Abstract
The gamma aminobutyric acid (GABA) neurotransmission system has been implicated in autism spectrum disorder (ASD). Molecular neuroimaging studies incorporating simultaneous acquisitions of GABA concentrations and GABAA receptor densities can identify objective molecular markers in ASD. We measured both total GABAA receptor densities by using [18F]flumazenil positron emission tomography ([18F]FMZ-PET) and GABA concentrations by using proton magnetic resonance spectroscopy (1H-MRS) in 28 adults with ASD and 29 age-matched typically developing (TD) individuals. Focusing on the bilateral thalami and the left dorsolateral prefrontal cortex (DLPFC) as our regions of interest, we found no differences in GABAA receptor densities between ASD and TD groups. However, 1H-MRS measurements revealed significantly higher GABA/Water (GABA normalized by water signal) in the left DLPFC of individuals with ASD than that of TD controls. Furthermore, a significant gender effect was observed in the thalami, with higher GABA/Water in males than in females. Hypothesizing that thalamic GABA correlates with ASD symptom severity in gender-specific ways, we stratified by diagnosis and investigated the interaction between gender and thalamic GABA/Water in predicting Autism-Spectrum Quotient (AQ) and Ritvo Autism Asperger’s Diagnostic Scale–Revised (RAADS-R) total scores. We found that gender is a significant effect modifier of thalamic GABA/Water’s relationship with AQ and RAADS-R scores for individuals with ASD, but not for TD controls. When we separated the ASD participants by gender, a negative correlation between thalamic GABA/Water and AQ was observed in male ASD participants. Remarkably, in female ASD participants, a positive correlation between thalamic GABA/Water and AQ was found.
Subject terms: Neuroscience, Biomarkers
Introduction
Autism spectrum disorder (ASD) is a highly heterogeneous neurodevelopmental disorder associated with over 900 genes [1] and many environmental factors [2]. There are no proven common pathophysiologic pathways that link these genetic and environmental factors. A pathophysiological model of ASD that has accumulated much evidence suggests that this condition is a result of an imbalance between excitation (E) and inhibition (I) in key neural systems [3]. While the major neurotransmitter involved in excitation is glutamate, the most abundant inhibitory neurotransmitter is gamma aminobutyric acid (GABA). Various animal models of ASD have been associated with evidence converging on a reduction of parvalbumin-positive GABAergic interneurons [4], which serve important neural functions including generation of γ oscillations [5] and mediation of synchrony of neural circuits [6]. Examination of postmortem brain samples of young adults with ASD and intellectual disability revealed decreased densities of GABAA and/or GABAB receptors in the anterior cingulate cortex (ACC) [7–9], hippocampus [10], fusiform gyrus [8], and superior frontal cortex (BA9), which contains part of the dorsolateral prefrontal cortex (DLPFC) [11–13]. Activation of the DLPFC is reduced in people with ASD as they perform spatial working memory [14] and executive function [15] tasks, suggesting that there could be an E/I imbalance in this region.
To interrogate the GABAergic system at the neurotransmitter receptor level in vivo, recent studies have employed positron emission tomography (PET). Using [11C]Ro15-4513, a radiotracer which binds selectively to α5 subunit-containing GABAA receptors, Horder et al. reported no differences in GABAA α5 subunit availability in any brain region of high-functioning men with ASD compared to age-matched and IQ-matched typically developing males [16]. Furthermore, using [11C]flumazenil, a radiotracer that binds to the α1, α2, α3, and α5 subunits of the GABAA receptor [17], the Horder group also reported that there were no differences in GABAA receptor availability in any brain region of adults with ASD compared to age-matched and IQ-matched typically developing adults [16].
In addition to the GABAA receptor, another crucial component of the GABAergic system is the neurotransmitter GABA. GABA concentrations have been measured successfully in individuals with ASD by proton magnetic resonance spectroscopy (1H-MRS) [18–23], and region-specific trends have emerged. GABA has been shown to be lower in the frontal lobes [19, 23], auditory cortex [21, 22], and motor cortex [21] of children and adolescents with ASD compared to typically developing (TD) controls. Other brain regions, such as the ACC [24], occipital cortex [25], and visual cortex [21], have shown no difference in GABA levels in ASD. Furthermore, none of the studies recently reviewed by Ajram et al. reported any regional differences in GABA levels in adults [26]. Looking at the relationship between neurotransmitter levels and ASD symptom severity, Cochran et al. revealed that GABA-to-creatine ratios in the ACC correlated positively with the social cognition subscale of the Social Responsiveness Scale, Second Edition (SRS-2) and negatively with the Reading the Mind in the Eyes score in adolescents with ASD [20]. Furthermore, Robertson et al. recently demonstrated an important relationship between GABA levels in the visual cortex and binocular rivalry (a basic visual function that is thought to rely on the E-I balance in the visual cortex) in neurotypical controls but not in adolescents and adults with ASD [18]. Collectively, these accumulating lines of evidence support the importance of the GABAergic system in the pathophysiology of ASD.
In addition to the differences in the GABAergic system in the cortical regions, we hypothesize that the GABAergic system in subcortical regions such as the thalamus is also aberrant. The thalamus is an anatomical structure that coordinates the synchronization of circuits connected to it. Aberrant GABAergic neurotransmission in thalamocortical circuits is supported by electroencephalogram (EEG) studies which revealed significantly shorter phase shift duration in the gamma frequency band in ASD subjects, as compared to age-matched control participants [27]. Alterations in connectivity between the thalami and various cortical regions have recently been found in high-functioning children with ASD by functional MRI and diffusion tensor imaging studies [28]. Furthermore, hyper-connectivity between the thalamus and parietal sensorimotor system were found in an analysis of 360 individuals with ASD (compared with 403 neurotypical controls) [29]. Although evidence of thalamocortical differences, as well as GABAergic dysfunction, in ASD is increasing, there has not yet been direct evaluation of the GABAergic system (i.e., GABA concentrations and GABAA receptor densities) in the thalamocortical network.
Sex/gender also impacts the function of the GABAergic system. The menstrual cycle has been shown to affect GABA levels in the prefrontal [30] and occipital cortices [31]. Furthermore, GABA in the DLPFC and the GABAA receptor α1 subunit in the superior temporal gyrus are both decreased in neurotypical women compared to men [32, 33]. Evidence suggests that these sex differences in the GABAergic system may also be relevant to ASD symptomatology. Focusing on adults with ASD, Kirkovski et al. found a positive correlation between GABA concentration in the superior temporal sulcus and ASD-related social impairments in women but not men [34]. These results suggest that there may be sex differences in the way the GABAergic system is impacted in ASD, and that these differences are region-specific.
Accordingly, the objectives of this innovative study are to determine simultaneously the GABAA receptor densities and GABA levels in the thalami and left DLPFC of adults with ASD using a state-of-the-art integrated PET-MR imaging system. Simultaneous PET-MR imaging allows for improvement in spatial alignment, temporal co-registration, and motion artifacts that would not be possible with sequential PET and MRI. Furthermore, GABA levels and GABAA receptor densities can change with time, and thus, the simultaneous acquisition of PET and MRS data can provide a more accurate assessment of the GABAergic system. To our knowledge, no previous study in the field of autism has been published examining receptor density and GABA levels in the same sample. We hypothesize that the GABAergic tone (GABAA receptor densities and/or GABA concentrations) in these regions will be different in individuals with ASD, compared to IQ-matched, age-matched, and gender-matched typically developing (TD) controls. We test our hypothesis through the simultaneous acquisition of GABAA receptor binding potentials (BPND) by [18F]flumazenil-PET ([18F]FMZ-PET) and GABA concentrations by 1H-MRS. Furthermore, we explore the roles of gender and specific brain regions in the GABAergic system of individuals with ASD.
Materials and methods
Participants
Twenty-eight individuals with ASD (mean[SD] 26.6[8.3] years; 11 females; IQ 102.1[16.5]) and 29 IQ-matched, gender-matched, and age-matched typically developing (TD; 27.7[7.4] years; 10 females; IQ 112.1[13.1]) individuals (Table 1) were recruited. Methodology of the study was approved by the Institutional Review Board of Stanford University. All participants provided written informed consent. Inclusion criteria for the ASD group included: (a) Diagnosis of ASD based on DSM-5 criteria as confirmed by a qualified clinician, and the administration of Autism Diagnostic Interview-Revised (ADI-R) [35] and Autism Diagnostic Observation Schedule, Second Edition-2 (ADOS-2) [36]. (b) Age 18 to 55. (c) Adults who are physically healthy. (d) No significant current psychosocial stressors per history. (e) Full scale IQ ≥ 70. Exclusion criteria for the ASD group included: (f) Pre-term birth (<34 weeks’ gestation). (g) Low birth weight (<2000g). (h) DSM-5 diagnosis of other severe psychiatric disorder such as bipolar disorder or schizophrenia. (i) Current use of benzodiazepines. (j) Use of other medications that directly modulate the binding of GABAA receptor [37] (e.g., flumazenil, zolpidem, zaleplon, eszopiclone) and active transport of GABA (e.g., tiagabine) within 4 weeks of scanning. (k) History of alcoholism or current substance abuse. (l) Active medical problems such as unstable seizures, congenital heart disease, endocrine disorders. (m) Significant sensory impairments such as blindness or deafness. (n) Contraindication for MRI or PET. (o) Pregnancy. (p) Evidence of any genetic syndrome. Inclusion criteria for the TD group included: Criteria (b) thru (e), as above. Exclusion Criteria: Criteria (f) thru (p). Additional exclusion criteria for the TD group included: (1) Current or past neurological disorders. (2) Current or past psychiatric disorders on the basis of clinical psychiatric evaluation. (3) History of significant perinatal difficulties or abnormal developmental milestones. In addition to the above inclusion and exclusion criteria, due to the effects of progesterone on the menstrual cycle, all female participants were scanned in the follicular phase when the progesterone level is low and stable. The follicular phase was estimated from the participants’ histories of menstrual cycles. All subjects were physically healthy post-pubertal adults.
Table 1.
Demographic data and selected findings from neuropsychological assessments in high-functioning adults with autism spectrum disorder (ASD) and typically developing adults (TD).
| ASD (N = 28) | TD (N = 29) | ASD male (N = 17) | ASD female (N = 11) | TD male (N = 19) | TD female (N = 10) | ANOVA F | ANOVA P | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 26.6 ± 8.3 | 27.4 ± 7.4 | 22.6 ± 4.1 | 32.7 ± 9.7 | 26.7 ± 7.2 | 28.6 ± 7.9 | 4.69 | 0.006** |
| FSIQ | 102.1 ± 16.5 | 112.1 ± 13.1 | 102.3 ± 16.8 | 101.7 ± 16.8 | 114.3 ± 12.6 | 108.5 ± 13.8 | 2.39 | 0.080 |
| VIQ | 104.4 ± 18.2 | 110.4 ± 14.1 | 99.1 ± 17.0 | 102.3 ± 21.1 | 112.9 ± 13.4 | 106.4 ± 14.9 | 1.00 | 0.402 |
| NVIQ | 99.8 ± 14.5 | 113.4 ± 11.9 | 105.7 ± 16.9 | 101.1 ± 13.1 | 115.4 ± 10.8 | 110.0 ± 13.3 | 4.54 | 0.007** |
| AQ–Total | 31.8 ± 6.5 | 17.3 ± 8.3 | 29.4 ± 5.3 | 35.5 ± 6.7 | 19.3 ± 9.1 | 13.5 ± 4.7 | 23.40 | <0.0001** |
| AQ–Social skills | 6.8 ± 2.5 | 3.0 ± 2.5 | 6.4 ± 2.5 | 7.3 ± 2.5 | 3.4 ± 2.8 | 2.3 ± 2.0 | 11.16 | <0.0001** |
| AQ–Attention switching | 7.4 ± 1.8 | 4.7 ± 2.3 | 6.6 ± 1.5 | 8.5 ± 1.6 | 5.1 ± 2.3 | 3.9 ± 2.0 | 12.42 | <0.0001** |
| AQ–Attention to details | 6.5 ± 2.4 | 5.1 ± 2.4 | 6.1 ± 2.3 | 7.1 ± 2.6 | 5.4 ± 2.4 | 4.7 ± 2.5 | 1.99 | 0.127 |
| AQ–Communication | 6.6 ± 2.2 | 2.5 ± 2.2 | 6.2 ± 1.9 | 7.2 ± 2.5 | 2.8 ± 2.6 | 1.8 ± 1.3 | 17.65 | <0.0001** |
| AQ–Imagination | 4.6 ± 2.0 | 2.1 ± 1.8 | 4.1 ± 2.1 | 5.4 ± 1.7 | 2.7 ± 1.9 | 0.8 ± 0.9 | 13.52 | <0.0001** |
| RAADS-R–Total | 126.6 ± 36.5 | 50.6 ± 39.5 | 116.5 ± 37.0 | 142.3 ± 31.1 | 59.6 ± 45.0 | 33.5 ± 17.2 | 22.79 | <0.0001** |
| RAADS-R–Social relatedness | 61.1 ± 20.2 | 25.3 ± 21.5 | 56.1 ± 21.8 | 68.8 ± 15.4 | 30.6 ± 24.6 | 15.2 ± 7.4 | 17.32 | <0.0001** |
| RAADS-R–Circumscribed interest | 24.7 ± 9.6 | 9.6 ± 6.8 | 22.6 ± 8.1 | 28.0 ± 11.1 | 11.1 ± 7.2 | 6.6 ± 5.1 | 18.61 | <0.0001** |
| RAADS-R–Language | 10.4 ± 4.2 | 4.8 ± 4.2 | 9.9 ± 4.4 | 11.1 ± 4.1 | 5.4 ± 4.7 | 3.7 ± 2.9 | 8.77 | <0.0001** |
| RAADS-R–Sensory motor | 30.2 ± 12.1 | 10.6 ± 10.0 | 27.8 ± 12.1 | 34.1 ± 11.5 | 12.5 ± 11.4 | 7.0 ± 5.5 | 16.80 | <0.0001** |
| SRS-2–Total | 69.3 ± 8.6 | 50.8 ± 9.3 | 68.5 ± 8.2 | 70.5 ± 9.4 | 53.3 ± 10.5 | 46.0 ± 3.2 | 22.95 | <0.0001** |
| SRS-2–Social awareness | 63.4 ± 9.0 | 49.3 ± 10.2 | 63.9 ± 8.9 | 62.5 ± 9.5 | 51.1 ± 11.4 | 46.0 ± 6.6 | 10.75 | <0.0001** |
| SRS-2–Social cognition | 64.4 ± 10.0 | 49.9 ± 8.7 | 61.0 ± 8.9 | 69.0 ± 10.2 | 52.1 ± 9.3 | 45.8 ± 5.9 | 15.22 | <0.0001** |
| SRS-2–Social communication | 67.4 ± 8.8 | 48.9 ± 9.4 | 67.4 ± 9.1 | 67.6 ± 8.8 | 51.7 ± 10.5 | 43.4 ± 2.8 | 22.85 | <0.0001** |
| SRS-2–Social motivation | 67.4 ± 10.5 | 54.4 ± 10.0 | 66.5 ± 10.8 | 68.9 ± 10.4 | 56.1 ± 10.9 | 51.3 ± 7.8 | 8.18 | 0.00014** |
| SRS-2–Repetitive behaviors | 73.1 ± 11.2 | 51.8 ± 8.7 | 72.5 ± 10.7 | 74.9 ± 12.4 | 54.2 ± 9.4 | 47.3 ± 4.7 | 23.18 | <0.0001** |
| SRS-2–Social information processing | 67.6 ± 8.4 | 50.8 ± 9.3 | 66.6 ± 8.0 | 69.3 ± 9.0 | 53.1 ± 10.7 | 45.8 ± 3.0 | 20.07 | <0.0001** |
| RBS-R–Total | 40.3 ± 35.7 | N/A | 51.1 ± 35.7 | 10.2 ± 7.6 | N/A | N/A | N/A | N/A |
| RBS-R–Stereotyped behavior | 5.4 ± 5.2 | N/A | 7.1 ± 4.9 | 0.4 ± 0.5 | N/A | N/A | N/A | N/A |
| RBS-R–Self-injurious behavior | 6.1 ± 6.3 | N/A | 8.0 ± 6.3 | 0.6 ± 0.9 | N/A | N/A | N/A | N/A |
| RBS-R–Compulsive behavior | 7.6 ± 6.9 | N/A | 9.3 ± 7.1 | 3.0 ± 3.3 | N/A | N/A | N/A | N/A |
| RBS-R–Ritualistic behavior | 5.7 ± 4.6 | N/A | 7.0 ± 4.7 | 2.0 ± 1.2 | N/A | N/A | N/A | N/A |
| RBS-R–Sameness | 11.0 ± 9.9 | N/A | 13.9 ± 9.9 | 2.6 ± 3.0 | N/A | N/A | N/A | N/A |
| RBS-R–Restricted behavior | 4.6 ± 4.1 | N/A | 5.7 ± 4.2 | 1.6 ± 1.1 | N/A | N/A | N/A | N/A |
| BEQ–Negative emotionality | 23.7 ± 8.4 | 21.6 ± 6.0 | 23.7 ± 6.0 | 23.6 ± 11.6 | 19.7 ± 5.1 | 25.1 ± 6.2 | 1.66 | 0.188 |
| BEQ–Positive emotionality | 19.5 ± 5.8 | 21.5 ± 5.0 | 17.9 ± 5.8 | 21.9 ± 4.9 | 20.7 ± 4.9 | 22.9 ± 4.9 | 2.34 | 0.084 |
| BEQ–Impulse strength | 29.6 ± 9.3 | 26.1 ± 7.7 | 25.7 ± 9.2 | 35.6 ± 5.4 | 23.7 ± 8.0 | 30.7 ± 4.5 | 6.88 | 0.0005** |
| BEQ–Emotional expressivity | 72.8 ± 19.2 | 69.1 ± 15.6 | 67.3 ± 18.1 | 81.2 ± 18.6 | 64.1 ± 15.0 | 78.7 ± 12.3 | 3.62 | 0.019* |
| SPAI–Social phobia | 110.8 ± 37.8 | 69.3 ± 40.1 | 106.7 ± 35.9 | 115.9 ± 41.3 | 73.0 ± 43.5 | 63.1 ± 35.2 | 3.78 | 0.017* |
| SPAI–Agoraphobia | 27.6 ± 18.0 | 16.3 ± 15.0 | 27.6 ± 20.6 | 27.6 ± 14.9 | 16.5 ± 14.9 | 16.0 ± 16.1 | 1.82 | 0.157 |
| SPAI–Difference | 83.2 ± 34.4 | 53.0 ± 32.4 | 79.1 ± 30.3 | 88.3 ± 40.0 | 56.5 ± 37.5 | 47.1 ± 22.1 | 3.54 | 0.022* |
Note: Values reported are mean ± SD. One-way ANOVA was performed between the four Diagnosis + Gender groups.
*P < 0.05, **P < 0.01.
FSIQ Full-scale IQ, VIQ Verbal IQ, NVIQ Non-verbal IQ, AQ Autism-Spectrum Quotient, RAADS-R Ritvo Autism Asperger Diagnostic Scale-Revised, SRS-2 Social Responsiveness Scale, 2nd Edition, RBS-R Repetitive Behavior Scale-Revised, BEQ Berkeley Expressivity Questionnaire, SPAI Social Phobia and Anxiety Inventory.
Socio-communicative functioning was assessed by the AQ, Ritvo Autism Asperger’s Diagnostic Scale–Revised (RAADS-R) [38], and SRS-2 [39]. Based on a recent systematic review of screening and diagnostic tools for adults with ASD of mean normal intelligence, AQ and RAADS-R were found to provide the most satisfactory psychometric properties [40]. Therefore, we have focused on these two measures in this report. Other emotional domains were measured by using the Berkeley Expressivity Questionnaire (BEQ) [41] and Social Phobia Anxiety Inventory (SPAI) [42]. Repetitive behaviors were assessed by the Repetitive Behavior Scale–Revised (RBS-R) [43]. The RBS-R is a rating scale completed by parents.
Among the 28 participants with ASD, 20 were taking at least 1 psychotropic medication, including serotonin reuptake inhibitors (N = 13), stimulants (N = 8), atypical antipsychotics (N = 4), non-stimulants (N = 3), and other medications (melatonin (N = 3), bupropion (N = 2), oxcarbazepine (N = 2), duloxetine (N = 1), hydroxyzine (N = 1)). Among the 29 TD participants, one was taking melatonin; another participant was taking a stimulant. Because of this group difference, psychotropic medication usage was included as a binary co-variate in generalized linear model (GLM) analyses (see “Statistical Analysis” section). No participants took benzodiazepines or other medications that directly modulate the binding of GABAA receptor within 4 weeks of the study.
Power analysis
When this study was first designed, there was no available [18F]FMZ-PET data measuring GABAA receptor BPND in the DLPFC or thalami of individuals with ASD. However, postmortem examination of the superior frontal cortex revealed lower levels of γ subunit of GABAA receptors in adults with ASD (0.255 ± 0.137), compared to neurotypical controls (0.198 ± 0.050) [12]. Using these results and assuming an α value of 0.05, 30 subjects per group would be needed to yield a power of 70% in a 1-way analysis of variance (ANOVA). Based on GABA data reported by Harada et al. [23], the GABA levels in the frontal lobe were 1.1 ± 0.23 and 1.5 ± 0.25. Using these results and assuming an α value of 0.05, 6 subjects per group would be needed to yield a power of 80% in a 2-way ANOVA. This number of participants needed was much lower than that estimated for the PET component of this study (N = 30 per group). Overall, we predicted that 30 participants would be needed to demonstrate significant group differences in BPND and GABA concentrations in the DLPFC.
Neuroimaging data acquisition
Acquisition of PET data with concurrent 1H-MRS and structural MRI was performed using a state-of-the-art simultaneous hybrid PET/MR imaging system (SIGNA PET/MR, GE Healthcare, Waukesha, WI) [44, 45]. The radiotracer employed for binding GABAA receptors was [18F]flumazenil ([18F]FMZ) [46]. Dynamic PET data were used in combination with 3D T1-weighted structural MR data to acquire the BPND of [18F]FMZ for the GABAA receptors [46]. The Ichise’s Original Multilinear Reference Tissue Model (MRTM0) [47] was employed for kinetic modeling. More detailed information on the synthesis of clinical grade [18F]FMZ, dynamic PET image acquisition, and PET data analyses can be found in supplementary materials.
In addition to region-based PET data analyses, we also performed whole-brain analyses. During PET data acquisition, a series of MR sequences were run, including a 3D T1-weighted protocol [repetition time (TR) = 7.9 ms; echo time (TE) = 2.9 ms; field of view (FOV) = 240 mm × 192 mm; matrix = 220 × 160; flip angle (FA) = 12°; axial plane; slice thickness (TH) = 1.4 mm; 128 slices] and two single-voxel 1H-MRS sequencing prescribed at the left DLPFC and bilateral thalami (Supplementary Fig. 1). The T1 was used for planning the positioning of the target voxels. The determination of brain levels of GABA and other metabolites was achieved by an Improved MEGA-SPECIAL sequence [TE = 80 ms; TR = 2000 ms; voxel size ~15 cm3; 15 min acquisition time] [48]. Based on 1D Image-Selected in Vivo Spectroscopy (ISIS) spatial localization and single spin echo, this editing technique allows much longer (30 ms) and more selective editing pulses than those used in MEGA-PRESS, enabling B0-inhomogeneity-insensitive GABA editing with macromolecule suppression. To reduce susceptibility and motion artifacts in the ISIS direction, out-of-voxel suppression was achieved using a 1D echo planar (EP) gradient during readout [48]. A full optimization of the acquisition of 1H-MRS data using Improved MEGA-SPECIAL performed in a 3T MR scanner without PET detector was recently reported [48]. This method was demonstrated to effectively suppress the macromolecule signal that typically interferes with the GABA signal. In this study, we employed the Improved MEGA-SPECIAL as the pulse sequence to acquire 1H-MRS data in the hybrid PET-MR scanner. In contrast to standalone MR scanners where the bed position is fixed within a pulse sequence but can be moved between pulse sequences, simultaneous PET and MR data acquisitions require that the position of the scanner bed be fixed during the PET scan.
Spectra of editing ON and editing OFF were reconstructed and the GABA edited spectrum was obtained by subtracting the editing OFF spectrum from the editing ON spectrum [48]. Total Cr (Cr + PCr), NAA, Cho, myoinositol (mI), and sum of glutamate (Glu) and glutamine (Gln) [Glx = Glu + Gln] were quantified from the editing OFF spectrum using LCModel and referenced to both the total Cr (Cr + PCr) and the unsuppressed water. Only spectra with CRLB lower than or equal to 20% for Cr + PCr, NAA, and Cho were included in the analysis. GABA levels were estimated from the integration of the 3ppm peak in the edited spectrum and were also referenced to both the total Cr (Cr + PCr) and the unsuppressed water.
The percentages of white matter, gray matter, and cerebrospinal fluid between ASD and TD groups were statistically indistinguishable (Supplementary Table 1); therefore, we chose to report concentrations of the metabolites without adjusting for tissue composition.
Primary hypotheses
We hypothesize that both BPND and GABA concentration in the DLPFC and thalamus will be reduced in ASD. We also hypothesize that there exists a correlation between both of these parameters and ASD symptom severity that may be modified by sex.
Statistical analysis
All analyses were run in R version 3.5.3. Participants’ demographic and neuropsychological assessment data were compared between the four Diagnosis + Gender groups—TD Male, ASD Male, TD Female, ASD Female—with one-way analysis of variance (ANOVA). Significance was set at P < 0.05. Demographic variables with significant group differences were identified as possible confounders and included as co-variates in subsequent analyses. Post-hoc comparisons to identify specific group-mean differences were performed using Tukey’s HSD test, with significance set at adjusted P < 0.05. To assess whether group differences in socio-communicative function could be driven by mood and anxiety differences in those same groups, Pearson’s correlations were run between AQ/RAADS-R/SRS-2 total scores and BEQ/SPAI scores.
For the MRS data, quality control parameters for magnetic resonance spectra determined from LCModel were compared between ASD and TD groups with Welch two-sample T-tests. Mean GABA/Water concentration at each of the two MRS voxels—bilateral thalami and left DLPFC—was compared between groups with two-way ANOVA that used Diagnosis and Gender as between-subject variables. Post-hoc analysis was run with a GLM at each of the voxels, using the significant Diagnosis, Gender, and/or interaction terms, as well as the demographic co-variates, as independent variables, and mean GABA/Water concentration as the dependent variable. Significance of the main effects or interaction effects was set at P < 0.05.
For the PET data, in an exploratory fashion, the mean BPND of [18F]FMZ at every PET region were compared between groups with the same two-way ANOVA as above. Findings from the PET regions that correspond to the MRS voxels—left thalamus, right thalamus, and left middle frontal gyrus—are reported.
To investigate possible correlations between MRS measurements of GABA levels and PET measurements of receptor density, Pearson’s correlation analysis was run between thalamic GABA/Water concentrations and [18F]FMZ BPND of both sides of the thalamus, as well as between GABA/Water at the left DLPFC and [18F]FMZ BPND of the left middle frontal gyrus.
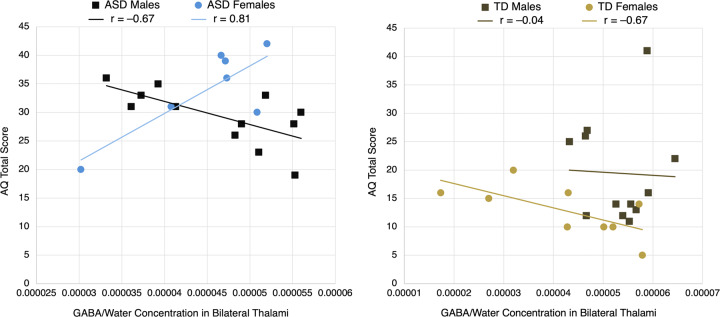
To investigate associations of GABA concentrations with AQ and RAADS-R total scores, participants were stratified by Diagnosis, then GLMs were run for the regions of interest that were identified by MRS to have significant group differences in GABA concentrations. The independent variables were Gender, GABA/Water concentration, the interaction term between Gender and GABA/Water concentration, and the demographic co-variates; the dependent variables were AQ and RAADS-R total scores. Significance was set at a P value less than 0.0125 to correct for 4 GLMs. Simple correlation coefficients (r) for each of the four Diagnosis + Gender groups’ trendlines in Fig. 3 are reported.
Fig. 3. Scatterplots, stratified by diagnosis, of AQ total score vs. thalamic GABA/Water concentration, with trendlines for each gender.
A significant interaction effect for ASD participants, but not TD participants, was found between gender and GABA in predicting AQ (P = 0.00071). Reported r values are simple correlation coefficients for each trendline.
Results
Demographics and clinical assessments
Table 1 shows the demographics of the participants and findings from neuropsychological assessments. Using one-way ANOVAs to compare means between the four groups separated by diagnosis and gender, we found significant group differences in age (F(3,53) = 4.69, P = 0.006) and non-verbal IQ (F(3,49) = 4.54, P = 0.007), as well as a near-significant group difference in full-scale IQ (F(3,49) = 2.39, P = 0.080). To account for possible confounding factors, we included age and full-scale IQ, along with medication usage, as co-variates in subsequent GLM analyses. Post-hoc comparisons with Tukey HSD demonstrated that ASD males were significantly younger than ASD females (P = 0.003); no other group differences in age were significant. Furthermore, ASD males had significantly lower non-verbal IQ than TD males (P = 0.008); no other group differences in non-verbal IQ were significant.
As expected, one-way ANOVA also revealed significant group differences in socio-communicative function (P < 0.0001 for AQ, RAADS-R, and SRS-2 total scores and almost all sub-scales). Post-hoc comparisons with Tukey HSD demonstrated that these significant group differences were not attributable to gender. There were no significant differences when comparing ASD males with ASD females, or when comparing TD males with TD females, with the one exception of AQ: Imagination (TD male vs. TD female adjusted P = 0.034). Instead, the diagnosis of ASD drove group differences. TD females differed significantly from ASD males and ASD females on all AQ, RAADS-R, and SRS-2 subscales (adjusted P < 0.01), except for AQ-Attention to Details and AQ-Imagination. TD males differed significantly from ASD males and ASD females on all AQ, RAADS-R, and SRS-2 subscales (adjusted P < 0.05), except for AQ: Attention to Details, Imagination, and Attention Switching.
In terms of other co-morbid symptoms, mood and anxiety were associated with both gender and diagnosis. Preliminary ANOVA identified significant group differences in BEQ-Impulse Strength (F(3,53) = 6.88, P = 0.0005) and BEQ-Emotional Expressivity (F(3,53) = 3.62, P = 0.019). Post-hoc comparisons with Tukey HSD demonstrated that ASD females scored significantly higher on BEQ-Impulse Strength than TD males (P < 0.001) and ASD males (P = 0.006), as well as significantly higher on BEQ-Emotional Expressivity than TD males (P = 0.037). No other significant group differences on the BEQ were found. ANOVA also identified significant group differences in SPAI-Social Phobia (F(3,45) = 3.78, P = 0.017) and SPAI-Difference (F(3,45) = 3.54, P = 0.022). Using Tukey HSD, we found only one significant difference in means: ASD females scored significantly higher on SPAI-Difference than TD females (P = 0.045).
To assess whether differences in socio-communicative function in ASD females could be driven by their underlying mood and anxiety differences, we used Pearson’s correlations to investigate if BEQ-Impulse Strength, BEQ- Emotional Expressivity, and SPAI-Difference scores correlated with the total scores of AQ, RAADS-R, and SRS-2. Importantly, we found no significant correlations (P > 0.10 for all). Therefore, any brain correlates of socio-communicative function in individuals with ASD described below were specific and not driven by underlying anxiety.
1H-MRS GABA concentrations
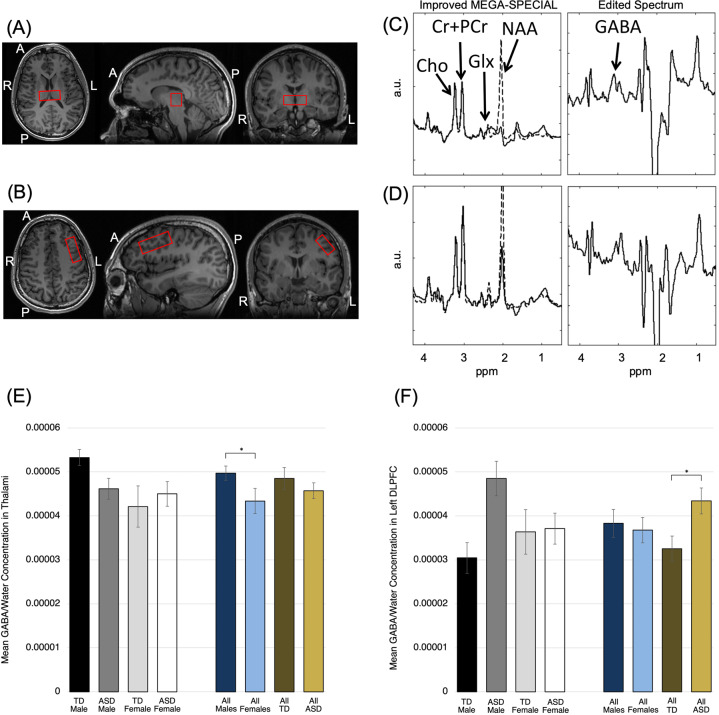
Figure 1 and Supplementary Fig. 1 show the location of voxel placements in the bilateral thalami and left DLPFC, as well as their corresponding proton magnetic resonance spectra. The mean concentrations of GABA/Water measured by 1H-MRS in these two regions are graphed by diagnosis and gender. In addition to GABA/Water, the concentrations of all other MRS-measured metabolites are presented in Table 2.
Fig. 1. Proton magnetic resonance spectroscopy (1H-MRS) data acquisition in adults with autism spectrum disorder (ASD) and typically developing (TD) controls.
Location of 1H-MRS voxel placement at the (a) bilateral thalami and (b) left DLPFC. Improved MEGA-SPECIAL spectra and corresponding edited spectra are shown for the (c) thalami and (d) left DLPFC. Group-mean GABA/Water concentrations by diagnosis and gender are shown for the (e) bilateral thalami and (f) left DLPFC. Error bars represent ± 1 SEM. Significant main effects of diagnosis or gender (P < 0.05 in primary two-way ANOVAs) are starred (*). After covarying for age, psychotropic medication usage, and IQ, the gender difference in thalamic GABA remained significant. The TD vs. ASD difference in DLPFC GABA remained significant after covarying for medication usage and IQ, but not after adjusting for age.
Table 2.
a. Concentrations of metabolites by group, as measured by proton magnetic resonance spectroscopy (1H-MRS) in bilateral thalami and left DLPFC.
| ASD | TD | ASD male | ASD female | TD male | TD female | Main effect of diagnosis P | Main effect of gender P | Interaction effect P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary analyses | |||||||||||||||
| Thalami | N | N | N | N | N | N | |||||||||
| GABA/Water (×10−5) | 19 | 4.57 ± 0.77 | 21 | 4.85 ± 1.16 | 12 | 4.62 ± 0.83 | 7 | 4.50 ± 0.74 | 12 | 5.33 ± 0.64 | 9 | 4.21 ± 1.41 | 0.49 | 0.049* | 0.11 |
| Left DLPFC | |||||||||||||||
| GABA/Water (×10−5) | 18 | 4.34 ± 1.25 | 20 | 3.25 ± 1.29 | 10 | 4.85 ± 1.24 | 8 | 3.71 ± 1.00 | 13 | 3.04 ± 1.26 | 7 | 3.63 ± 1.34 | 0.027* | 0.51 | 0.041* |
| Secondary analyses | |||||||||||||||
| Thalami | N | N | N | N | N | N | |||||||||
| GABA/Cr + PCr | 19 | 5.08 ± 0.81 | 21 | 5.44 ± 1.51 | 12 | 5.06 ± 0.78 | 7 | 5.12 ± 0.93 | 12 | 5.70 ± 1.07 | 9 | 5.09 ± 1.96 | |||
| GABA/Glx | 18 | 5.73 ± 0.81 | 14 | 5.97 ± 2.33 | 11 | 5.67 ± 1.43 | 7 | 5.84 ± 1.61 | 7 | 5.56 ± 1.64 | 7 | 6.38 ± 2.94 | |||
| NAA/Cr + PCr | 26 | 1.54 ± 0.21 | 26 | 1.49 ± 0.31 | 15 | 1.61 ± 0.17 | 11 | 1.45 ± 0.24 | 16 | 1.43 ± 0.32 | 10 | 1.58 ± 0.27 | |||
| Glx/Cr + PCr | 21 | 0.98 ± 0.34 | 17 | 1.00 ± 0.29 | 12 | 1.06 ± 0.43 | 9 | 0.88 ± 0.13 | 10 | 1.05 ± 0.22 | 7 | 0.94 ± 0.37 | |||
| Cr + PCr (×108) | 27 | 1.49 ± 0.80 | 26 | 1.45 ± 0.59 | 16 | 1.37 ± 0.75 | 11 | 1.66 ± 0.88 | 16 | 1.46 ± 0.64 | 10 | 1.44 ± 0.54 | |||
| Left DLPFC | |||||||||||||||
| GABA/Cr + PCr | 18 | 3.39 ± 0.99 | 20 | 2.92 ± 1.71 | 10 | 3.51 ± 1.07 | 8 | 3.23 ± 0.92 | 13 | 2.41 ± 1.13 | 7 | 3.87 ± 2.26 | |||
| GABA /Glx | 14 | 2.83 ± 1.06 | 18 | 2.04 ± 1.12 | 9 | 2.88 ± 1.08 | 5 | 2.72 ± 1.13 | 12 | 1.82 ± 1.22 | 6 | 2.47 ± 0.86 | |||
| NAA/Cr + PCr | 24 | 1.42 ± 0.23 | 24 | 1.32 ± 0.37 | 14 | 1.39 ± 0.25 | 10 | 1.46 ± 0.22 | 16 | 1.28 ± 0.41 | 8 | 1.41 ± 0.25 | |||
| Glx/Cr + PCr | 18 | 1.30 ± 0.33 | 21 | 2.14 ± 2.73 | 12 | 1.32 ± 0.39 | 6 | 1.25 ± 0.19 | 14 | 1.71 ± 0.89 | 7 | 3.02 ± 4.66 | |||
| Cr + PCr (×108) | 26 | 1.82 ± 1.05 | 28 | 1.72 ± 0.80 | 16 | 1.75 ± 1.06 | 10 | 1.93 ± 1.08 | 18 | 1.73 ± 0.72 | 10 | 1.70 ± 0.98 | |||
| b. Quality control parameters for magnetic resonance spectra determined from LCModel | |||
|---|---|---|---|
| Cramer Rao lower bound (CRLB) | |||
| Thalami | ASD | TD | P |
| Cho | 3.95 ± 1.28 | 4.25 ± 2.02 | 0.588 |
| Glx | 21.24 ± 10.55 | 21.01 ± 18.83 | 0.798 |
| mI | 23.71 ± 11.65 | 18.78 ± 8.52 | 0.081 |
| NAA | 6.86 ± 3.90 | 6.75 ± 3.53 | 0.932 |
| Cr + PCr | 4.24 ± 1.09 | 4.94 ± 3.77 | 0.423 |
| Left DLPFC | |||
| Cho | 5.29 ± 2.39 | 5.93 ± 3.39 | 0.606 |
| Glx | 15.82 ± 12.10 | 19.95 ± 20.76 | 0.241 |
| mI | 14.41 ± 7.23 | 15.29 ± 7.35 | 0.935 |
| NAA | 8.41 ± 4.62 | 7.27 ± 3.69 | 0.272 |
| Cr + PCr | 4.12 ± 1.90 | 5.00 ± 2.90 | 0.341 |
| Signal-to-noise ratio (SNR) and full width at half maximum (FWHM) | |||
|---|---|---|---|
| Thalami | ASD | TD | P |
| SNR | 16.59 ± 6.65 | 15.59 ± 6.11 | 0.559 |
| FWHM | 0.071 ± 0.018 | 0.077 ± 0.026 | 0.270 |
| Left DLPFC | |||
| SNR | 15.56 ± 6.35 | 17.75 ± 8.09 | 0.299 |
| FWHM | 0.096 ± 0.029 | 0.114 ± 0.043 | 0.105 |
Note: a: Values reported are mean ± SD. P values reported are from two-way ANOVAs: Thalami: F(3,36) = 2.78; Left DLPFC: F(3,34) = 4.18. *P < 0.05.
TD typically developing, Cr + PCr creatine and phosphocreatine, GABA gamma aminobutyric acid, Glx glutamine and glutamate, mI myo-inositol, NAA N-acetylaspartate.
Note: b: Values reported are mean ± SD. P values are from Welch two-sample T-test performed between ASD and TD groups. Cho choline, Glx sum of glutamine and glutamate, mI myo-inositol, NAA N-acetylaspartate, Cr + PCr creatine + phosphocreatine.
In the thalami, two-way ANOVA using diagnosis and gender as between-subject variables did not identify a significant interaction, but did identify an effect of gender (F(3,36) = 2.78, P = 0.049). Post-hoc GLM analysis that included gender, age, medication usage, and FSIQ as the independent variables identified significantly higher GABA/Water in males than in females (F(4,34) = 2.19, P = 0.043).
In the left DLPFC, two-way ANOVA identified a significant Diagnosis × Gender interaction effect (F(3,34) = 4.18, P = 0.041) and a significant main effect of diagnosis (P = 0.027). Post-hoc GLM analysis adjusting for medication usage and IQ retained the significance of the interaction (F(5,30) = 2.39, P = 0.046); however, including age in the model made the term insignificant.
PET GABAA receptor densities
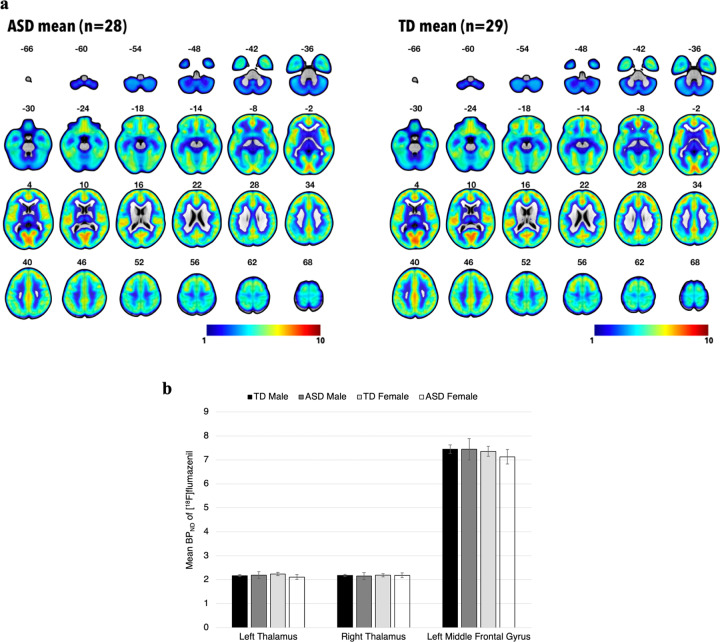
We investigated GABAA receptor densities, as represented by BPND of [18F]FMZ, in both left and right thalami, as well as left middle frontal gyrus (within which lies the left DLPFC), using two-way ANOVA. We found no significant differences between participants grouped by diagnosis and gender (Fig. 2b).
Fig. 2. Positron emission tomography (PET) imaging with [18F]flumazenil in adults with autism spectrum disorder (ASD) and typically developing (TD) individuals.
a Group-mean parametric maps derived from PET data in standard MNI space. Color bar represents non-displaceable binding potential (BPND) of [18F]flumazenil. Mean parametric maps do not differ significantly between groups. b Group-mean BPND of [18F]flumazenil in the thalami and left DLPFC, as detected by PET. Error bars represent ±1 SEM. Mean BPND in these regions of interest do not differ significantly between groups.
The BPND’s of other regions of interest were also compared between groups with exploratory two-way ANOVA, and no significant differences were found (Supplementary Table 2). Whole-brain voxel-based analysis of BPND’s also revealed neither any significant main effects of Diagnosis (Fig. 2a) or Gender, nor a Diagnosis × Gender interaction effect.
Possible correlations between MRS measurements of GABA levels and PET measurements of receptor density at the thalami and left DLPFC / left middle frontal gyrus were investigated using Pearson’s correlation analysis. No significant correlations between GABA/Water concentrations and [18F]FMZ BPND were found at these regions.
Gender modifies thalamic GABA–symptom severity relationship
Having shown that thalamic GABA/Water concentrations differ between genders, we tested the hypothesis that thalamic GABA correlates with ASD symptom severity in gender-specific ways. Stratifying by diagnosis—the dominant predictor of AQ and RAADS—we used four total GLMs covarying for age, medication usage, and IQ in order to investigate the interaction between gender and thalamic GABA/Water in predicting AQ and RAADS-R total scores.
For ASD participants, a significant interaction effect was noted between gender and thalamic GABA in predicting AQ total score (F(6,12) = 4.76, P = 0.00071) and RAADS-R total score (F(6,12) = 4.76, P = 0.0019). For TD participants, on the other hand, there was no significant interaction effect for either behavioral measure. Figure 3 presents scatterplots of the relationships between AQ total score and thalamic GABA/Water concentrations, with participants separated by diagnosis and gender.
Discussion
In a comprehensive manner, we studied both GABAA receptor densities and GABA concentrations in the left DLPFC and bilateral thalami in high-functioning adults (HFA) with ASD. Our results provide evidence for region-dependent and gender-specific differences in GABA concentrations, but not GABAA receptor binding densities, between HFA with ASD and TD adults. The latter result further replicated the findings in a recent report [16], which examined GABAA receptor densities but not GABA concentrations.
While previous studies have reported lower GABA levels in cortical regions (frontal lobes [19, 23], auditory cortex [21, 22], and motor cortex [21]) in children and adolescents with ASD as compared to age-matched TD controls, this study found higher GABA levels in the left DLPFC of HFA with ASD as compared to TD adults. It is not clear what contributes to the discrepancy in GABA levels in the cortical regions. However, higher resting levels of GABA have been shown to negatively correlate with the BOLD response in various brain regions [49–51], including the DLPFC [52]. Increased GABAergic (inhibitory) tone in the DLPFC could thus explain why this region exhibits decreased activation during working memory tasks in adults with ASD [14]. We also speculate that higher cortical GABA levels may be the result of compensation for primary defects occurring elsewhere in the GABAergic signaling pathway. Compensatory models have been proposed to explain why, for instance, despite having alterations in the E-I ratio, several mouse models of ASD have relatively normal synaptic depolarization and spiking [53]. One possibility is that increased neurotransmitter production could compensate for abnormalities in GABA receptor function or localization rather than density, as seen in cerebellar basket cells in ASD [54]. Although we did not find group differences in GABAA receptor density, our study cannot rule out that GABAA receptors are functionally impaired in ASD, as prior studies have suggested [12]. Furthermore, our study does not examine GABAB receptors, and several studies have indicated that this receptor subtype may be dysfunctional in ASD [8, 13, 55].
Compared to the cortical regions, sub-cortical brain regions have been studied much less. Harada et al. reported that the GABA levels in the lenticular nucleus of the basal ganglia of children and adolescents with ASD and age-matched controls were statistically indistinguishable [23]. This study represents the first study investigating the GABA levels in the thalami of adults with ASD. When all participants were included, we found no group difference in thalamic GABA levels. It is interesting to find region-specific differences in GABA levels. We speculate that cortical regions tend to be more plastic and are therefore more able to compensate for the deficits in GABAergic tone by increasing the levels of GABA over time. However, the thalami may not be as plastic as the cortical regions.
In addition to region-dependent GABA concentration alterations, we also found region-dependent and gender-specific correlations between GABA concentrations and socio-communicative function. Our findings complement previous research on the relationship between GABA in the right superior temporal sulcus (STS) and socio-communicative function [34]. Specifically, Kirkovski et al. found a significant positive correlation between GABA concentrations at the right STS and social relatedness subscale of RAADS-R in females with ASD but not in males with ASD.
The gender difference in the correlations between thalamic GABA levels and socio-communicative function (negative correlation in ASD males and positive correlation in ASD females) may translate to different pharmacologic effects and behavioral outcomes between males and females with ASD. Our results suggest that medications that modulate GABA levels throughout the brain will normalize the GABA levels in some brain regions but potentially disturb the GABA levels in other brain regions, depending on gender. Such an idea is consistent with studies that show ASD symptomatology can vary by gender [56]. Potential mechanisms to explain these differences remain speculative, but evidence suggests that females with ASD may have distinct neuroanatomical and neurophysiological signatures [57, 58]. For instance, Kirkovski et al. found decreased activity in the superior temporal sulcus in ASD males compared to controls while processing social information, but no difference when comparing ASD females to controls [59]. Furthermore, the direction of the relationship between GABA and social impairments in ASD has been shown to vary by gender in previous literature, consistent with our own findings. In a separate study examining GABA and social functioning in ASD, Kirkovski found a positive relationship between GABA concentrations at the superior temporal sulcus and social impairment in females with ASD, but not males [34]. In contrast, Brix et al. found a negative relationship in boys when assessing GABA levels in the anterior cingulate cortex [60]. Collectively, these results, in conjunction with our current findings, indicate the importance of investigating gender differences in future ASD studies.
Limitations
Our study has several limitations. One major limitation of this study is that the age between males and females with ASD was not well matched. ASD females were, on average, 10 years older than ASD males. Second, the FSIQ for the ASD group is lower than the TD group; this difference is more pronounced in males. Third, although our overall sample size is larger than most studies involving PET, it is relatively small when we separated males from females in our investigation on gender effects. (However, at α level of 0.05, we did achieve 94% power when comparing left DLPFC GABA/Water levels between ASD males and TD males.) Fourth, some participants in this study were taking medications. For example, some antipsychotic medications are known to modulate the GABAergic system. (This is unlikely to affect the results significantly, as only four participants took antipsychotics. Furthermore, no participants took benzodiazepines.) Finally, the success rate for GABA concentration determination by 1H-MRS was only about 70% in the PET-MR scanner; therefore, we did not have measurable GABA concentrations for every participant. Given these limitations, in order to further translate the findings in this study to the clinic, we will need to replicate the results in a larger sample with improved matches in age and IQ.
Conclusions
To our knowledge, this is the first study to examine both GABA concentrations and GABAA receptor binding densities simultaneously in any psychiatric population. It is also the first neuroimaging study to investigate the role of the GABAergic system in regions of the thalamocortical network, as it relates to HFA with ASD. We show that, despite no group differences in GABAA receptor densities, GABA concentrations in the left DLPFC are higher in HFA with ASD, compared to TD controls. Furthermore, GABA concentrations in the thalami correlate with AQ and RAADS-R scores in a gender-specific manner in HFA with ASD, but not in TD controls. Remarkably, higher thalamic GABA concentrations are associated with lower socio-communicative symptom severity in males with ASD, and with higher symptom severity in females with ASD. We conclude that thalamic and prefrontal GABA levels are altered in a region-dependent and gender-specific manner in HFA with ASD. Our findings are important steps toward identifying molecular neuroimaging markers of socio-communicative function in individuals with ASD, thus aiding the development of assessment tools to evaluate neural circuits and interventions targeting core symptoms of ASD.
Supplementary information
Acknowledgements
We thank Bin Shen and Jessa Castillo for synthesizing the radiotracer [18F]flumazenil, Harsh Gandhi and Dawn Holley for acquiring the PET and MR data, and Dr. Praveen Gulaka for coordinating the acquisition of the PET and MR data. Dr. Shyam Srinivas was the nuclear medicine physician supporting this project. Dr. Greg Zaharchuk provided useful comments on the acquisition of MR data. Research reported in this publication was supported by the National Institute of Mental Health (K08MH111750 awarded to LKF; R01MH110683 awarded to DS), American Academy of Child and Adolescent Psychiatry (awarded to LKF), National Institute of Child and Human Development (R01HD084214 awarded to FTC), and GE Healthcare (awarded to FTC).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Frederick T. Chin, Antonio Y. Hardan
Supplementary information
The online version of this article (10.1038/s41380-020-0756-y) contains supplementary material, which is available to authorized users.
References
- 1.Vorstman JAS, Parr JR, Moreno-De-Luca D, Anney RJL, Nurnberger JI, Jr., Hallmayer JF. Autism genetics: opportunities and challenges for clinical translation. Nat Rev Genet. 2017;18:362–76. doi: 10.1038/nrg.2017.4. [DOI] [PubMed] [Google Scholar]
- 2.Hertz-Picciotto I, Schmidt RJ, Krakowiak P. Understanding environmental contributions to autism: causal concepts and the state of science. Autism Res. 2018;11:554–86. doi: 10.1002/aur.1938. [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–67. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gogolla N, Leblanc JJ, Quast KB, Sudhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009;1:172–81. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–71. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 6.Bonifazi P, Goldin M, Picardo MA, Jorquera I, Cattani A, Bianconi G, et al. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science. 2009;326:1419–24. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- 7.Oblak A, Gibbs TT, Blatt GJ. Decreased GABA(A) receptors and benzodiazepine binding sites in the anterior cingulate cortex in autism. Autism Res. 2009;2:205–19. doi: 10.1002/aur.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oblak AL, Gibbs TT, Blatt GJ. Decreased GABA(B) receptors in the cingulate cortex and fusiform gyrus in autism. J Neurochem. 2010;114:1414–23. doi: 10.1111/j.1471-4159.2010.06858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oblak AL, Gibbs TT, Blatt GJ. Reduced GABAA receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Res. 2011;1380:218–28. doi: 10.1016/j.brainres.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Autism Dev Disord. 2001;31:537–43. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- 11.Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) receptor downregulation in brains of subjects with autism. J Autism Dev Disord. 2009;39:223–30. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatemi SH, Reutiman TJ, Folsom TD, Rustan OG, Rooney RJ, Thuras PD. Downregulation of GABAA receptor protein subunits alpha6, beta2, delta, epsilon, gamma2, theta, and rho2 in superior frontal cortex of subjects with autism. J Autism Dev Disord. 2014;44:1833–45. doi: 10.1007/s10803-014-2078-x. [DOI] [PubMed] [Google Scholar]
- 13.Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD. Expression of GABA(B) receptors is altered in brains of subjects with autism. Cerebellum. 2009;8:64–69. doi: 10.1007/s12311-008-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luna B, Minshew NJ, Garver KE, Lazar NA, Thulborn KR, Eddy WF, et al. Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology. 2002;59:834–40. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- 15.Sawa T, Kodaira M, Oiji A, Sasayama D, Iwadare Y, Ushijima H, et al. Dysfunction of orbitofrontal and dorsolateral prefrontal cortices in children and adolescents with high-functioning pervasive developmental disorders. Ann Gen Psychiatry. 2013;12:31. doi: 10.1186/1744-859X-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horder J, Andersson M, Mendez MA, Singh N, Tangen A, Lundberg J, et al. GABAA receptor availability is not altered in adults with autism spectrum disorder or in mouse models. Sci Transl Med 2018;10:pii: eaam8434. [DOI] [PubMed]
- 17.Sieghart W. Structure and pharmacology of gamma-aminobutyric acid A receptor subtypes. Pharm Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 18.Robertson CE, Ratai EM, Kanwisher N. Reduced GABAergic action in the autistic brain. Curr Biol. 2016;26:80–85. doi: 10.1016/j.cub.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Kubas B, Kulak W, Sobaniec W, Tarasow E, Lebkowska U, Walecki J. Metabolite alterations in autistic children: a 1H MR spectroscopy study. Adv Med Sci. 2012;57:152–6. doi: 10.2478/v10039-012-0014-x. [DOI] [PubMed] [Google Scholar]
- 20.Cochran DM, Sikoglu EM, Hodge SM, Edden RA, Foley A, Kennedy DN, et al. Relationship among glutamine, gamma-aminobutyric acid, and social cognition in autism spectrum disorders. J Child Adolesc Psychopharmacol. 2015;25:314–22. doi: 10.1089/cap.2014.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, et al. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage. 2014;86:1–9. doi: 10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rojas DC, Singel D, Steinmetz S, Hepburn S, Brown MS. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage. 2014;86:28–34. doi: 10.1016/j.neuroimage.2013.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H, et al. Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. J Autism Dev Disord. 2011;41:447–54. doi: 10.1007/s10803-010-1065-0. [DOI] [PubMed] [Google Scholar]
- 24.Brix MK, Ersland L, Hugdahl K, Dwyer GE, Grüner R, Noeske R, et al. Within- and between-session reproducibility of GABA measurements with MR spectroscopy. J Magn Reson Imaging. 2017;46:421–30. doi: 10.1002/jmri.25588. [DOI] [PubMed] [Google Scholar]
- 25.Drenthen GS, Barendse EM, Aldenkamp AP, van Veenendaal TM, Puts NA, Edden RA, et al. Altered neurotransmitter metabolism in adolescents with high-functioning autism. Psychiatry Res Neuroimaging. 2016;256:44–49. doi: 10.1016/j.pscychresns.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ajram LA, Pereira AC, Durieux AMS, Velthius HE, Petrinovic MM, McAlonan GM. The contribution of [1H] magnetic resonance spectroscopy to the study of excitation-inhibition in autism. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:236–44. doi: 10.1016/j.pnpbp.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Thatcher RW, North DM, Neubrander J, Biver CJ, Cutler S, Defina P. Autism and EEG phase reset: deficient GABA mediated inhibition in thalamo-cortical circuits. Dev Neuropsychol. 2009;34:780–800. doi: 10.1080/87565640903265178. [DOI] [PubMed] [Google Scholar]
- 28.Nair A, Treiber JM, Shukla DK, Shih P, Muller RA. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136(Pt 6):1942–55. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K, et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 2014;19:659–67. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Bondt T, De Belder F, Vanhevel F, Jacquemyn Y, Parizel PM. Prefrontal GABA concentration changes in women-Influence of menstrual cycle phase, hormonal contraceptive use, and correlation with premenstrual symptoms. Brain Res. 2015;1597:129–38. doi: 10.1016/j.brainres.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 31.Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59:851–8. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- 32.Pandya M, Palpagama TH, Turner C, Waldvogel HJ, Faull RL, Kwakowsky A. Sex- and age-related changes in GABA signaling components in the human cortex. Biol Sex Differ. 2019;10:5. doi: 10.1186/s13293-018-0214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J Magn Reson Imaging. 2011;33:1262–7. doi: 10.1002/jmri.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkovski M, Suo C, Enticott PG, Yucel M, Fitzgerald PB. Short communication: sex-linked differences in gamma-aminobutyric acid (GABA) are related to social functioning in autism spectrum disorder. Psychiatry Res Neuroimaging. 2018;274:19–22. doi: 10.1016/j.pscychresns.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 36.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism diagnostic observation schedule. 2nd edn. Los Angeles: Western Psychological Services; 2012. [Google Scholar]
- 37.Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome. A focus on non-benzodiazepine GABAergic medications. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1106–17. doi: 10.1016/j.pnpbp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Ritvo RA, Ritvo ER, Guthrie D, Ritvo MJ, Hufnagel DH, McMahon W, et al. The Ritvo Autism Asperger Diagnostic Scale-Revised (RAADS-R): a scale to assist the diagnosis of Autism Spectrum Disorder in adults: an international validation study. J Autism Dev Disord. 2011;41:1076–89. doi: 10.1007/s10803-010-1133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427–33. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 40.Baghdadli A, Russet F, Mottron L. Measurement properties of screening and diagnostic tools for autism spectrum adults of mean normal intelligence: a systematic review. Eur Psychiatry. 2017;44:104–24. doi: 10.1016/j.eurpsy.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Gross JJ, John OP. Revealing feelings: facets of emotional expressivity in self-reports, peer ratings, and behavior. J Personal Soc Psychol. 1997;72:435–48. doi: 10.1037//0022-3514.72.2.435. [DOI] [PubMed] [Google Scholar]
- 42.Beidel DC, Borden JW, Turner SM, Jacob RG. The social phobia and anxiety inventory: concurrent validity with a clinic sample. Behav Res Ther. 1989;27:573–6. doi: 10.1016/0005-7967(89)90093-4. [DOI] [PubMed] [Google Scholar]
- 43.Bodfish JW, Symons FJ, Lewis MH. The repetitive behavior scale: a test manual. 1998.
- 44.Grant AM, Deller TW, Khalighi MM, Maramraju SH, Delso G, Levin CS. NEMA NU 2-2012 performance studies for the SiPM-based ToF-PET component of the GE SIGNA PET/MR system. Med Phys. 2016;43:2334. doi: 10.1118/1.4945416. [DOI] [PubMed] [Google Scholar]
- 45.Levin CS, Maramraju SH, Khalighi MM, Deller TW, Delso G, Jansen F. Design features and mutual compatibility studies of the time-of-flight PET capable GE SIGNA PET/MR system. IEEE Trans Med Imaging. 2016;35:1907–14. doi: 10.1109/TMI.2016.2537811. [DOI] [PubMed] [Google Scholar]
- 46.Odano I, Halldin C, Karlsson P, Varrone A, Airaksinen AJ, Krasikova RN, et al. [18F]flumazenil binding to central benzodiazepine receptor studies by PET–quantitative analysis and comparisons with [11C]flumazenil. NeuroImage. 2009;45:891–902. doi: 10.1016/j.neuroimage.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Ichise M, Ballinger JR, Golan H, Vines D, Luong A, Tsai S, et al. Noninvasive quantification of dopamine D2 receptors with iodine-123-IBF SPECT. J Nucl Med. 1996;37:513–20. [PubMed] [Google Scholar]
- 48.Gu M, Hurd R, Noeske R, Baltusis L, Hancock R, Sacchet MD, et al. GABA editing with macromolecule suppression using an improved MEGA-SPECIAL sequence. Magn Reson Med. 2018;79:41–47. doi: 10.1002/mrm.26691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10:1515–7. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- 50.Donahue MJ, Near J, Blicher JU, Jezzard P. Baseline GABA concentration and fMRI response. Neuroimage. 2010;53:392–8. doi: 10.1016/j.neuroimage.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 51.Bednařík P, Tkáč I, Giove F, DiNuzzo M, Deelchand DK, Emir UE, et al. Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. J Cereb Blood Flow Metab. 2015;35:601–10. doi: 10.1038/jcbfm.2014.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michels L, Martin E, Klaver P, Edden R, Zelaya F, Lythgoe DJ, et al. Frontal GABA levels change during working memory. PLoS ONE. 2012;7:e31933. doi: 10.1371/journal.pone.0031933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antoine MW, Langberg T, Schnepel P, Feldman DE. Increased excitation-inhibition ratio stabilizes synapse and circuit excitability in four autism mouse models. Neuron. 2019;101:648–61.e644. doi: 10.1016/j.neuron.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yip J, Soghomonian JJ, Blatt GJ. Increased GAD67 mRNA expression in cerebellar interneurons in autism: implications for Purkinje cell dysfunction. J Neurosci Res. 2008;86:525–30. doi: 10.1002/jnr.21520. [DOI] [PubMed] [Google Scholar]
- 55.Silverman JL, Pride MC, Hayes JE, Puhger KR, Butler-Struben HM, Baker S, et al. GABAB receptor agonist R-baclofen reverses social deficits and reduces repetitive behavior in two mouse models of autism. Neuropsychopharmacology. 2015;40:2228–39. doi: 10.1038/npp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moseley RL, Hitchiner R, Kirkby JA. Self-reported sex differences in high-functioning adults with autism: a meta-analysis. Mol Autism. 2018;9:33. doi: 10.1186/s13229-018-0216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferri SL, Abel T, Brodkin ES. Sex differences in autism spectrum disorder: a review. Curr Psychiatry Rep. 2018;20:9. doi: 10.1007/s11920-018-0874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirkovski M, Enticott PG, Fitzgerald PB. A review of the role of female gender in autism spectrum disorders. J Autism Dev Disord. 2013;43:2584–603. doi: 10.1007/s10803-013-1811-1. [DOI] [PubMed] [Google Scholar]
- 59.Kirkovski M, Enticott PG, Hughes ME, Rossell SL, Fitzgerald PB. Atypical neural activity in males but not females with autism spectrum disorder. J Autism Dev Disord. 2016;46:954–63. doi: 10.1007/s10803-015-2639-7. [DOI] [PubMed] [Google Scholar]
- 60.Brix MK, Ersland L, Hugdahl K, Gruner R, Posserud MB, Hammar A, et al. Brain MR spectroscopy in autism spectrum disorder-the GABA excitatory/inhibitory imbalance theory revisited. Front Hum Neurosci. 2015;9:365. doi: 10.3389/fnhum.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.