly, our analysis of spinal cord tissue from patients with sALS revealed that the sequestration of Ub into inclusions was not associated with a compensatory increase in Ub expression. In fact, compared with control tissue, there was a decrease in the expression of UBB along with the decreased expression of Ub ligases and DUBs. Modulation of Ub levels can also be achieved through overexpression or removal of Ub from protein aggregates. Overexpression of the DUB USP14 increases the amount of free Ub in cells (Hyrskyluoto et al., 2014), and increasing free Ub levels through Ub overexpression was shown to improve the structural dysfunction in the neuromuscular junction of mice expressing a mutation in USP14 (Vaden et al., 2015). Similarly, an ataxia-associated mutation in the UCHL1 gene has been associated with decreased levels of monomeric Ub in the brains of mice, and overexpression of UCHL1 results in increased levels of free Ub and improvements in synaptic function (Osaka et al., 2003; Gong et al., 2006). Overexpression of Ub has also been shown to enhance UPS function and reduce the cytosolic accumulation of TDP-43 and aggregation associated with UBQLN2 overexpression (Picher-Martel et al., 2019). Collectively, these studies demonstrate that modulation of cellular Ub pools play an important role in the pathogenesis of neurodegenerative diseases such as ALS. Moreover, although perturbations to Ub homeostasis appear to be a unifying feature of ALS, it is likely treatment will have to be stratified based on the root cause of the disruption.
In conclusion, we observe that the expression of mutant TDP-43 and mutant FUS causes UPS dysfunction, where the aggregation of these proteins is associated with the redistribution of Ub and depletion of the free Ub pool. Together with our previous observations in a SOD1 model, our findings suggest that perturbations in Ub homeostasis may represent a common molecular pathway underlying neurodegeneration in ALS across genetically distinct forms of the disease.
Limitations of the Study
Although NSC-34 cells exhibit many motor neuron characteristics and are routinely used to model ALS, it should be noted that they are an immortalized mouse cell line. Furthermore, we are using a transient transfection model to ectopically overexpress fluorescently tagged proteins. This type of model recapitulates disease pathology but may not accurately reflect endogenous levels of protein in post-mitotic neurons and restricts the bulk analysis of cell populations (e.g., by western blot), as non-transfected cells influence the results. This work forms the basis of future research using human iPSC-derived motor neurons and animal models to further investigate the importance of Ub homeostasis in ALS.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to the lead contact, Professor Justin Yerbury (jyerbury@uow.edu.au).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
Access to data and CellProfiler pipelines can be made available upon reasonable request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
J.J.Y. was supported by a University of Wollongong Professorship in Neurodegenerative Diseases, and J.J.Y., I.P.B. and N.E.F. were supported by an National Health and Medical Research Council, Australia Dementia Teams Grant (1095215). L.M. was supported by a Motor Neuron Disease Research Institute of Australia Bill Gole Postdoctoral Fellowship. C.G.C was supported by a University of Wollongong-Yerbury Family Scholarship. We thank the New South Wales Brain Bank and Queensland Brain Bank (Australian Brain Bank Network) for access to postmortem tissue.
Author Contributions
Conceptualization: N.E.F., D.N.S., J.J.Y.; Methodology: N.E.F., L.M., K.L.V., D.N.S., J.J.Y.; Validation: N.E.F., Formal analysis: N.E.F., L.M., J.S.L., C.G.C., K.L.V., D.N.S., J.J.Y.; Investigation: N.E.F., L.M., J.S.L., S.T.W., D.N.S., J.J.Y.; Resources: D.N.S., J.J.Y.; Data curation: N.E.F., L.M., J.J.Y.; Writing - original draft: N.E.F., J.J.Y.; Writing - review & editing: N.E.F., L.M., J.S.L., C.G.C., I.P.B., K.L.V., D.N.S., J.J.Y.; Visualization: N.E.F., J.J.Y.; Supervision: K.L.V., D.N.S., J.J.Y.; Project administration: K.L.V., J.J.Y.; Funding acquisition: K.L.V., D.N.S., J.J.Y.
Declaration of Interests
The authors declare no competing interests.
Published: November 20, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101700.
Supplemental Information
References
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D.E., Schlessinger J., Elson E., Webb W.W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 1976;16:1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran P., Shaw C., Guthrie S. TDP-43 causes neurotoxicity and cytoskeletal dysfunction in primary cortical neurons. PLoS One. 2018;13:e0196528. doi: 10.1371/journal.pone.0196528. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bax M., Mckenna J., Do-Ha D., Stevens C.H., Higginbottom S., Balez R., Cabral-Da-Silva M.E.C., Farrawell N.E., Engel M., Poronnik P. The ubiquitin proteasome system is a key regulator of pluripotent stem cell survival and motor neuron differentiation. Cells. 2019;8:581. doi: 10.3390/cells8060581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker L.A., Huang B., Bieri G., Ma R., Knowles D.A., Jafar-Nejad P., Messing J., Kim H.J., Soriano A., Auburger G. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature. 2017;544:367–371. doi: 10.1038/nature22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Yehuda A., Risheq M., Novoplansky O., Bersuker K., Kopito R.R., Goldberg M., Brandeis M. Ubiquitin accumulation on disease associated protein aggregates is correlated with nuclear ubiquitin depletion, histone de-ubiquitination and impaired DNA damage response. PLoS One. 2017;12:e0169054. doi: 10.1371/journal.pone.0169054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair I.P., Williams K.L., Warraich S.T., Durnall J.C., Thoeng A.D., Manavis J., Blumbergs P.C., Vucic S., Kiernan M.C., Nicholson G.A. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J. Neurol. Neurosurg. Psychiatry. 2010;81:639–645. doi: 10.1136/jnnp.2009.194399. [DOI] [PubMed] [Google Scholar]
- Brettschneider J., Arai K., Del Tredici K., Toledo J.B., Robinson J.L., Lee E.B., Kuwabara S., Shibuya K., Irwin D.J., Fang L. TDP-43 pathology and neuronal loss in amyotrophic lateral sclerosis spinal cord. Acta Neuropathol. 2014;128:423–437. doi: 10.1007/s00401-014-1299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington A., Ning K., Heath P.R., Wood E., Kirby J., Fusi N., Lawrence N., Wharton S.B., Ince P.G., Shaw P.J. Unravelling the enigma of selective vulnerability in neurodegeneration: motor neurons resistant to degeneration in ALS show distinct gene expression characteristics and decreased susceptibility to excitotoxicity. Acta Neuropathol. 2013;125:95–109. doi: 10.1007/s00401-012-1058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.C., Bhattacharyya B.J., Hanna J., Minkel H., Wilson J.A., Finley D., Miller R.J., Wilson S.M. Ubiquitin homeostasis is critical for synaptic development and function. J. Neurosci. 2011;31:17505–17513. doi: 10.1523/JNEUROSCI.2922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Sayana P., Zhang X., Le W. Genetics of amyotrophic lateral sclerosis: an update. Mol. Neurodegener. 2013;8:28. doi: 10.1186/1750-1326-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.J., Sun L.J. Nonproteolytic functions of ubiquitin in cell signaling. Mol. Cell. 2009;33:275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Cheroni C., Marino M., Tortarolo M., Veglianese P., De Biasi S., Fontana E., Zuccarello L.V., Maynard C.J., Dantuma N.P., Bendotti C. Functional alterations of the ubiquitin-proteasome system in motor neurons of a mouse model of familial amyotrophic lateral sclerosis. Hum. Mol. Genet. 2009;18:82–96. doi: 10.1093/hmg/ddn319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.S., Bollinger S.A., Prada L.F., Scavone F., Yao T., Cohen R.E. High-affinity free ubiquitin sensors for quantifying ubiquitin homeostasis and deubiquitination. Nat. Methods. 2019;16:771–777. doi: 10.1038/s41592-019-0469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli E.T., Lasseigne B.N., Petrovski S., Sapp P.C., Dion P.A., Leblond C.S., Couthouis J., Lu Y.F., Wang Q., Krueger B.J. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;347:1436–1441. doi: 10.1126/science.aaa3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciryam P., Tartaglia G.G., Morimoto R.I., Dobson C.M., Vendruscolo M. Widespread aggregation and neurodegenerative diseases are associated with supersaturated proteins. Cell Rep. 2013;5:781–790. doi: 10.1016/j.celrep.2013.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciryam P., Kundra R., Morimoto R.I., Dobson C.M., Vendruscolo M. Supersaturation is a major driving force for protein aggregation in neurodegenerative diseases. Trends Pharmacol. Sci. 2015;36:72–77. doi: 10.1016/j.tips.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciryam P., Lambert-Smith I.A., Bean D.M., Freer R., Cid F., Tartaglia G.G., Saunders D.N., Wilson M.R., Oliver S.G., Morimoto R.I. Spinal motor neuron protein supersaturation patterns are associated with inclusion body formation in ALS. Proc. Natl. Acad. Sci. U S A. 2017;114:E3935–E3943. doi: 10.1073/pnas.1613854114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Erchia A.M., Gallo A., Manzari C., Raho S., Horner D.S., Chiara M., Valletti A., Aiello I., Mastropasqua F., Ciaccia L. Massive transcriptome sequencing of human spinal cord tissues provides new insights into motor neuron degeneration in ALS. Sci. Rep. 2017;7:10046. doi: 10.1038/s41598-017-10488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantuma N.P., Groothuis T.A., Salomons F.A., Neefjes J. A dynamic ubiquitin equilibrium couples proteasomal activity to chromatin remodeling. J. Cell Biol. 2006;173:19–26. doi: 10.1083/jcb.200510071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H.X., Chen W., Hong S.T., Boycott K.M., Gorrie G.H., Siddique N., Yang Y., Fecto F., Shi Y., Zhai H. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477:211–215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhan S.M.K., Howrigan D.P., Abbott L.E., Klim J.R., Topp S.D., Byrnes A.E., Churchhouse C., Phatnani H., Smith B.N., Rampersaud E. Publisher Correction: Exome sequencing in amyotrophic lateral sclerosis implicates a novel gene, DNAJC7, encoding a heat-shock protein. Nat. Neurosci. 2020;23:295. doi: 10.1038/s41593-019-0570-5. [DOI] [PubMed] [Google Scholar]
- Farrawell N.E., Lambert-Smith I.A., Warraich S.T., Blair I.P., Saunders D.N., Hatters D.M., Yerbury J.J. Distinct partitioning of ALS associated TDP-43, FUS and SOD1 mutants into cellular inclusions. Sci. Rep. 2015;5:13416. doi: 10.1038/srep13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrawell N.E., Lambert-Smith I., Mitchell K., Mckenna J., Mcalary L., Ciryam P., Vine K.L., Saunders D.N., Yerbury J.J. SOD1(A4V) aggregation alters ubiquitin homeostasis in a cell model of ALS. J. Cell Sci. 2018;131:jcs209122. doi: 10.1242/jcs.209122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecto F., Yan J., Vemula S.P., Liu E., Yang Y., Chen W., Zheng J.G., Shi Y., Siddique N., Arrat H. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch. Neurol. 2011;68:1440–1446. doi: 10.1001/archneurol.2011.250. [DOI] [PubMed] [Google Scholar]
- Giordana M.T., Piccinini M., Grifoni S., De Marco G., Vercellino M., Magistrello M., Pellerino A., Buccinna B., Lupino E., Rinaudo M.T. TDP-43 redistribution is an early event in sporadic amyotrophic lateral sclerosis. Brain Pathol. 2010;20:351–360. doi: 10.1111/j.1750-3639.2009.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B., Cao Z., Zheng P., Vitolo O.V., Liu S., Staniszewski A., Moolman D., Zhang H., Shelanski M., Arancio O. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126:775–788. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Groothuis T.A., Dantuma N.P., Neefjes J., Salomons F.A. Ubiquitin crosstalk connecting cellular processes. Cell Div. 2006;1:21. doi: 10.1186/1747-1028-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardiman O., Al-Chalabi A., Chio A., Corr E.M., Logroscino G., Robberecht W., Shaw P.J., Simmons Z., Van Den Berg L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers. 2017;3:17085. doi: 10.1038/nrdp.2017.85. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Huang Q., Figueiredo-Pereira M.E. Ubiquitin/proteasome pathway impairment in neurodegeneration: therapeutic implications. Apoptosis. 2010;15:1292–1311. doi: 10.1007/s10495-010-0466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyer M.L., Milhollen M.A., Ciavarri J., Fleming P., Traore T., Sappal D., Huck J., Shi J., Gavin J., Brownell J. A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat. Med. 2018;24:186–193. doi: 10.1038/nm.4474. [DOI] [PubMed] [Google Scholar]
- Hyrskyluoto A., Bruelle C., Lundh S.H., Do H.T., Kivinen J., Rappou E., Reijonen S., Waltimo T., Petersen A., Lindholm D., Korhonen L. Ubiquitin-specific protease-14 reduces cellular aggregates and protects against mutant huntingtin-induced cell degeneration: involvement of the proteasome and ER stress-activated kinase IRE1alpha. Hum. Mol. Genet. 2014;23:5928–5939. doi: 10.1093/hmg/ddu317. [DOI] [PubMed] [Google Scholar]
- Johnson J.O., Mandrioli J., Benatar M., Abramzon Y., Van Deerlin V.M., Trojanowski J.Q., Gibbs J.R., Brunetti M., Gronka S., Wuu J. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68:857–864. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamelgarn M., Chen J., Kuang L., Jin H., Kasarskis E.J., Zhu H. ALS mutations of FUS suppress protein translation and disrupt the regulation of nonsense-mediated decay. Proc. Natl. Acad. Sci. U S A. 2018;115:E11904–E11913. doi: 10.1073/pnas.1810413115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe H., Brose N. The role of ubiquitylation in nerve cell development. Nat. Rev. Neurosci. 2011;12:251–268. doi: 10.1038/nrn3009. [DOI] [PubMed] [Google Scholar]
- Lai S.L., Abramzon Y., Schymick J.C., Stephan D.A., Dunckley T., Dillman A., Cookson M., Calvo A., Battistini S., Giannini F. FUS mutations in sporadic amyotrophic lateral sclerosis. Neurobiol. Aging. 2011;32:550.e1-4. doi: 10.1016/j.neurobiolaging.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh P.N., Whitwell H., Garofalo O., Buller J., Swash M., Martin J.E., Gallo J.M., Weller R.O., Anderton B.H. Ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosismorphology, distribution, and specificity. Brain. 1991;114:775–788. doi: 10.1093/brain/114.2.775. [DOI] [PubMed] [Google Scholar]
- Mabb A.M., Ehlers M.D. Ubiquitination in postsynaptic function and plasticity. Annu. Rev. Cell Dev. Biol. 2010;26:179–210. doi: 10.1146/annurev-cellbio-100109-104129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H., Morino H., Ito H., Izumi Y., Kato H., Watanabe Y., Kinoshita Y., Kamada M., Nodera H., Suzuki H. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465:223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- McAlary L., Aquilina J.A., Yerbury J.J. Susceptibility of mutant SOD1 to form a destabilized monomer predicts cellular aggregation and toxicity but not in vitro aggregation propensity. Front. Neurosci. 2016;10:499. doi: 10.3389/fnins.2016.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.C., Mcgoldrick P., Vance C., Hortobagyi T., Sreedharan J., Rogelj B., Tudor E.L., Smith B.N., Klasen C., Miller C.C. Overexpression of human wild-type FUS causes progressive motor neuron degeneration in an age- and dose-dependent fashion. Acta Neuropathol. 2013;125:273–288. doi: 10.1007/s00401-012-1043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K., Fujita T., Ozaki T., Kato C., Kurose Y., Sakamoto M., Kato S., Goto T., Itoyama Y., Aoki M., Nakagawara A. NEDL1, a novel ubiquitin-protein isopeptide ligase for dishevelled-1, targets mutant superoxide dismutase-1. J. Biol. Chem. 2004;279:11327–11335. doi: 10.1074/jbc.M312389200. [DOI] [PubMed] [Google Scholar]
- Neumann M., Rademakers R., Roeber S., Baker M., Kretzschmar H.A., Mackenzie I.R. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132:2922–2931. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Nguyen H.P., Van Broeckhoven C., Van Der Zee J. ALS genes in the genomic era and their implications for FTD. Trends Genet. 2018;34:404–423. doi: 10.1016/j.tig.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Nonaka T., Arai T., Buratti E., Baralle F.E., Akiyama H., Hasegawa M. Phosphorylated and ubiquitinated TDP-43 pathological inclusions in ALS and FTLD-U are recapitulated in SH-SY5Y cells. FEBS Lett. 2009;583:394–400. doi: 10.1016/j.febslet.2008.12.031. [DOI] [PubMed] [Google Scholar]
- Nonaka T., Masuda-Suzukake M., Arai T., Hasegawa Y., Akatsu H., Obi T., Yoshida M., Murayama S., Mann D.M., Akiyama H., Hasegawa M. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 2013;4:124–134. doi: 10.1016/j.celrep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Osaka H., Wang Y.L., Takada K., Takizawa S., Setsuie R., Li H., Sato Y., Nishikawa K., Sun Y.J., Sakurai M. Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Hum. Mol. Genet. 2003;12:1945–1958. doi: 10.1093/hmg/ddg211. [DOI] [PubMed] [Google Scholar]
- Park C.W., Ryu H.W., Ryu K.Y. Locus coeruleus neurons are resistant to dysfunction and degeneration by maintaining free ubiquitin levels although total ubiquitin levels decrease upon disruption of polyubiquitin gene Ubb. Biochem. Biophys. Res. Commun. 2012;418:541–546. doi: 10.1016/j.bbrc.2012.01.063. [DOI] [PubMed] [Google Scholar]
- Park C.W., Ryu K.Y. Cellular ubiquitin pool dynamics and homeostasis. BMB Rep. 2014;47:475–482. doi: 10.5483/BMBRep.2014.47.9.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.K., Hong J.Y., Arslan F., Kanneganti V., Patel B., Tietsort A., Tank E.M.H., Li X., Barmada S.J., Liebman S.W. Overexpression of the essential Sis1 chaperone reduces TDP-43 effects on toxicity and proteolysis. PLoS Genet. 2017;13:e1006805. doi: 10.1371/journal.pgen.1006805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picher-Martel V., Renaud L., Bareil C., Julien J.P. Neuronal expression of UBQLN2(P497H) exacerbates TDP-43 pathology in TDP-43(G348C) mice through Interaction with ubiquitin. Mol. Neurobiol. 2019;56:4680–4696. doi: 10.1007/s12035-018-1411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart C.M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Ryu H.W., Park C.W., Ryu K.Y. Restoration of cellular ubiquitin reverses impairments in neuronal development caused by disruption of the polyubiquitin gene Ubb. Biochem. Biophys. Res. Commun. 2014;453:443–448. doi: 10.1016/j.bbrc.2014.09.103. [DOI] [PubMed] [Google Scholar]
- Ryu K.Y., Garza J.C., Lu X.Y., Barsh G.S., Kopito R.R. Hypothalamic neurodegeneration and adult-onset obesity in mice lacking the Ubb polyubiquitin gene. Proc. Natl. Acad. Sci. U S A. 2008;105:4016–4021. doi: 10.1073/pnas.0800096105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons F.A., Menendez-Benito V., Bottcher C., Mccray B.A., Taylor J.P., Dantuma N.P. Selective accumulation of aggregation-prone proteasome substrates in response to proteotoxic stress. Mol. Cell. Biol. 2009;29:1774–1785. doi: 10.1128/MCB.01485-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotter E.L., Vance C., Nishimura A.L., Lee Y.B., Chen H.J., Urwin H., Sardone V., Mitchell J.C., Rogelj B., Rubinsztein D.C., Shaw C.E. Differential roles of the ubiquitin proteasome system and autophagy in the clearance of soluble and aggregated TDP-43 species. J. Cell Sci. 2014;127:1263–1278. doi: 10.1242/jcs.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton C.F., Tang A.A., Kulkarni A., West J., Brooks M., Stubblefield J.J., Liu Y., Zhang M.Q., Green C.B., Huber K.M. Activity-dependent FUS dysregulation disrupts synaptic homeostasis. Proc. Natl. Acad. Sci. U S A. 2014;111:E4769–E4778. doi: 10.1073/pnas.1406162111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M.Y., Goldberg A.L. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29:15–32. doi: 10.1016/s0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- Tashiro Y., Urushitani M., Inoue H., Koike M., Uchiyama Y., Komatsu M., Tanaka K., Yamazaki M., Abe M., Misawa H. Motor neuron-specific disruption of proteasomes, but not autophagy, replicates amyotrophic lateral sclerosis. J. Biol. Chem. 2012;287:42984–42994. doi: 10.1074/jbc.M112.417600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticozzi N., Ratti A., Silani V. Protein aggregation and defective RNA metabolism as mechanisms for motor neuron damage. CNS Neurol. Disord. Drug Targets. 2010;9:285–296. doi: 10.2174/187152710791292585. [DOI] [PubMed] [Google Scholar]
- Turner M.R., Hardiman O., Benatar M., Brooks B.R., Chio A., De Carvalho M., Ince P.G., Lin C., Miller R.G., Mitsumoto H. Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol. 2013;12:310–322. doi: 10.1016/S1474-4422(13)70036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaden J.H., Watson J.A., Howard A.D., Chen P.C., Wilson J.A., Wilson S.M. Distinct effects of ubiquitin overexpression on NMJ structure and motor performance in mice expressing catalytically inactive USP14. Front. Mol. Neurosci. 2015;8:11. doi: 10.3389/fnmol.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eersel J., Ke Y.D., Gladbach A., Bi M., Gotz J., Kril J.J., Ittner L.M. Cytoplasmic accumulation and aggregation of TDP-43 upon proteasome inhibition in cultured neurons. PLoS One. 2011;6:e22850. doi: 10.1371/journal.pone.0022850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Kaneko K., Yamanaka K. Accelerated disease onset with stabilized familial amyotrophic lateral sclerosis (ALS)-linked mutant TDP-43 proteins. J. Biol. Chem. 2013;288:3641–3654. doi: 10.1074/jbc.M112.433615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K.L., Topp S., Yang S., Smith B., Fifita J.A., Warraich S.T., Zhang K.Y., Farrawell N., Vance C., Hu X. CCNF mutations in amyotrophic lateral sclerosis and frontotemporal dementia. Nat. Commun. 2016;7:11253. doi: 10.1038/ncomms11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Simonsen A. The elimination of accumulated and aggregated proteins: a role for aggrephagy in neurodegeneration. Neurobiol. Dis. 2011;43:17–28. doi: 10.1016/j.nbd.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., Nonaka T., Hirai S., Miwa A., Okado H., Arai T., Hosokawa M., Akiyama H., Hasegawa M. Distinct pathways leading to TDP-43-induced cellular dysfunctions. Hum. Mol. Genet. 2014;23:4345–4356. doi: 10.1093/hmg/ddu152. [DOI] [PubMed] [Google Scholar]
- Yerbury J.J., Farrawell N.E., Mcalary L. Proteome homeostasis dysfunction: a unifying principle in ALS pathogenesis. Trends Neurosci. 2020;43:274–284. doi: 10.1016/j.tins.2020.03.002. [DOI] [PubMed] [Google Scholar]
- Yerbury J.J., Kumita J.R. Protein chemistry of amyloid fibrils and chaperones: implications for amyloid formation and disease. Curr. Chem. Biol. 2010;4:89–98. [Google Scholar]
- Yerbury J.J., Ooi L., Dillin A., Saunders D.N., Hatters D.M., Beart P.M., Cashman N.R., Wilson M.R., Ecroyd H. Walking the tightrope: proteostasis and neurodegenerative disease. J. Neurochem. 2016;137:489–505. doi: 10.1111/jnc.13575. [DOI] [PubMed] [Google Scholar]
- Yerbury J.J., Ooi L., Blair I.P., Ciryam P., Dobson C.M., Vendruscolo M. The metastability of the proteome of spinal motor neurons underlies their selective vulnerability in ALS. Neurosci. Lett. 2019;704:89–94. doi: 10.1016/j.neulet.2019.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to data and CellProfiler pipelines can be made available upon reasonable request.