Abstract
Identifying the mechanisms linking early experiences, genetic risk factors, and their interaction, with later health consequences is central to the development of preventive interventions and identifying potential boundary conditions for their efficacy. In the current investigation of 412 African American adolescents followed across a 20-year period, we examined change in BMI across adolescence as one possible mechanism linking childhood adversity and adult health. We found associations of childhood adversity with objective indicators of young adult health, including a Cardiometabolic risk index, a methylomic aging index, and a count of chronic health conditions. Childhood adversities were associated with objective indicators indirectly through their association with gains in BMI across adolescence and early adulthood. We also found evidence of an association of genetic risk with weight gain across adolescence and young adult health, as well as genetic moderation of childhood adversity’s effect on gains in BMI, resulting in moderated mediation. These patterns indicated that genetic risk moderated the indirect pathways from childhood adversity to young adult health outcomes and childhood adversity moderated the indirect pathways from genetic risk to young adult health outcomes through effects on weight gain during adolescence and early adulthood.
Keywords: Childhood adversity, obesity, genetic risk, health disparities, African American
African Americans are at increased risk for early onset of a range of chronic illnesses (Bellatore, Finch, Do, Bird, & Beck, 2011; Nuru-Jeter et al., 2011; Simons et al., 2018), leading to increased levels of morbidity and mortality across the lifespan. Although there are many likely contributing causes to these disparities, one potential source is increased exposure to a range of adversities during childhood. Two qualitative reviews of the links between adult health outcomes and childhood adversity concluded that childhood adversities are robustly associated with later adult health and are likely causal in their impact (Shonkoff, Boyce, & McEwen, 2009; Miller, Chen, & Parker, 2011), leading to calls for enhanced policy initiatives to reduce toxic stress during childhood as a means to reduce health disparities (Shonkoff & Bales, 2011; Shonkoff et al., 2012) and focusing attention on exposure to childhood adversity as a factor that contributes to elevated levels of chronic health problems among young adult African Americans, including increased risk for obesity (e.g., Wall et al., 2019).
Elevated obesity also has the potential to influence health outcomes and explain health disparities. Obesity predicts earlier onset of various facets of cardiometabolic disease, including elevated blood pressure, type 2 diabetes mellitus, and coronary heart disease (e.g., Manson et al., 1990; Rimm et al., 1995; Carey et al, 1997; Chan et al., 1994; Eckel, Kahn, Robertson, & Rizza, 2006; Li et al., 2006; 2010). In addition, obesity that begins at earlier points in the life course appears to have more adverse effects (e.g., Thompson, Edelsbery, Colditz, Bird, & Oster, 1999; Visscher & Siedell, 2001), suggesting the importance of developmental timing. At the same time, because obesity has an even stronger effect on the development of chronic illnesses than on mortality (Visscher & Siedell, 2001), obesity is also associated with an increased number of years spent “unhealthy.” That is, overweight individuals develop disabilities at a younger age and experience reduced quality of life over a longer period of time relative to those who are not overweight (Felson et al., 1997; Rissanen et al., 1990; Lean, Han, & Seidell, 1998.). These patterns have particular relevance for African American youth, who show elevated levels of obesity relative to non-Hispanic Whites at every age (Ogden, Carroll, Kit, & Flegal, 2014). During adolescence and early adulthood, a substantial number of African American youth show increases in BMI that place them at risk for negative health outcomes (Chen et al., 2018), potentially contributing to premature death (Hoyert & Xu, 2012; Mozaffarian et al., 2016; Olshansky et al., 2005) and increased health care costs (Lutz, Sanderson, & Scherbov, 2008). Accordingly, elevated weight gain, particularly that emerging in adolescence and young adulthood, has the potential to be a mechanism linking adversity to health disparities among African Americans.
Finally, genetic risk for obesity is an important risk factor in developmental models of adult health outcomes (cf. Sankar et al., 2004) and has been increasingly studied, resulting in genetic risk indices suitable for use among African American samples (Domingue et al., 2014; Monda et al., 2013). By including genetic risk in developmental models we can rule out genetic risk as a potential source of spuriousness in the association between childhood adversity and health outcomes, and also better test a series of competing models to describe the interplay between genetic risk and childhood adversity in the prediction of young adult health outcomes. Of particular interest is the possibility that genetic risk may accentuate the impact of childhood adversity on obesity (or vice versa), and/or whether genetic risk serves as an additive factor in developmental models of etiology.
We propose a model integrating effects of childhood adversity, changes in obesity across adolescence, and genetic predisposition for obesity on adult health. Specifically, we hypothesize childhood adversity affects adult health indirectly through its effects on weight gain across adolescence, with these indirect effects more likely for those with elevated genetic risk for obesity. We review the empirical support for the components of this model below, noting that change in BMI across adolescence and young adulthood is temporally well-situated to explain the connection between early adversity (i.e., adversity occurring before age 10) and young adult indices of health (i.e., at age 29). Adding plausibility to the proposed model, a number of the adversities associated with later increased health problems also have been found to confer risk of elevated BMI. Because of complexities in the literature, we also review research on our choice of genetic risk index and briefly review the objective health indicators we use as dependent variables. Finally, we present the full theoretical model to be tested.
Multiple Aspects of Adversity are Important
There are a number of types of potential adversity in the early social environment of children that appear to be important in understanding and predicting later adult morbidity and elevated risk for chronic illness in adulthood. Exposure to chronic stress, relative deprivation, lower socioeconomic position, and family-related adversities early in development have been associated with elevated HbA1c, elevated glucose, and high blood pressure in adulthood (e.g., Elgar, Pfortner, White, & Pickett, 2016; Gouin, Glaser, Malarkey, Beversdorf, & Kiecolt-Glaser, 2012) as well as with indicators of allostatic load (Solis et al., 2015; Turner, Thomas, & Brown, 2016). Likewise, exposure to childhood maltreatment is associated with the development of a range of cardiometabolic problems in adulthood, even after controlling for sociodemographic and later adult health risk behaviors (Basu, McLaughlin, Misra, & Koenen, 2017; but see also Turner et al., 2016). Accordingly, a number of different childhood adversities appear to be relevant to the prediction of poorer adult health (Wickrama, Lee, & O’Neal, 2015).
Paralleling the literature on the role of childhood adversities in predicting elevated risk for chronic illness and poor health, there is an emerging literature indicating that exposure to a broad range of childhood adversities is also associated with increased risk for obesity (e.g., Wall et al., 2019). For example, Björntorp (2001) and Garasky, Stewart, Gundersen, Lohman, and Eisenmann (2009) found that early adversities related to family problems, such as exposure to abusive family interactions, were predictive of overweight in children and adolescents, and a comprehensive meta-analytic review of 41 studies by Danese and Tan (2014) found a relationship between childhood maltreatment and obesity that was robust to definitions of abuse and SES-related covariates or current health behaviors. Similarly, more general disruptions in early family structure, repeated housing changes, and changes in household composition that resulted in overall family instability were also predictive of obesity (Garasky et al., 2009; Björntorp, 2001). Further, childhood adversities originating outside the family but related to chronic fear, such as those produced by exposure to experiences of childhood or adolescent bullying (Baldwin, 2016; Gini & Pozzoli, 2009), or experiences of discrimination (e.g., Stepanikova, Baker, Simoni et al., 2017) also convey increased risk for obesity. Accordingly, the broad array of childhood adversities associated with later poor health in adulthood likely overlap with the range of adversities predictive of elevated obesity.
SES and the Interconnected Nature of Adversity.
Two broad concerns confront most efforts to identify an association between childhood adversity and obesity or later adult health. One concern is that the childhood adversities typically found to be associated with adult obesity and negative adult health outcomes are likely to be associated with childhood poverty and childhood SES, and so may be a reflection of childhood SES rather than a contribution of adversity that goes beyond the effects of childhood SES. Because childhood SES has been linked to poorer cardiometabolic and other health outcomes for both men and women, even after controlling for adult SES (Cohen, Janicki-Deverts, Chen, & Matthews, 2010; Kittleson et al., 2006; Tamayo, Chrinstian, & Rathmann, 2010), confounding of childhood adversity with childhood SES effects may muddy the examination of unique effects of childhood adversity. In addition, because of racial disparities in wealth and education, SES effects are relevant to understanding health disparities, with African Americans more likely than non-Hispanic Whites to suffer the stresses associated with low SES.
Several lines of research suggest that there are effects of childhood adversity that go beyond those of childhood and adult SES. Findings related to discrimination, bullying, and family stability indicate associations with health among African Americans that are additive to SES effects (e.g., Phelan & Link, 2005). Geronimus (1992), in particular, has argued that exposure to chronic adversities, including chronic discrimination and marginalization, may lead to health consequences for African Americans, finding evidence that African Americans have higher scores than Whites on cardiometabolic biomarkers even after controlling for SES (Geronimus et al., 2006a,b). Similarly, Duru, Harawa, Kermah, and Norris (2012) found higher mortality among African Americans even after controlling SES and health behavior. Neighborhood factors also have been found to contribute to biomarkers beyond the effect of SES (Bellatorre et al., 2011; Simons et al., 2018).
A second broad challenge in the examination of childhood adversity effects on obesity and adult health is the large number of different types of adversities that have been implicated, as well as their tendency to co-occur (Kessler et al., 2010; Slopen et al., 2010; Dong et al., 2004; Radford, Corral, Bradley, & Fisher, 2013; Felitti et al., 1998). As noted above, the lists of adversities purported to influence health and obesity are broad, including adversities arising inside the family, within the community, within school settings, and those reflective of broader social adversity; and adversities appear to accumulate to predict adult obesity and health outcomes (Dong et al., 2004). These findings suggest that an overly narrow examination of sources of adversity may underestimate effects on obesity and health; and, so it is necessary to utilize an index of adversity to capture the range of childhood adversities contributing to risk. At the same time, it should be noted that combining adversity types into a single measure limits more nuanced conclusions about the impact of specific types of adversity that are often useful in deriving implications for intervention.
Measurement of Childhood Adversity via Retrospective Report.
A separate concern in the examination of the association between childhood adversity and adult health is methodological in nature: retrospective measurement of childhood adversity potentially introduces a range of measurement-related concerns having to do with the introduction of potential recall biases. As a consequence, there has been considerable discussion regarding the reliability and validity of retrospective reports to capture exposure to adversity (e.g., Hardt & Rutter, 2004; Brewin, Andrews, & Gotlib, 1993), concluding that it is important to avoid recalled adversities that require subjective judgments, and noting the potential problem of under-reporting of adversity. In a meta-analytic examination of sixteen studies, Baldwin Reuben, Newbury, and Danese (2019) computed agreement between prospective and retrospective report of childhood maltreatment, finding that overall the agreement across studies was significant, but poor, with better agreement when retrospective reports of childhood maltreatment were the result of interviews rather than questionnaires, with little effect of age at the time of the report or the sex composition of the sample. Likewise, recent work by Reuben and colleagues (2016) found that both prospective and retrospective reports of childhood adversity were linked to adult health outcomes and that agreement was stronger for events that required little interpretation, such as the loss of a parent, but was minimal for those requiring the application of subjective criteria such as whether one experienced emotional abuse. An implication of this body of work is that retrospective indices of childhood adversity may be more readily interpretable to the extent they focus on relatively objective facets of adversity and exclude those, like emotional abuse, that require greater subjective interpretation. In addition, although data provided in an interview format may be more consistent with prospective reports, it is likely that prospective and retrospective reports identify somewhat different “at risk” groups.
Prior research also suggests that compared to prospective measures, retrospective reports may underestimate the impact of adversity on objective, non-self-report health outcomes but may overestimate effects on subjective health indicators (e.g. Reuben, et al., 2015; Osborn & Widom, 2019). It is also possible that associations with subjective health outcomes are inflated by strong associations between recalled childhood adversity and concurrent depression (Gee & Casey, 2015; Reuben et al., 2016) or other behavioral risk factors at the time of the report that influence recall. Because depressive symptoms and other factors may be associated with changes in the recollection of events (Hitchcock, Rees, & Dalgleish, 2017; Hitchcock et al, 2019), and with a tendency toward less specific and negatively overgeneralized recall of personal history (Hitchcock, Nixon, & Weber, 2014; Sumner, Griffith, & Mineka, 2010; van Vreeswijk & de Wilde, 2004; Williams et al., 2007), controlling the impact of depressive symptoms measured at the time of recall should help reduce potential recall bias. Accordingly, to provide conservative estimates of the association between childhood adversity and later adult health, excluding effects due to depression and other potential confounds at the time of recall, we will focus on objective indicators of adult health as outcomes and control for the impact of concurrent depressive symptoms on recalled adversity (Reuben et al., 2016).
Stability and Change in BMI
Higher BMI in childhood is an important predictor of chronic adult obesity and associated health outcomes (Belsky et al., 2012; Katzmarzyk, Pérusse, Malina, & Bouchard, 1999). At the same time, there can be substantial increases in BMI across adolescence and into early adulthood for African American youth (Chen et al., 2018; Kimm et al., 2002), supporting the view that change in BMI across adolescence, perhaps related to childhood experiences, may be an important risk factor in the development of adult obesity and obesity-related health problems. Further supporting this hypothesis, Zhang and colleagues (2019) found that the association between adult obesity and weight gain occurring in adolescence was particularly strong. Thus, to the extent that elevated levels of child adversity are associated with increased gain in BMI across adolescence and early adulthood, it could confer substantial indirect effects on eventual adult obesity and health.
Examining the Role of Genetic Risk
Models examining the impact of childhood adversities on obesity or health have not typically considered the potential role of genetic risk. This omission would have little impact on tests of the association between childhood adversity and later obesity if genetic risk had only a minor influence on obesity and/or only exerted additive effects on obesity and health outcomes. However, the high heritability inferred from twin studies (Silventoinen et al., 2017) and the observed effects when genetic risk is measured (El-Sayed & Froguel, 2013; Belsky et al., 2012) suggest that genetic risk has the potential to play a substantial role in the development of obesity. Across 40 twin cohorts, Silventoinen and colleagues (2017) found evidence of strong genetic influence on BMI, especially in early adulthood, with degree of genetic influence decreasing in older samples. Likewise, in a diverse sample, Belsky and colleagues (2012) found that higher genetic risk was associated with higher BMI at a younger age as well as with chronic obesity in adulthood, with earlier obesity accounting for much of the increased risk for later adult obesity.
Underexamined to date is whether they are interaction effects involving genetic risk. Given a genetic risk index for obesity that is valid for the population and outcome of interest, it is possible to examine the effect of genetic risk on obesity and health, and then examine whether psychosocial variables, such as childhood adversity, amplify these effects. Similarly, given an adversity index that is appropriate for the population and outcome of interest, we can examine the effect of childhood adversity on obesity and health, and then examine whether effects are amplified among those with greater genetic risk for obesity. To address these questions in the current investigation we examine genetically moderated indirect effects of childhood adversity on objective adult markers of poor health or risk for chronic illness. We also examine similar models testing adversity moderated indirect effects of genotype on young adult outcomes.
Which Genetic Markers Have Been Validated?
GWAS studies have identified a number of potential single-nucleotide polymorphisms (SNPs) that are associated with increased risk for obesity (El-Sayed & Froguel, 2013). Such markers have been found to be associated with greater increase in weight earlier in life, accounting for their association with later adult BMI (Belsky et al., 2012). However, because most SNPs have small effects when considered individually, it is necessary to aggregate them as a composite genetic index to provide sufficient signal to probe etiology and to examine their moderating effect on potential environmental contributors, such as adverse childhood events. This is particularly true in the context of longitudinal studies that typically do not have sufficient power to examine effects of individual genes (Speliotes et al., 2010; Plomin, Haworth, & Davis, 2009; Dudbridge, 2013).
Complicating studies of genetic risk in African American samples, GWAS-derived genetic indices using White samples may not always work as well, or in exactly the same way, when used for prediction in African American samples (cf. Belsky, Moffitt, Sugden, et al., 2013; Domingue et al. 2014; Monda et al. 2013). Problems in cross-ethnic use of genetic risk scores arise because GWAS-identified SNPs are usually not “causal” variants, but only proxies that are correlated with the true causal variants. Because patterns of linkage disequilibrium often vary across racial and ethnic groups (Price et al., 2006)], a SNP that is in linkage disequilibrium and so provides a good marker for a causal variant in a European American sample may not be in linkage disequilibrium for African Americans. In that case, the SNP that worked well for a European American sample would not be a good risk marker for African Americans. This problem is further compounded when, as often happens, a SNP needs to be replaced because the original SNP is not available for the new sample. In addition, causal variants that are not present for samples with European ancestry but that are present in other racial or ethnic groups create potential problems generalizing from samples with European ancestry to other groups (Wojcik et al., 2019).
Researchers have only recently begun the important task of expanding polygenetic risk score development to include African American and other non-European samples (Khoury, Gwinn, Bowen, & Dotson, 2012). For the current research, we use an eight-locus, BMI-associated index identified in a meta-analysis of individuals of African ancestry (Monda et al. 2013), and subsequently replicated (Domingue et al. 2014), to characterize genetic risk for obesity among African Americans. Nonetheless, it cannot be assumed that this index captures all relevant genetic influences on BMI among African Americans.
Which Pattern of Gene – Outcome Effects?
Following Belsky, Moffit, and Caspi (2013), we examine evidence for three broad models of association linking genetic factors in combination with childhood adversity to the prediction of weight gain across adolescence and early adulthood beyond the contribution of SES and potential confounders: 1) additive effects, 2) correlated effects (rGE), and 3) moderated effects (G × E). These alternative models are of particular interest with regard to designing preventive intervention, and also of interest in the context of better characterizing the impact of adversity.
Support for an additive-effect model would suggest separate processes that can be considered in relative isolation. An rGE effect would reflect genotypic influence on experience or recall of childhood adversity, such as might occur if there was a genotypic effect on the salience of adverse events either at the time they occurred or in memory. If such effects resulted in an rGE effect this would be important, and would suggest possible mediation of genetic effects or else a confounding of gene and childhood adversity effects on health outcomes, potentially obviating the need for examination of G × E effects. Significant G × E effects on BMI in young adulthood or change across adolescence could reflect genotypic effects that vary depending on level of childhood adversity, suggesting potential sources of resilience and vulnerability processes that moderate genetic risk. Conversely, G × E effects could reflect significant childhood adversity effects that vary depending on level of genotypic risk. Both additive and interactive models suggest the value of increased attention to the prevention of childhood adversity and its indirect effects on health outcomes through changes in obesity. Unique to interaction models that suggest diathesis-stress is the suggestion that some part of the population is resilient, either to childhood adversity’s impact on obesity and its later health effects, or to genetic risk effects on obesity and its later health effects. In either case, a significant GxE effect would help focus future research on mechanisms compatible with the genetic risk factors identified by the GxE effect. Because the presence of rGE may confound tests for G × E interaction, we examine simple correlations to identify any rGE correlations prior to any examination of G × E effects.
Choosing Objective Indicators of Health and Early Onset of Diseases of Aging.
Because objective indicators of health are less susceptible to artifactual association with retrospective recall of childhood adversity (Reuben et al. 2016), we identified three objective indicators of poor health in early adulthood. First, we obtained participants’ reports of physician-diagnosed common chronic illnesses (Lei, Beach, & Simons, 2018; Simons et al., 2017). Second, we examined an index of Cardiometabolic health that captures elevated levels of glycosolated hemogoblin as well as elevated blood pressure and elevated adult obesity. To ensure that identified patterns were robust, we also examined the Cardiometabolic health index without including adult BMI. Finally, we selected a recently-developed methylomic index of phenotypic aging specifically designed to capture risk for the development of problematic biomarkers of poor health and early onset of chronic illness, DNAm PhenoAge (Levine et al. 2018). Each outcome is described in detail in the Method section.
Hypothesized Model Linking Childhood Adversity to Young Adult Health
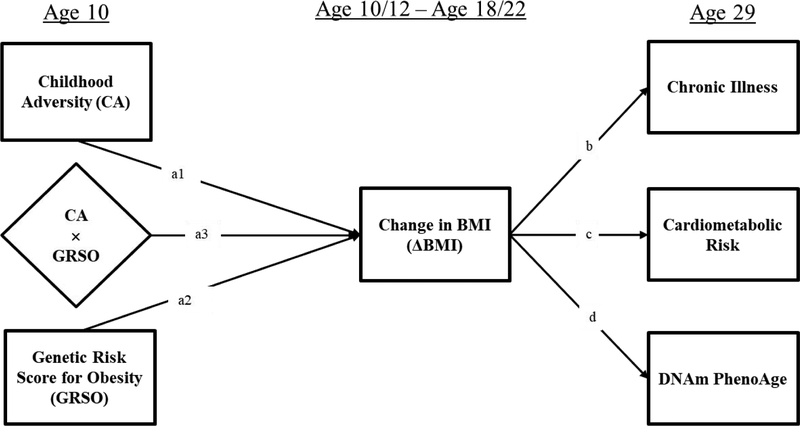
The basic theoretical model emerging from our review of the literature is presented in Figure 1. The model highlights the potential effects of childhood adversity and genetic risk and their potential interaction on change in BMI, controlling for effects attributable to childhood SES as well as depressive symptoms, attained adult SES and adult health behaviors at the time of recalled adversity (not shown). The model further highlights the potential effect of change in BMI on objective health indicators. By controlling for obesity that has already developed in childhood (i.e., BMI at the baseline assessment), the model isolates the effect of childhood adversity and genetic risk on weight gain across adolescence and young adulthood, identifying the extent to which they have an impact on weight gain occurring after the experience of adversity (Kittleson et al., 2006; Miller et al., 2011) and before the young adult health outcomes of interest.
Figure 1.
Theoretical model showing the effect of Childhood Adversity (CA) and Genetic Risk (GRSO) on change in BMI (ΔBMI) across adolescence, with the effect of CA amplified for those at greater genetic risk, resulting in moderated mediation of indirect pathways from Childhood Adversity to objective indicators of young adult health (age 29) net of effects due to childhood SES, adult SES, and adult depression, as well as adult health behaviors, and variation in DNAm due to cell type variation (not shown in Figure).
The model suggests that childhood adversity has the potential to influence gains in BMI across adolescence, after controlling for the effect of BMI already attained by late childhood. In addition, the hypothesized model suggests that childhood adversity should predict change in BMI across adolescence and young adulthood net of effects attributable to genetic predisposition to obesity. Additionally, if the conditional effects proposed in Figure 1 are supported, we should also observe moderated mediation. Specifically, individuals higher in genetic propensity for obesity should show stronger indirect effects of childhood adversity on later objective indictors of adult poor health through BMI relative to those with lower genetic propensity. Similarly, the model suggests stronger indirect effects of genetic risk on later objective indicators of adult poor health for those with elevated childhood adversity relative to those with less childhood adversity. We test the following specific hypotheses:
-
A. A retrospective index reflecting multiple domains of childhood adversity will be associated with change in BMI (ΔBMI) across adolescence and early adulthood.
B. A genetic risk index will be associated with ΔBMI across adolescence and early adulthood.
C. ΔBMI across adolescence and early adulthood will be associated with increased risk for objective indicators of poor adult health, including methylomic and cardiometabolic indicators, as well as physician-diagnosed chronic illness.
Childhood adversity and genetic risk will both be associated with ΔBMI across adolescence and early adulthood after the introduction of controls for childhood SES, and both will be indirectly associated with objective indicators of health through ΔBMI.
There will be an interaction of genetic risk and childhood adversity resulting in greater ΔBMI for those at greater genetic risk and also elevated childhood adversity, with the interaction taking the form of a diathesis stress effect.
-
A. There will be significant moderated mediation of pathways to objective health outcomes, such that those at higher genetic risk for obesity will show significantly enhanced indirect effects of childhood adversity on objective indicators of poor adult health through their effect on weight gain across adolescence and young adulthood relative to those with lower genetic risk.
B. Similarly, those that with greater childhood adversity will show significantly enhanced indirect effects of genetic risk on objective indicators of poor adult health through effects on increased weight gain across adolescence and young adulthood relative to those with lower childhood adversity.
Method
Participants
We tested all hypotheses using data from the FACHS sample (Beach et al., 2017) derived from a longitudinal study initiated in 1997 with a sampling strategy designed to generate families representing a range of socioeconomic status and neighborhood settings in Iowa and Georgia. The protocol and all study procedures were approved by the University of Georgia Institutional Review Board. At baseline (1997–1998), the FACHS sample consisted of 889 African American fifth-grade children. Their mean age was 10.56 years (SD = .63; range 9–13). At that time, the average family per capita income reported by children’s primary caregivers was $6,956, with 36% of the families below the poverty line and 51% of the respondents self-identified as single parents. Data used in the current study was also collected in 1999–2000, 2004–2005, 2007–2008, and 2015–2016, when the participants in the current study were, on average, aged 12.48, 18.72, 21.49, and 28.67 respectively. In the 2015–2016, data collection we also included blood draws allowing genetic analyses, as well as objective indicators of health. Of the 889 targets interviewed at baseline, 779 were re-interviewed in 1999–2000; 714 in 2004–2005; 687 in 2007–2008. The 2015–2016 data collection included blood draws, resulting in the inclusion only of those members of the sample residing in Georgia, Iowa, or a contiguous state who could be visited at home by phlebotomists. After also excluding persons who were deceased, incarcerated, or otherwise unreachable, we were left with a potential pool of 556 individuals, 470 of whom (182 men and 288 women) provided blood. Of these, 412 (88%) were successfully assayed and comprise the sample for the current analyses. In the current study, analyses are based on the 412 respondents (160 men and 252 women) who provided blood samples at age 29 and for whom objective health indicators could be computed. Comparisons of this subsample with those who were not included in the analysis did not reveal any significant differences with regard to major study variables or covariates (e.g., sex, education, income, family poverty, single-parent family, healthy diet, and exercise; table available in supplemental materials: Supplemental Table S1).
Procedure
African American university students and community members served as field researchers to collect data. Prior to data collection, all field researchers received one month of training in the administration of the interview to increase validity and enhance rapport and cultural understanding. The interview was administered in the respondent’s home and took on average about 2 hours to complete. Primary caregivers were interviewed concurrently with youth. Some of the instruments administered in later waves (after 2008) included questions regarding illegal or potentially embarrassing sexual activities. Hence, in an effort to further enhance anonymity, we used audio-enhanced, computer-assisted, self-administered interviews (ACASI). Using this procedure, the respondent sat in front of a computer and responded to questions as they were presented visually on the screen, and also auditorily, via earphones. Data on BMI was collected at baseline as well as for the 1999–2001, 2005–2007, 2008–2009, and 2015–2016 data collections. In 2015–2016, data collection included blood draws allowing genetic analyses, as well as objective indicators of health. After blood was drawn it was shipped via courier to a laboratory at the University of Iowa to allow assessment of Hemoglobin A1c (HbA1C), a marker of elevated blood sugar, as well as assessment of methylation patterns as described below. Biometric assessment of BMI and blood pressure was also conducted at this wave, as was assessment of childhood adversity and other variables detailed below. Mean age was 28.67 years (SD = .80; range: 27–31) at the time of the blood draw.
Measures
Childhood adversity (CA) was assessed retrospectively at age 29 (during the 2015–2016 data collection) using a 23-item questionnaire created for the current investigation that included questions about family physical abuse and neglect as well as sexual abuse, along with other potential sources of adversity, such as family instability, neighborhood safety, being bullied at school, and racial discrimination. The instrument asked respondents to report (1 = yes, 0 = no) whether they experienced each specific adversity before the age of 10 years (e.g., prior to age 10, would you say … I was punished with a belt, a board, a cord, or some other hard object; People in my family hit me so hard that it left me with bruises or marks; Someone in my family tried to touch me in a sexual way, or tried to make me touch them; There was a lot of violence in my neighborhood; I was sometimes bullied at school; The number of adults in your home shifted; family or close friends were treated unfairly just because of their race or ethnic background?). Coefficient alpha for this scale was .78. The scale did not assess emotional abuse or emotional neglect. All items and item-total correlations are listed in the supplemental materials (Table S2).
Childhood and young adult BMI.
At ages 10 and 12 youth height and weight were reported to the interviewer. At age 19, 22, and 29, the respondents’ height and weight were measured at the time of the home visit. At all ages the Centers for Disease Control calculator (https://www.cdc.gov/healthyweight/bmi/calculator.html) was used to calculate BMI as weight in kilograms divided by the square of height in meters. Mean BMI at age 10 was M = 21.6 (SD = 5.96); at age 12 M = 23.6 (SD = 5.98); at age 18 M = 26.9 (SD = 6.58); at age 22 M= 28.9 (SD = 8.37). We used regressed change to capture the difference between BMI in childhood (ages 10 and 12) (M = 22.6, SD = 5.44) and mean BMI in young adulthood (ages 19 and 22) (M = 27.8, SD = 7.33), i.e. ΔBMI.
Cardiometabolic risk (CR) was assessed by combining three biomarkers measured at age 29. (1) Each persons’ body mass index (BMI) score at age 29 was calculated as weight in kilograms divided by the square of height in meters, with mean BMI at age 29 of 31.36 (SD = 8.30). (2) Resting diastolic and systolic blood pressure (BP) was monitored with Dinamap Pro 100 while the participants sat reading quietly. Three readings were taken, one every 2 minutes, and the average of the last two readings was used as the resting index. Mean arterial BP (MAP) was calculated according to the following formula: [(systolic BP) + (2 × diastolic BP)]/3. Mean MAP in the current sample was 93.37 (SD = 11.63). (3) Hemoglobin A1c (HbA1c) was assessed at the University of Iowa using antecubital serum samples drawn by certified phlebotomists. HbA1c provides an indication of average blood glucose concentrations over the preceding 2 to 3 months. Mean HbA1c was 5.32 (SD = .76), with 2.2% of the sample having HbA1c above 6.5, the cutoff for type II diabetes (The International Expert Committee). Given that these three biomarkers are characterized by a skewed distribution, we applied a log transformation to normalize the distribution. CVD risk was calculated by summing the standardized log-transformed scores of BMI, MAP, and HbA1c. Models involving CR were also rerun excluding age 29 BMI from the composite to ensure that results were not dependent on the BMI component of the cardiometabolic risk index.
Methylomic Index of Accelerated Phenotypic Aging (DNAm PhenoAge).
DNA methylation was assessed using the Illumina HumanMethylation EPIC array, under contract by the University of Minnesota Genome Center, and using the protocol specified by the manufacturer. This provided Genome-wide DNA methylation characterization of whole blood drawn at age 29 and allowed us to calculate methylomic phenotypic aging using the epigenetic index recently developed by Levine et al. (2018) (DNAm PhenoAge), as well as calculate cell-type correction factors. The DNAm PhenoAge index produces a score based on an individual’s methylation pattern that is designed to capture morbidity and mortality risk. To control for variability in target age, we regressed the methylomic risk index score on chronological age and used the residual. In addition, because cell type distribution is correlated with observed methylation patterns, we controlled for cell type variation when the methylomic risk index was the dependent variable using a procedure to characterize cell-type variation across individuals described by Horvath (2013). Controlling cell-type variation yields index values relatively free of influences from cell-type variation and so represents “intrinsic” phenotypic aging. Positive values indicate accelerated phenotypic aging and elevated risk for morbidity and mortality, and negative values indicate decelerated phenotypic aging. It should be noted that controls for cell-type variation are only approximations and do not entirely rule out effects due specific cell types.
We focus on DNAm PhenoAge, rather than earlier “epigenetic clocks” such as those proposed by Horvath (2013) and Hannum et al. (2013) because DNAm PhenoAge was designed to overcome some limitations of the first generation of measures. Unfortunately, acceleration of the earlier epigenetic clocks was not found to be consistently related to cardiovascular disease or early onset of chronic illness (Jyhava et al. 2017). Further complicating use of the initially proposed clocks, the newer EPIC 850 Beadchip, widely used for measurement of methylation patterns, omitted several of the methylation sites used in earlier arrays. In response, a new epigenetic measure of accelerated phenotypic aging (DNAm PhenoAge) was developed (Levine et al. 2018; Horvath & Raj, 2018) using both age and clinical measures so that it would better predict individual differences in lifespan and healthspan. The index is based upon 513 CpG sites that reflect several known aging pathways (Horvath & Raj, 2018; Levine et al., 2018). Accordingly, it provides an objective marker of elevated risk for early onset morbidity and chronic illness.
Chronic illness count (CI).
Self-reported chronic illness was measured at age 29. Respondents were asked, “Have you even been diagnosed with any of the following health illnesses?” The list of health problems consisted of seven illnesses and an “other” category: coronary heart disease, hypertension, diabetes, peptic ulcer, kidney disease, liver disease, thyroid disease, and other disease. For each illness, “no” was coded as 0 and “yes” was coded as 1. Items were summed to form an index of chronic illness that ranged from 0 to 8. The mean score for this variable was 0.22 (SD = 0.52), with roughly 18.7% of the sample reporting that they had at least one diagnosed chronic disease.
Genetic Risk Score for Obesity (GRSO) index.
A weighted genetic risk score based on genotyping at age 29 was calculated for each participant based on the eight BMI-associated SNPs identified by Monda et al. (2013) for individuals of African ancestry, and subsequently replicated (Domingue et al. 2014). The eight SNPs included were located on eight different genes (rs543874 on SEC16B; rs6545800 on ADCY3; rs348495 on GNPDA2; rs7708584 on GALNT10; rs974417 on KLHL32; rs10261878 on MIR148A-NFE2L3; rs17817964 on FTO; and rs6567160 on MC4R). The weighted risk score weights the number of risk alleles present at each SNP (0,1,2) by its corresponding effect size estimated using the original association study (Monda et al. 2013). The current investigation used the Illumina Infinium Multi-Ethnic Global beadchip to genotype participants. Out of the eight BMI-associated SNPs previously identified by Monda and colleagues (2013), seven were present on our genotyping platform. For the one SNP (rs7586879) that was absent, we used a proxy SNP, rs6545800, which is in complete linkage disequilibrium with the target SNP (r2 = 1 in the 1000 Genomes Project YRI population). Among the eight SNPs used in our analysis, only three had any missing data; two SNPs (rs348495 and rs7708584) had missing data for one individual (i.e., missing rate = 0.22%) while one SNP was missing for four individuals (missing rate = 0.88%). In terms of sample-level missing rate, out of 449 participants with genetic data, only six had missing data at one of the eight SNPs. For individuals with any missing genetic data, we calculated a pro-rated genetic risk score by dividing the calculated genetic risk score by the number of SNPs with available calls and multiplying by the total number of SNPs in the score. Accordingly, no participants were excluded solely due to missing genetic data. The eight SNPs used in this analysis are described in Supplemental Table S3, which provides their frequencies, weights, and their individual correlations with childhood obesity, young adult obesity, ΔBMI, and childhood adversity in the current sample. All SNPs were found to be in Hardy-Weinberg equilibrium.
Depressive Symptoms age 29.
Age 29 depression was assessed using the nine-item Composite International Diagnostic Interview (UM-CIDI; Kessler, et al., 1994) measure of depressive symptoms. Respondents were asked to report (0 = no; 1 = yes) whether they experienced symptoms of depression (e.g., “felt sad, empty, or depressed most of the day” and “lost interest in things”) for at least a 2-week period in the past year. All respondents were asked all 9 items, and items were summed to create a measure of depressive symptoms. Cronbach alpha for the scale was .86. Depressive symptoms at age 29 are controlled for CA and all Age 29 outcomes, controlling potential effects of recall bias
Childhood SES.
To examine the potential effect of early SES risk on BMI and objective indicators of health, we examined SES risk at age 10. Caregiver reports across six indicators were used to create our measure of socio-economic risk. Risk indicators were (a) family poverty, defined as being below the poverty level, taking into account both family income and number of family members; (b) primary caregiver non-completion of high school or an equivalent; (c) primary caregiver unemployment; (d) single-parent family structure; (e) family receipt of Temporary Assistance for Needy Families; and (f) income rated by the primary caregiver as not adequate to meet all needs. Each indicator was scored dichotomously (0 if absent, 1 if present). SES risk was defined as the number of SES-related indicators, summing items to form an index with a theoretical range of 0 to 6 (M = 1.84, SD = 1.54), with larger numbers indicating greater SES risk (i.e., lower SES). Childhood SES effects are controlled for ΔBMI and all age 29 outcomes isolating the impact of CA on outcomes and mediational pathways.
Other Covariates.
To account for plausible rival explanations of adult health outcomes, we controlled for several additional covariates at age 29. Specifically, all analyses statistically controlled for participants’ annual income at age 29 and educational level at age 29, to control for potential effects of adult SES on health outcomes (see Cockerham, Hamby & Oates, 2017; Minkler, Fuller-Thompson, Guralnik, 2006). Similarly, all analyses controlled for sex (1 = male), and health-behaviors assessed at age 29 (including substance use, diet, and exercise) to control for factors in adulthood that might influence health outcomes (Beach et al, 2015; Mills et al, 2019; Rezende, et al, 2014; Schwingshackl et al., 2013). Items assessing level of substance use at age 29 included alcohol consumption (“How many alcoholic drinks have you consumed during the past month?”), cigarette use (“How many cigarettes have you smoked in the last 3 months?”), and marijuana use (“How many times have you used marijuana during the past month?”). Items were standardized and summed to create the substance use index. Healthy diet at age 29 was assessed using two items that asked about frequency of fruit and vegetable consumption during the previous 7 days. Responses ranged from 1 (none) to 6 (more than once every day) and were averaged to form the healthy diet variable. Exercise at age 29 was measured with two items (e.g., “On how many of the past 7 days did you exercise or participate in physical activity for at least 30 min that made you breathe hard such as running or riding a bicycle hard?”). The response categories ranged from 1 (0 days) to 5 (all 7 days). Scores on the two items were averaged to form the exercise variable.
Analytic Plan
After examining zero-order correlations along with means and SDs for all primary study variables, we examined proposed indirect effects from both CA and genetic risk using path modeling in Mplus (Version 8;Muthen & Muthen, 2017). Mean weight during late childhood (ages 10–12) was used as a covariate in the analyses to allow examination of the effect of childhood adversity and genetic risk on change in young adult BMI (i.e., ΔBMI). In turn, indirect effects on objective indicators of adult health outcomes through ΔBMI were examined. To characterize goodness-of-fit of each model, we report the standardized root mean square residual (good fit SRMR < .05) and the comparative fit index (good fit CFI >.90) along with Chi-square and degrees of freedom. When direct effects between stages of the model were significant, indirect effects for IV and DV combinations are presented in tabular form along with the 95% confidence interval (CI) estimated using bias-corrected and accelerated bootstrapping with 1,000 resamples.
We also examined the interaction of genetic risk and childhood adversity in predicting ΔBMI. After presenting the analysis of moderation in multiple regression format, controlling childhood SES, we explicated significant interaction effects graphically. To provide comprehensive characterization of interactions, we plotted effects with both childhood adversity and the genetic risk index on the x-axis, showing effects from IV to ΔBMI with confidence intervals around the regression lines for those high vs. low on the moderating variable. We then examined moderated mediation in a final Mplus model and present a table of conditional indirect effects. Using Hayes’ (2015) index of moderated mediation, we also examined the significance of mediated moderation for each indirect pathway. Finally, we tested for any differences between the models for female and male participants using the multiple group analysis option in Mplus.
As described earlier, to account for measures that could provide plausible rival explanations, all analyses of mediation statistically controlled for health-behavior covariates reported at age 29, adult SES indicators (education and income), and adult depressive symptoms. Sex and childhood SES at wave 1 were also controlled in all analyses.
Results
H1: Correlation of childhood adversity, genetic risk, objective health indicators in young adulthood, and ΔBMI
Table 1 presents means, standard deviations, and intercorrelations for childhood adversity, genetic risk, objective health indicators at age 29, as well as demographic and control variables (all correlations based on N = 412). As shown, there were significant correlations of childhood adversity with depression (r = .35, p = .000), ΔBMI (r = .18, p = .000) and objective health indicators of cardiometabolic risk (r = .17, p = .001), and chronic illness (r = .10, p = .043), but not with DNAm PhenoAge (r = .08, NS). In addition, depression was related to chronic illness (r = .17, p = .001), cardiometabolic risk (r=10, p =.036), ΔBMI (r = .13, p = .011), sex (r = −.15, p = .003), and substance use (r = .18, p = .000). The index of genetic risk for obesity (GRSO) was significantly correlated with both DNAm PhenoAge (r = .13, p = .013) and ΔBMI (r = .15, p = .003), but not with chronic illness, cardiometabolic risk, or childhood SES risk. In addition, there was no significant correlation between GRSO and childhood adversity, providing no evidence of an rGE in which elevated genetic risk led to increased exposure to childhood adversity (or increased recall of adversity), nor was GRSO significantly associated with depression or SES in childhood or adulthood. All control variables showed significant or marginal associations with other variables in the full model, suggesting the value of retaining them as controls in the analyses.
Table 1.
Correlations, Means, and Standard Deviations for Main Predictors (Childhood Adversity and Genetic Risk for Obesity), Mediator ΔBMI, Primary Objective Health Outcomes (Chronic Illness, Cardiometabolic Risk, DNAm PhenoAge), and Covariates (Childhood SES, Adult Depressive Symptoms, Sex, Substance Use, Healthy Diet, Exercise, Education at Age 29, Income Age 29) (N = 412)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Chronic Illness | — | |||||||||||||
| 2. Cardiometabolic Risk | .38** | — | ||||||||||||
| 3. DNAm PhenoAge | .08† | .17** | — | |||||||||||
| 4. ΔBMI | .18* | .54** | .18** | — | ||||||||||
| 5. Childhood Adversity | .10* | .17** | .08 | .18** | — | |||||||||
| 6. GRSO | .08 | .01 | .13* | .15** | .07 | — | ||||||||
| 7. Depression Age 29 | .16** | .10* | .06 | .13* | .35** | .02 | — | |||||||
| 8. Gender (Male=1) | −.02 | .01 | −.09† | −.14** | −.05 | .00 | −.15** | — | ||||||
| 9. Education Age 29 | .05 | .02 | −.09† | .04 | .07 | .00 | −.02 | −.05 | — | |||||
| 10. Childhood SES Age 10 | .08 | .07 | .01 | .02 | .09† | −.04 | .03 | −.08 | −.22** | — | ||||
| 11. Substance Use Age 29 | .02 | −.01 | −.01 | −.00 | .26** | .07 | .18** | .08† | −.02 | −.02 | — | |||
| 12. Healthy Diet Age 29 | −.01 | −.02 | .01 | .06 | .08 | .13** | −.00 | −.17** | .17** | −.01 | −.02 | — | ||
| 13. Exercise Age 29 | −.07 | −.10* | −.11* | −.02 | .10* | .01 | −.04 | .17** | .12* | −.07 | .12* | .19** | — | |
| 14. Income Age 29 | −.15** | .00 | −.08 | .02 | .15** | −.03 | −.07 | .11* | .30** | −.15** | .04 | .03 | .15** | — |
| Mean | .27 | −.01 | −.05 | .04 | 3.46 | .27 | 1.84 | .38 | 13.10 | 1.84 | .01 | 6.67 | 4.97 | 442.46 |
| SD | .58 | 2.06 | 5.33 | 5.94 | 3.10 | .08 | 2.32 | .48 | 1.75 | 1.54 | .71 | 2.43 | 2.29 | 337.07 |
Note:
≤ 0.10;
p ≤ .05;
p ≤ .01 (two-tailed tests).
H2: Association of childhood adversity and genetic risk with ΔBMI and indirect associations with objective indicators of health through ΔBMI
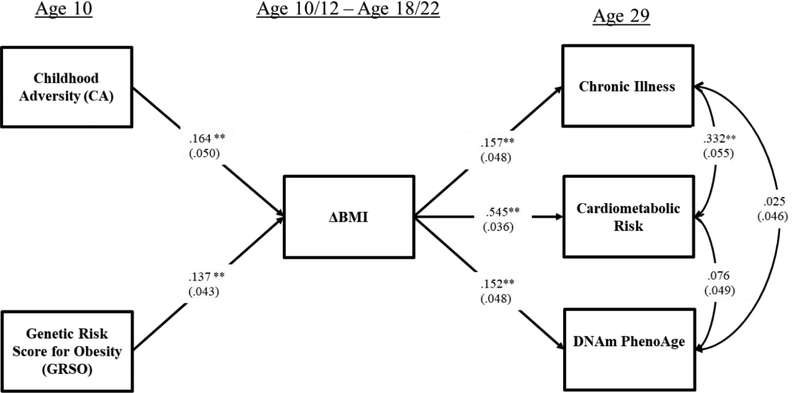
As shown in Figure 2, controlling for gender and childhood SES, childhood adversity continued to be significantly and positively associated with ΔBMI (β = .16, p = .001). Additionally, ΔBMI was significantly associated with each of the objective indicators of adult health, including intrinsic DNAm PhenoAge (β = .15, p = .002), cardiometabolic health (β = .54, p = .000), and diagnoses of chronic ailments by a physician at age 29 (β = .15, p = .001). These results indicate that the hypothesized associations were robust to covariates and potential confounds. The indirect effects model showed good fit to the data with CFI = 0.911 and SRMR=0.029; Chi-square = 75.653, df = 34, p = .0001.
Figure 2.
The unconditional indirect effects model showing the association of childhood adversity with chronic illness, cardiometabolic risk, and DNAm PhenoAge through change in body mass index.
Note: Chi-square = 75.653, df = 34, p = .0001; CFI = 0.911; SRMR= 0.029. Values are standardized parameter estimates, and standard errors are in parentheses. Depression age 29 is controlled for in CA and Age 29 health outcomes, controlling potential recall bias; gender and childhood socioeconomic status age 10 are controlled for in ΔBMI and age 29 outcomes, isolating CA effects; education age 29, substance use age 29, healthy diet age 29, exercise age 29, income age 29 are controlled for in all age 29 health outcomes to control alternative influences on health; and cell types are controlled for DNAm PhenoAge to yield intrinsic PhenoAge. Control variable effects are not shown in the figure. DNAm PhenoAge is residualized on chronological age and so represents age acceleration..
**p ≤ .01; *p ≤ .05 (two-tailed tests), n = 412.
Because the cardiometabolic index included BMI at age 29, potentially inflating effect estimates, we also examined the association of ΔBMI with the cardiometabolic index excluding BMI (i.e., considering only blood pressure and HbA1c). The reduced index of cardiometabolic risk was also significantly associated with ΔBMI (β = .31, p = .000), and showed the same pattern of results as the full cardiometabolic risk variable. Results using the reduced cardiometabolic risk index can be seen in supplemental figure S1.
Indirect effects of childhood adversity (CA) on adult objective indicators of health through ΔBMI are presented in Table 2. As expected, the significant associations of CA with ΔBMI and of ΔBMI with objective outcomes (DNAm PhenoAge; cardiometabolic risk; and chronic illness) resulted in significant indirect effects of childhood adversity on health outcomes via ΔBMI, estimated using bootstrapping with 1,000 replications. Each pathway was significant, with none of the 95% confidence intervals containing “0” (DNAm PhenoAge: IE = .025, 95% CI = [.009,.059]; cardiometabolic health: IE = .089, 95% CI = [.038,.140]; chronic diseases of aging: IE = .026, 95% CI = [.010,.056]). Similarly, there was a significant indirect effect from childhood adversity to cardiometabolic health even when BMI at age 29 was excluded from the cardiometabolic health index (cardiometabolic health: IE = .051, 95% CI = [.024,.090]).
Table 2.
Unconditional Indirect Effects to Objective Indicators of Young Adult Health from Childhood Adversity (CA) and Genetic Risk Score for Obesity (GRSO) through ΔBMI
| Paths | Effect | 95% CI | p |
|---|---|---|---|
| Effects from CA to Outcome | |||
| CA → ΔBMI → Chronic Illness | .026* | [.010,.056] | .026 |
| CA → ΔBMI → Cardiometabolic Risk | .089** | [.038,.140] | .000 |
| CA → ΔBMI → DNAm PhenoAge | .025* | [.009,.059] | .036 |
| Effects from GRSO to Outcome | |||
| GRSO → ΔBMI → Chronic Illness | .022* | [.006,.046] | .030 |
| GRSO → ΔBMI → Cardiometabolic Risk | .075** | [.030,.123] | .001 |
| GRSO → ΔBMI → DNAm PhenoAge | .021* | [.007,.050] | .035 |
Note:
p ≤ .01;
p ≤ .05 (two-tailed tests), n = 412. CI = Confidence interval; BMI = Body mass index. GRSO = Genetic risk score for obesity. Diagnostic VIF scores for all variables were below 10, ranging from 1.08 to 1.52, indicating no evidence of multicollinearity among the study variables.
The indirect effect of genetic risk (GRSO) on outcomes via ΔBMI are also shown in Table 2, controlling for gender, diet, exercise, substance use, and adult markers of attained SES (i.e., education level and income at age 29), as well as depression at age 29 and childhood SES. Each of the indirect pathways was significant (DNAm PhenoAge: IE = .21, 95% CI = [.007,.050]; cardiometabolic health: IE = .075, 95% CI = [.030,.123]; chronic diseases of aging: IE = .022, 95% CI = [.006,.046]). Similarly, there was a significant indirect effect from GRSO to cardiometabolic health even when BMI at age 29 was excluded from the index (cardiometabolic health: IE = .043, 95% CI = [.018,.074]).
H3: The interaction of genetic risk with CA predicting ΔBMI
As noted above, there was not a significant correlation between genetic risk (GRSO) and childhood adversity (CA), suggesting an absence of gene-environment correlation. In addition, there was no significant correlation between CA and childhood BMI or between GRSO and childhood BMI, suggesting that effects on BMI emerged after childhood. Accordingly, we proceeded to examine the possibility that CA and GRSO might interact to predict ΔBMI (i.e. that there might be a GxE effect). As shown in Table 3, Model 1, there was support for the additive main effects model with a significant effect for CA (β = .164, p = .001, 95% CI = [.073, .258]) and a significant effect for GRSO (β = .137, p = .002, 95% CI = [.053, .224]), even after accounting for effects attributable to covariates. In Model 2, we show that the interaction of CA and GRSO also accounted for significant additional variance in ΔBMI (β = .167, p = .014, 95% CI = [.023,.292]).
Table 3.
Regression Analysis showing the Association of Childhood Adversity, Genetic Risk and their Interaction with ΔBMI
| ΔBMI | ||||
|---|---|---|---|---|
| Model 1 | β [95% CI] | Model 2 | β [95% CI] | |
| Main effect | ||||
| Childhood Adversity | .164** | [.073, .258] | .150** | [.063, .241] |
| GRSO | 137** | [.053, .224] | .014 | [−.112,.157] |
| Two-way interaction | ||||
| Childhood Adversity × GRSO | .167** | [.023,.292] | ||
| Control variables | ||||
| Gender | −.128** | [−.209, −.038] | −.122** | [−.201,−.030] |
| Childhood Socioeconomic Status | −.004 | [−.096, .079] | .004 | [−.086, .087] |
| Depression at Age 29 | .345** | [.233,.434] | .345** | [.233,.434] |
| Constant | −.070 | [−.267,.131] | −.076 | [−.275,.128] |
Note:
p ≤ .01;
p ≤ .05 (two-tailed tests); n = 412. CI = Confidence interval; BMI = Body mass index. GRSO = Genetic risk score for obesity.
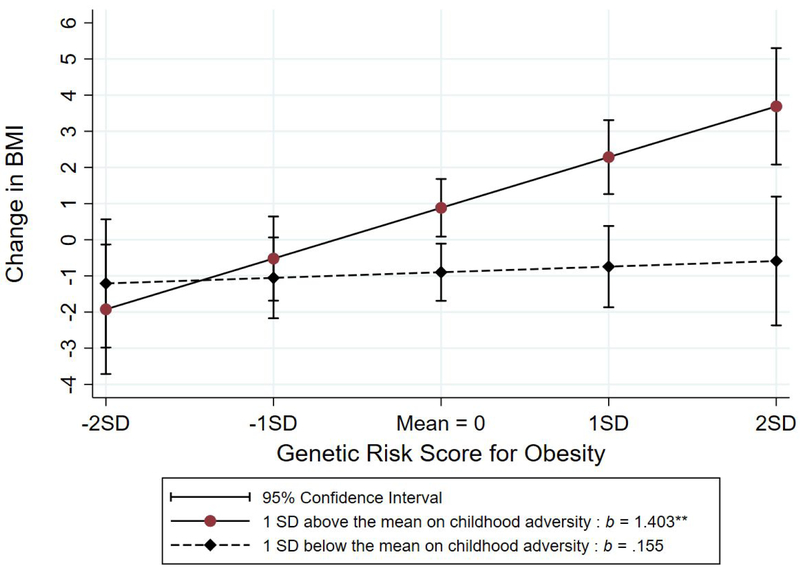
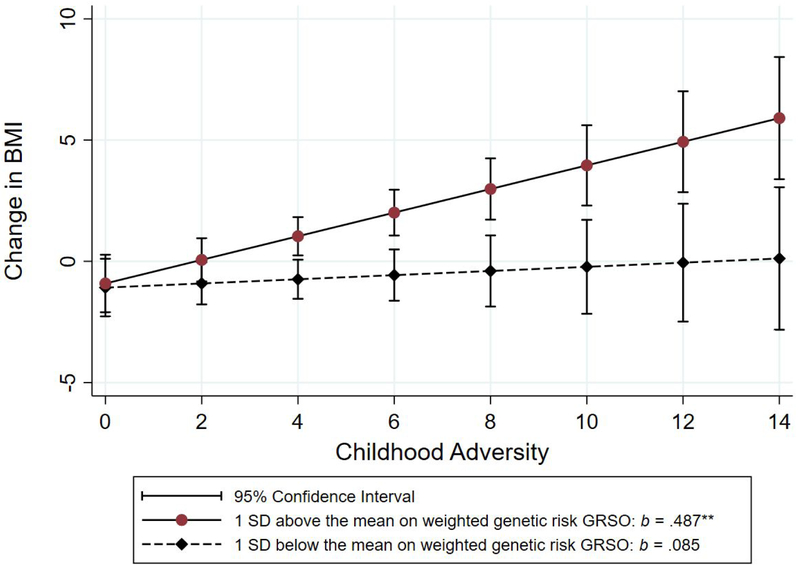
To better characterize the shape of the interaction effect, we plotted and compared the simple slopes for the association of Childhood Adversity with ΔBMI at +1 and − 1 SD of GRSO. The effect of CA on ΔBMI among those with low genetic risk was weak and non-significant (b = .085, NS). Conversely, the effect of CA on ΔBMI among those high in genetic risk was strong and significant (b = .487, p = .000). In addition, the 95% confidence intervals around the slopes do not overlap for those scoring above the mean on childhood adversity (i.e., those scoring 4 or greater on Childhood Adversity). As shown in Figure 3B, when the interaction is plotted differently, with CA as the moderator of the impact of GRSO on ΔBMI, the pattern of effects is similar. The effect of GRSO on ΔBMI among those low on childhood adversity is non-significant (b = .155, NS) but the slope among those high on childhood adversity is strong and significant (b = 1.403, p = .000). In addition, the 95% confidence intervals around the slopes do not overlap for those scoring above the mean on GRSO.
Figure 3b.
Joint effect of childhood adversity and weighted genetic risk (GRSO) on ΔBMI for those one SD above vs. one SD below the mean on the genetic risk index
H4: Significant moderated mediation of pathways to objective health outcomes
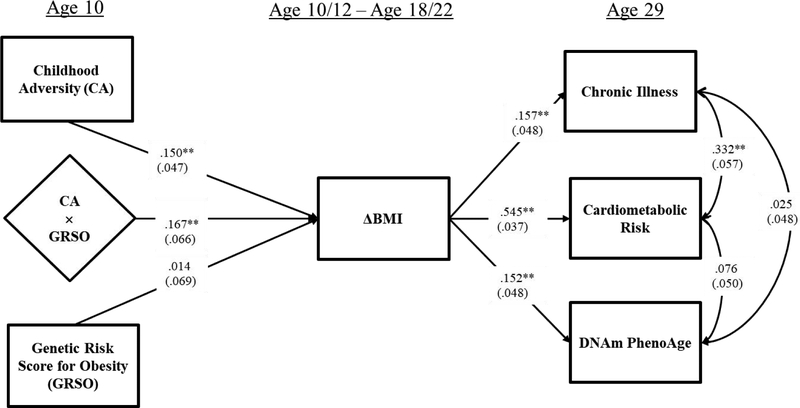
In Figure 4 we display the full model, showing the moderated impact of childhood adversity on ΔBMI, and significant indirect effects on all outcomes. The full model provided a good fit to the observed data with CFI = 0.904; SRMR= 0.029; Chi-square = 83.656, df = 38, p = .0000.
Figure 4.
The full conditional indirect effects of Childhood Adversity, Genetic Risk, and their interaction, on Chronic Illness at age 29, Cardiometabolic Risk at age 29, and DNAm PhenoAge at age 29 through ΔBMI moderated by a weighted genetic risk score for obesity. Note: Chi-square = 83.656, df = 38, p = .0000; CFI = 0.904; SRMR= 0.029. Values are standardized parameter estimates, and standard errors are in parentheses. Depression age 29 is controlled for in CA and Age 29 health outcomes, controlling potential recall bias; gender and childhood socioeconomic status age 10 are controlled for in ΔBMI and age 29 outcomes, isolating CA effects; education age 29, substance use age 29, healthy diet age 29, exercise age 29, income age 29 are controlled for in all age 29 health outcomes to control alternative influences on health; and cell types are controlled for DNAm PhenoAge to yield intrinsic PhenoAge. Control variable effects are not shown in the figure. DNAm PhenoAge is residualized on chronological age and so represents age acceleration.
**p ≤ .01; *p ≤ .05 (two-tailed tests), n = 412.
Given results in Figure 2 showing significant mediation of the effect of childhood adversity on all outcomes through ΔBMI, the results shown in Figure 3 indicating significant moderation, and the good fit of the model in Figure 4, we directly tested the conditional indirect effects from independent variables to the three objective indicators of adult health outcomes. The conditional indirect results of CA and GRSO on health outcomes are presented in Table 4, showing that CA has a significant indirect effect on each objective health outcome through ΔBMI when GRSO is elevated, but a non-significant effect among individuals with low GRSO. Similarly, GRSO has a significant indirect effect on each objective health outcome through ΔBMI when CA is elevated but a non-significant effect among those with low CA. Further, as shown in Table 5, this pattern results in a significant coefficient of moderated mediation (Hayes, 2015) for each health outcome, showing that indirect pathways from childhood adversity are moderated significantly by GRSO and that indirect pathways from GRSO are moderated significantly by CA. Follow-up tests to examine potential sex differences in indirect pathways from childhood adversity to outcomes showed no significant sex differences.
Figure 3a.
Joint effect of childhood adversity and weighted genetic risk (GRSO) on ΔBMI for those one SD above vs. one SD below the mean on the genetic risk index
Table 4.
Conditional Indirect Effects of Childhood Adversity (CA) and Genetic Risk Score for Obesity (GRSO) on Health Outcomes (Chronic Illness; Cardiometabolic Risk; DNAm PhenoAge) via Residualized Change in Body Mass Index (ΔBMI).
| Paths | Effect | 95% CI | p |
|---|---|---|---|
| Low GRSO (−1sd) | |||
| CA → ΔBMI → Chronic Illness by Low GRSO | .001 | [−.002,.005] | .587 |
| CA → ΔBMI → CMR by Low GRSO | .016 | [−.021, .054] | .410 |
| CA → ΔBMI → DNAm PhenoAge by Low GRSO | .012 | [−.012, .052] | .471 |
| Low C4 (−1sd) | |||
| GRSO → ΔBMI → Chronic Illness by Low CA | .002 | [−.008,.017] | .766 |
| GRSO → ΔBMI → CMR by Low CA | .029 | [−.099,.190] | .707 |
| GRSO → ΔBMI → DNAm PhenoAge by Low CA | .021 | [−.075,.174] | .753 |
| High GRSO (+1sd) | |||
| CA → ΔBMI → Chronic Illness by High GRSO | .008** | [.003,.015] | .008 |
| CA → ΔBMI → CMR by High GRSO | .092** | [.042,.140] | .000 |
| CA → ΔBMI → DNAm PhenoAge by High GRSO | .067* | [.025,.158] | .047 |
| High CA (+1sd) | |||
| GRSO → ΔBMI → Chronic Illness by High CA | .022** | [.009,.043] | .011 |
| GRSO → ΔBMI → CMR by High CA | .266** | [.144,.375] | .000 |
| GRSO → ΔBMI → DNAm PhenoAge by High CA | .193** | [.075,.374] | .011 |
Note:
p ≤ .01;
p ≤ .05 (two-tailed tests), n = 412. Values are unstandardized parameter estimates and confidence intervals. CA = Childhood Adversity; ΔBMI = Change in body mass index; CMR = Cardiometabolic Risk; GRSO = Genetic risk score for obesity
Table 5.
Hayes Index of Moderated Mediation Indicating Significant Moderated Mediation for each of the Three Objective Indicators of Young Adult Health
| β | 95%CI | |
|---|---|---|
| CA × GRSO × Chronic Illness | .003* | [.001, .007] |
| CA × GRSO × Cardiometabolic Risk | .038** | [.005, .068] |
| CA × GRSO × DNAm PhenoAge | .028* | [.005, .063] |
Note: CA = Childhood adversity; GRSO = Genetic risk score for obesity.
p ≤ .05;
p ≤ .01 (two-tailed tests).
Exploratory and Supplemental Analyses.
To provide evidence that the model was robust to a range of analytic decisions, we ran the same model several ways. First, as noted above, we excluded BMI at age 29 from the cardiometabolic index (results shown in Supplemental Figure S1), and found the same pattern of significant indirect and moderated effects. Second, we reran the model excluding all control variables, other than those for cell-type variation on DNAm PhenoAge, and found the same pattern of significant indirect and moderated effects (see Supplemental Figure S2 and Supplemental Table 4). Third, we reran the model including all controls but excluding controls for cell-type variation to see if effects on DNAm PhenoAge were due to controls for cell-type variation, and found the same pattern of effects (See Supplemental Figure S3 and Table 5). Fourth, because comparison of observed indirect and moderated pathways for DNAm PhenoAge with those obtained using other potential methylomic aging indices is of potential interest, we also ran a comparative analysis, showing that similar significant indirect and moderated effects would be observed using the Hannam methylomic aging measure (Hannum et al., 2013) or the Horvath methylomic aging measure (Horvath, 2013) as dependent variables (see Supplemental Figure S4 and Supplemental Table S6). We also show that there was not a significant indirect or moderated effect using the GRIM (Lu et al., 2019), a methylomic aging measure designed to enhance prediction of increased mortality risk. Examination of intercorrelation of the several methylomic aging indices shows relatively low correlations, on average (See Supplemental Table S7).
Discussion
There is a need for integrative models that highlight mechanisms connecting childhood adversity and biological mediators to health outcomes in adulthood (Brody, Yu, & Beach, 2016; Suglia et al., 2018). Such models have the potential to highlight risk and resilience processes to guide future prevention efforts (Brody et al., 2013; Deighton, Nevillea, Puschb, & Dobson, 2018). Likewise, there is a need for research that expands our understanding of genetic risk factors, potentially identifying ways such risk is moderated by social environments. To address these gaps, in the current investigation we examined the association of childhood adversity and genetic risk for obesity, as well their interaction, in the prediction of adult health conditions. The pattern of observed results suggests that increased weight gain during adolescence and young adulthood is an indirect pathway connecting childhood adversity to a range of objective indicators of adult health for African American young adults, including cardiometabolic health, early onset of chronic illness, and methylomic indicators of aging phenotypes. In addition, elevated genetic risk for obesity confers increased risk for the adverse health effects of childhood adversity, at least in part, because it strengthens the impact of child adversity on increases in BMI across adolescence and young adulthood. The use of objective indicators of adult health in the current investigation is also important because it limits potential artifactual connections between reports of childhood adversity and health. Likewise, the use of a broad range of childhood adversities with little subjective content, and representative of the range of childhood conditions previously found to be associated with the development of obesity, strengthens our conclusions.
The most central prediction for the proposed model was that childhood adversity would be associated with elevation in risk markers for cardiometabolic illness in early adulthood. African Americans have greater prevalence and earlier onset of cardiovascular disease (Hozawa et al. 2007), are significantly more likely to die of CVD (Carnethon et al., 2017), and are twice as likely as Whites to develop type 2 diabetes and to be affected by its complications including heart disease, blindness, amputations, stroke, and death (Konen, Summerson, Bell, & Curtis. 1999), making this a key area of investigation to explain broader health disparities. Nonetheless, similar racial differences exist for a number of age-related chronic diseases (Geronimus, 2006a,b; Williams, 2012), with African Americans disproportionately suffering the burden of age-related disease and having earlier onset of chronic illness (Gaillard & Ose, 2010). The current results were supportive of the expectation that the pathway from childhood adversity to accelerated weight gain in adolescence and early adulthood would be useful in describing a plausible biological pathway linking childhood adversity to elevated risk for cardiometabolic outcomes.
An important benefit of hypothesizing that ΔBMI might serve as a mechanism of biological embedding, and using SNPs drawn from a widely utilized genetic platform to index GRSO, is that the model proposed in Figure 1 can be examined and confirmed or disconfirmed in multiple existing data sets with African Americans, or can be tested with appropriate changes for samples using other ethnic groups. The fact that we observed effects of childhood adversity beyond GRSO and a variety of control variables in our main effects model is also consistent with prior reports regarding the impact of childhood adversity on obesity (Wall et al., 2019; Björntorp, 2001; Garasky et al. 2009), and extends those reports by controlling for additional sources of potential spuriousness. Further suggesting non-spurious effects, both childhood adversity and genetic risk showed similar patterns of indirect effects through ΔBMI, providing additional support for the hypothesis that childhood adversity and genetic effects are important in the prediction of change in BMI across adolescence and ultimately in predicting objective indicators of young adult health.
The effect of childhood adversity on our indicators of young adult health was fully accounted for by ΔBMI, as was the effect of GRSO on indicators of young adult health, with significant indirect effects in each case. Thus, the straightforward interpretation of our results would be that the observed associations between recalled childhood adversities and young adult health is non-spurious, and this association can be accounted for by ΔBMI. However, it should be noted that the observed pattern does not preclude concurrent operation of alternative biological mechanisms. In particular, it is likely that there are other biological and psychosocial pathways linking childhood adversity to later adult health outcomes. For example, childhood adversity was associated with both substance use and depressive symptoms in the current sample, suggesting potential behavioral pathways to poorer long-term adult health. Likewise, there may be associations of childhood adversity with greater inflammatory response to depression (Beach, et., 2017; Miller & Cole, 2012), increased chronic inflammation (Hostinar, et al., 2015; Nusslock & Miller, 2016), or increased allostatic load (Brody et al., 2013), or changes in other cryptic biological processes that may further explain emerging health problems in later in adulthood, or that may co-occur with increased weight gain. Of importance to future theorizing, our focus was on predictors of morbidity but not mortality. Accordingly, it is likely that prediction of mortality risk may need to include somewhat different variables and may identify somewhat different processes than were the focus in the current investigation (Mills et al., 2019). At a minimum, however, ΔBMI across adolescence and young adulthood appears to be an important and useful marker of a biological embedding process associated with elevated childhood adversity.
As we developed our model, we confronted several methodological issues which will require additional attention in future efforts to test the proposed model. Although assessment of childhood adversity via retrospective reports is useful for many purposes, such reports may be limited by factors that influence self-report, or introduce bias, suggesting the value of using control variables to reduce potential threats to validity. In line with prior results, retrospective report of childhood adversity was correlated with concurrent depressive symptoms. This suggests that when depression is not the focus of the investigation, it will often be desirable to control for depressive symptoms when retrospective report of childhood adversity is used as a predictor (e.g., Reuben et al., 2016). Prospective assessment of childhood adversity should also be examined in relationship to change in BMI and objective indicators of young adult health. As has been noted in other contexts, such examination may yield different insights, highlight different mechanisms of change, and identify somewhat different “at risk” populations (Baldwin et al., 2019). Because they tend to assess different time frames (blocks of time vs. a specific snapshot of time) and because they are likely subject to different influences and memory processes, it cannot be assumed that prospective and retrospective assessment of childhood adversity will converge strongly.
An additional methodological issue is that genetic risk indices need to be validated for African American samples in order to avoid potential problems that can emerge for some genetic risk indices due to cross-ethnic differences in linkage disequilibrium (cf. Belsky et al., 2012). Similarly, identifying the contributions of childhood adversity to later health problems of African American youth may require developmentally and contextually sensitive assessments of adversity (cf. Brody et al., 2016). In the current investigation, for example, discrimination was included in the index of childhood adversities commonly experienced by African American youth prior to age 10. This was informed by a large body of research on African American youth and their exposure to discrimination at an early age (Sanders-Phillips, 2009). However, research is emerging with regard to the effects of discrimination against other ethnic, religious, and sexual minorities and it may be that, with appropriate changes, discrimination-focused assessment could contribute to prediction of obesity and longer-term health outcomes in other groups.
Despite our focus on weight gain across adolescence and young adulthood among African Americans, the current findings should not be taken as minimizing the likely value of examining influences on weight gain during other, even earlier developmental stages, including gestation, early infancy, and early childhood (Dietz, 1994). Our focus on weight gain across adolescence was driven by our desire to isolate the impact of childhood adversity and focus on changes that could have plausibly occurred after the period of reported childhood adversity. In the current investigation influences attributable to weight gain during earlier developmental stages were controlled, but these other potential developmental windows for change in BMI are very likely important in their own right and warrant future study. A related possibility, deserving attention in future research, is that genetic risk markers may exert developmentally specific effects (cf. Justice, et al., 2019; North et al, 2010, Sovio et al., 2011), increasing risk for weight gain only during particular developmental windows or in response to specific classes of risk. The current results are consistent with this speculation given that genetic risk only exerted a significant effect on weight gain for those with greater than average adversity.
Our findings provide insights with regard to the risk posed by childhood adversity and genetic risk factors for objective indicators of adult health. Although we controlled for confounds potentially inflating associations between recall of childhood adversity and health outcomes, we observed indirect effects of both childhood adversity and genetic risk on objective indicators of poor health at age 29. Better characterizing these risks may help in the development of both biological and social intervention strategies likely to improve long-term outcomes for youth exposed to childhood adversities. There appear to be opportunities to identify those at increased risk and to develop programs that may interrupt weight gain across adolescence, with potential beneficial effects for African American youth. In particular, it may be that family-based interventions delivered in late childhood may have a role to play in interrupting biological embedding of earlier adversity (e.g., Brody et al., 2016; Chen et al., 2018).
Limitations of the current investigation have been touched upon previously, but deserve additional mention. Childhood adversity effects need to be further elaborated and clarified by examining their connection to prospective reports of the social environment. In particular, some types of childhood adversity may be more common and/or consequential for adolescent weight gain than others, making them better targets of preventive intervention. Future work may also identify prospectively reported challenges and adversities that are highly correlated with retrospective reports of adversity, or that predict weight gain across adolescence and young adulthood. Coverage of the full range of relevant sources of adversity may be difficult to obtain prospectively, and valid reports during childhood may also be difficult to obtain for some types of adversity (Baldwin et al., 2019). It should also be noted that our polygenic risk score was not very powerful, suggesting that it does not capture all available genetic variance in BMI. This may be why it was not more strongly associated with chronic illness and cardiometabolic risk. Likewise, our measure of BMI at ages 10 and 12 was based on reported rather than measured height and weight, introducing an unknown amount measurement error. Finally, our conclusions are limited because the current data set does not allow us to examine change in objective indicators of health. However, this limitation is lessened somewhat given that the objective indicators of health we used as primary outcomes are unlikely to show much variation in late childhood, suggesting that having a measure of, for example chronic illness in childhood on which almost everyone scored “0”, would not change observed results appreciably.
It is also worth noting that observed effect sizes in the current study were relatively small, suggesting that the current results are preliminary. If replicated, these effects provide an important starting point for the identification of potential targets of preventive intervention. In particular, significant GxE effects allow for more in-depth examination of processes linking the environment and outcomes by focusing attention on mechanisms that may involve the identified genes. At the same time, there are likely additional genes of importance in understanding risk for obesity and these may operate in a complex manner and interact with developmental stage. It is also noteworthy that the GxE interaction effect was successful in identifying a group who, on average, did not show increased BMI across adolescence in response to childhood adversity, and a group who did, suggesting a basis for future applications. Likewise, the small, significant, indirect effects linking early precursors to young adult outcomes via changes occurring across adolescence support continued examination of adolescence as a key time frame when malleable predictors of later health outcomes for African American youth can be identified. Future investigations using experimental designs to probe potential points of preventive intervention may provide stronger and more specific tests of causal hypotheses (Brody, et al., 2013; Howe et al., 2016).
In sum, the current investigation provides support for the hypothesis that childhood adversity is related to adult health outcomes indirectly through its effect on weight gain across adolescence and young adulthood. It seems likely that accelerated weight gain across adolescence and young adulthood may be an important and potentially modifiable risk factor for later health problems among African American youth, and account for outcomes beyond risk due to childhood SES or those conferred by earlier childhood weight gain. In addition, the indirect and moderated relationships implied by our theoretical model were robust to a range of analytic choices, with the largest effects observed for cardiometabolic health. Supplemental analyses showed that effects found for PhenoAge generalized to other previously used measures of methylomic aging, but not to a recently derived methylomic predictor of mortality, and that effects on PhenoAge were robust to controls for cell-type variation. Nonetheless, all observed effects require replication in other samples to see if the observed patterns generalize to a) other samples of African American youth, b) other minority samples confronting elevated levels of childhood adversity, and c) European American samples confronting varying levels of childhood adversity. The evidence linking obesity to health problems in adulthood is quite strong (Manson et al., 1990), making the periods of increased vulnerability for the development of obesity, especially vulnerability during adolescence and young adulthood (Zhang et al., 2019), a particularly attractive target for preventive intervention. Better understanding the way that genetic risk may moderate the contribution of adversities at these periods of increased risk both to young adult obesity and ultimately to health consequences later in adulthood is important for enhanced models of lifespan health development.
Supplementary Material
Acknowledgments
This research was supported by Award Number R01 HD080749 from the National Institute of Child Health and Human Development, Award Number R01 CA220254 from the National Cancer Institute, Award Number R01 HL118045 from the National Heart, Lung, and Blood Institute, Award Number R01 AG055393 from the National Institute on Aging, and Award Number P30 DA027827 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Footnotes
The genetic data reported in the current manuscript have not been used previously.
References
- Baldwin JR, Arseneault L, Odgers C, Belsky DW, Mathews T, Ambler, …. Danese A (2016). Childhood bullying victimization and overweight in young adulthood: A cohort study. Psychosomatic Medicine, 78, 1094–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin JR, Reuben A, Newbury JB, & Danese A (2019). Agreement between prospective and retrospective measures of childhood maltreatment: A systematic review and meta-analysis. JAMA Psychiatry, 76(6), 584–593. doi: 10.1001/jamapsychiatry.2019.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, McLaughlin KA, Misra S, & Koenen KC (2017). Childhood maltreatment and health impact: The examples of cardiovascular disease and type 2 diabetes mellitus in adults. Clinical Psychology: Science and Practice, 24, 125–139. doi: 10.1111/cpsp.12191. Epub 2017 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SRH, Dogan MV, Lei MK, Cutrona CE, Gerrard M, Gibbons FX, Simons RL, Brody GH, Philibert RA (2015). Methylomic aging as a window on lifestyle impact: Tobacco and alcohol use alter rate of biological aging. Journal of the American Geriatrics Society, 63, 2519–2525. doi: 10.1111/jgs.13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SRH, Lei MK, Simons RL, Barr AB, Simons LG, Ehrlich K, Brody GH, & Philibert RA (2017). When inflammation and depression go together: The longitudinal effects of parent-child relationships. Development and Psychopathology, 29, 1969–1986. doi: 10.1017/S0954579417001523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellatorre A, Finch B, Do DP, Bird C & Beck A (2011). Contextual predictors of cumulative biological risk: Segregation and allostatic load. Social Science Quarterly, 92 (5), 1338–1362. Doi: 10.1111/j.1540-6237.2011.00821.x. [DOI] [Google Scholar]
- Belsky DW, Moffitt TE, & Caspi A (2013). Genetics in population health science: Strategies and opportunities. American Journal of Public Health, 103(Suppl 1), S73–83. doi: 10.2105/AJPH.2012.301139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Houts R, Bennett GG, Biddle AK, et al. (2012). Polygenic risk, rapid childhood growth, and the development of obesity: Evidence from a 4-decade longitudinal study. Archives of Pediatric and Adolescent Medicine, 166(6), 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Sugden K, Williams B, Houts R, McCarthy J, Caspi A (2013). Development and evaluation of a genetic risk score for obesity. Biodemography and Social Biology, 59, 85–100. doi: 10.1080/19485565.2013.774628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, & Beach SRH (2016). Resilience to adversity and the early origins of disease. Development and Psychopathology, 28(4), 1347–1365. doi: 10.1017/S0954579416000894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Beach SRH, Hill KG, Howe GW, Prado G, Fullerton SM (2013). Using genetically informed, randomized prevention trials to test etiological hypotheses about child and adolescent drug use and psychopathology. American Journal of Public Health, 103 Suppl 1:S19–24. doi: 10.2105/AJPH.2012.301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björntorp P (2001). Do stress reactions cause abdominal obesity and comorbidities? Obesity Review, 2, 73–86. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, & Gotlib IH (1993). Psychopathology and early experience: a reappraisal of retrospective reports. Psychological Bulletin, 113, 82–98. [DOI] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen E, Miller GE, Kogan SM, & Beach SR (2013). Is resilience only skin deep?: Rural African Americans’ socioeconomic status-related risk and competence in preadolescence and psychological adjustment and allostatic load at age 19. Psychological Science, 24, 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey VJ, Walters EE, Colditz GA et al. (1997). Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women: The Nurses’ Health Study. American Journal of Epidemiology, 145, 614–619. [DOI] [PubMed] [Google Scholar]
- Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA, Willis M, & Yancy CW (2017). Cardiovascular health in African Americans a scientific statement from the American Heart Association. Circulation, 136, e393–e423. DOI: 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- Chan JM, Rimm EB, Colditz GA, Stampfer MJ, & Willett WC (1994). Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care, 179, 61–69. [DOI] [PubMed] [Google Scholar]
- Chen E, Yu T, Miller GE, & Brody GH (2018). Substance use and obesity trajectories in African Americans entering adulthood. American Journal of Preventive Medicine. 55, 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerham W, Hamby BW, & Oates GR (2017). The social determinants of chronic disease. American Journal of Preventative Medicine, 52, S5–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, & Matthews KA (2010). Childhood socioeconomic status and adult health. Annals of the New York Academy of Science, 1186, 37e55. [DOI] [PubMed] [Google Scholar]
- Deightona S, Nevillea A, Puschb D, & Dobson K (2018). Biomarkers of adverse childhood experiences: A scoping review. Psychiatry Research, 269, 719–732. [DOI] [PubMed] [Google Scholar]
- Dietz WH (1994). Critical periods in childhood for the development of obesity. American Journal of Clinical Nutrition, 59, 955–959. [DOI] [PubMed] [Google Scholar]
- Domingue BW, Belsky DW, Harris KM, Smolen A, McQueen MB, et al. (2014) Polygenic risk predicts obesity in both White and Black young adults. PLoS ONE, 9(7), e101596. doi: 10.1371/journal.pone.0101596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, Loo CM, & Giles WH (2004). The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse & Neglect, 28, 771–784. [DOI] [PubMed] [Google Scholar]
- Dudbridge F (2013). Power and predictive accuracy of polygenic risk scores. PLoS Genetics, 9/3: e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duru OK, Harawa NT, Kermah D, & Norris KC (2012). Allostatic load burden and racial disparities in mortality. Journal of the National Medical Association, 104(1–2), 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Kahn R, Robertson RM & Rizza RA (2006). Preventing cardiovascular disease and diabetes. A call to action from the American Diabetes Association and the American Heart Association. Circulation, 113, 2943–2946. [DOI] [PubMed] [Google Scholar]
- Elgar FJ, Xie A, Pfortner TK, White J, & Pickett KE (2016). Relative deprivation and risk factors for obesity in Canadian adolescents. Social Science and Medicine, 152, 111e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed MJS, & Froguel P (2013). From obesity genetics to the future of personalized obesity therapy. National Review of Endocrinology, 9, 402–413. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine, 14(4), 245–58. [DOI] [PubMed] [Google Scholar]
- Felson DT, Zhang Y, Hannan MT, et al. (1997). Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis and Rheumatism, 40, 728–733. [DOI] [PubMed] [Google Scholar]
- Gaillard TR, & Ose K (2010). Ethnic differences in lipids/lipoproteins/apo lipoproteins and proinflammatory markers in non-diabetic African American and White postmenopausal women. Diabetes, 59, A607–A607. [Google Scholar]
- Garasky S, Stewart S, Gundersen C, Lohman B, & Eisenmann J (2009). Family stressors and child obesity. Social Science Research, 38, 755–766. [DOI] [PubMed] [Google Scholar]
- Gee DG, & Casey BJ (2015). The impact of developmental timing for stress and recovery. Neurobiology of Stress, 1, 184–194. doi: 10.1016/j.ynstr.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT (1992). The weathering hypothesis and the health of African-American women and infants: Evidence and speculations. Ethnicity and Disease, 2(3), 207–221. [PubMed] [Google Scholar]
- Geronimus AT, Bound J, Keene D, & Hicken M (2006a). Black-white differences in age trajectories of hypertension prevalence among adult women and men, 1999–2002. Ethnicity & Disease, 16(1), 40–48. [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, & Bound J (2006b). “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health, 96(5), 826–833. doi: 10.2105/ajph.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gini G, & Pozzoli T (2009). Association between bullying and psychosomatic problems: a meta-analysis. Pediatrics, 123, 1059–65. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Glaser R, Malarkey WB, Beversdorf D, & Kiecolt-Glaser J (2012). Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychology, 31, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S,…. & Zhang K (2013). Genome-Wide methylation profiles reveal quantitative views of human aging rates. Molecular Cell 49(2), 359–367. doi: 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, & Rutter M (2004). Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. Journal of Child Psychology and Psychiatry, 45 (2), 260–273. doi: org/10.1111/j.1469-7610.2004.00218.x [DOI] [PubMed] [Google Scholar]
- Hayes AF (2015). An index and test of linear moderated mediation. Multivariate Behavioral Research, 50, 1–22. [DOI] [PubMed] [Google Scholar]
- Hitchcock C, Rodrigues E, Rees C, Gormley S, Dritschel B, & Dalgleish T (2019). Misremembrance of things past: Depression is associated with difficulties in the recollection of both specific and categoric autobiographical memories. Clinical Psychological Science, 7, 693–700. 10.1177/2167702619826967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock C, Nixon RDV, & Weber N (2014). A review of over general memory in child psychopathology. British Journal of Clinical Psychology, 53, 170–193. doi: 10.1111/bjc.12034 [DOI] [PubMed] [Google Scholar]
- Hitchcock C, Rees C, & Dalgleish T (2017). The devil’s in the detail: Accessibility of specific personal memories supports rose-tinted self-generalizations in mental health and toxic self-generalizations in clinical depression. Journal of Experimental Psychology: General, 146, 1286–1295. doi: 10.1037/xge0000343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S (2013). DNA methylation age of human tissues and cell types. Genome Biology, 14(10), R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, & Raj K (2018). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews: Genetics, 19(6), 371–384. Doi.org/10.1038/s415/6-018-0004-3. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Lachman ME, Mroczek DK, Seeman TE, & Miller GE (2015). Additive contributions of childhood adversity and recent stressors to inflammation at midlife: Findings from the MIDUS study. Developmental Psychology, 51(11), 1630–1644. 10.1037/dev0000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe G, Beach SRH, Brody GH, Wyman PA (2016). Translating genetic research into prevention and treatment: The baseline target moderated mediator design. Frontiers in Psychology, 6, 1911. doi: 10.3389/fpsyg.2015.01911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyert DL, & Xu J (2012). Deaths: Preliminary data for 2011. National Vital statistics, Report 61, 1–51. [PubMed] [Google Scholar]
- Hozawa A, Folsom AR, Sharrett AR, & Chambless LE (2007). Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects: Atherosclerosis Risk in Communities Study. Archives of Internal Medicine, 167, 573–579. doi: 10.1001/archinte.167.6.573. [DOI] [PubMed] [Google Scholar]
- Justice AE Chittoor G, Blanco E, Graff M, Wang Y, Albala C, Santos JL, Angel B, Lozoff B, Voruganti VS, North KE, & Gahagan S(2019). Genetic determinants of BMI from early childhood to adolescence: the Santiago Longitudinal Study. Pediatric Obesity, 14(3), e12479. doi: 10.1111/ijpo.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyhava J, Pedersen N, & Hagg S (2017). Biological age predictors. EBioMedicine, 21, 29–36. 10.1186/1753-2000-4-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmarzyk PT, Pérusse L, Malina RM, & Bouchard C (1999). Seven-year stability of indicators of obesity and adipose tissue distribution in the Canadian population. The American Journal of Clinical Nutrition, 69(6), 1123–1129. 10.1093/ajcn/69.6.1123 [DOI] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, et al. (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. The British Journal of Psychiatry, 197, 378–385. doi; org/10.1192/bjp.bp.110.080499PMID:21037215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H-U, & Kendler KS (1994). Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry, 51, 8–19. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Gwinn M, Bowen MS, & Dotson WD (2012). Beyond base pairs to bedside: a population perspective on how genomics can improve health. American Journal of Public Health, 102, 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimm SY, Barton BA, Obarzanek E, Mcmahon RP, Kronsberg SS, Waclawiw MA, Morrison JA, Schreiber GB, Sabry ZI, & Daniels SR (2002). Obesity development during adolescence in a biracial cohort: The NHLBI growth and health study. Pediatrics, 110, e54. [DOI] [PubMed] [Google Scholar]
- Kittleson MM, Meoni LA, Wang N-Y, Chu AY, Ford DE, & Klag MJ (2006). Association of childhood socioeconomic status with subsequent coronary heart disease in physicians. Archives of Internal Medicine, 166, 2356e2361. [DOI] [PubMed] [Google Scholar]
- Konen JC, Summerson JH, Bell RA, & Curtis LG (1999). Racial differences in symptoms and complications in adults with type 2 diabetes mellitus. Ethnicity and Health, 4, 39–49. [DOI] [PubMed] [Google Scholar]
- Lean MEJ, Han TS, & Seidell JC (1998). Impairment of health and quality of life in people with large waist circumference. Lancet, 351, 853–856. [DOI] [PubMed] [Google Scholar]
- Lei MK, Beach SRH, & Simons RL (2018). Childhood trauma, pubertal timing, and cardiovascular risk in adulthood. Health Psychology. Health Psychology, 37, 613–617. doi: 10.1037/hea0000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, … & Horvath S (2018). An epigenetic biomarker of aging for lifespan and healthspan. Aging, 10(4), 573–591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TY et al. (2006). Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation, 113, 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhao JH, Luan J, Ekelund U, Luben RN, Khaw KT, … & Loos RJ (2010). Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk prospective population study. PLoS Medicine, 7/8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, … Horvath S (2019). DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging, 11(2), 303–327. doi: 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz W, Sanderson W, & Scherbov S, (2008). The coming acceleration of global population ageing. Nature, 451(7179), 716–719. [DOI] [PubMed] [Google Scholar]
- Manson JE, Colditz GA, Stampfer MJ et al. (1990). A prospective study of obesity and risk of coronary heart disease in women. New England Journal of Medicine, 322, 882–889. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137, 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE & Cole SW (2012). Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry, 72(1), 34–40. doi: 10.1016/j.biopsych.2012.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J, Beach SRH, Dogan M, Simons RA, Gibbons FX, Long J, & Philibert RA (2019). A direct comparison of the relationship of epigenetic aging and epigenetic substance consumption markers to mortality in the Framingham Heart Study. Genes. 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkler M, Fuller-Thompson E, & Guralnik JM (2006). Gradient 36 Biological consequences of socioeconomic inequalities of Disability across the Socioeconomic Spectrum in the United States. New England Journal of Medicine, 355(7), 695–703. [DOI] [PubMed] [Google Scholar]
- Monda KL, Chen GK, Taylor KC, Palmer C, Edwards TL, Lange LA…& Haiman CA (2013). A meta-analysis identifies new loci associated with body mass index in individuals of African ancestry. Nature Genetics, 45(6), 690–698. doi: 10.1038/ng.2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. (2016). Executive summary: Heart disease and stroke statistics-2016 update: A report from the American heart association. Circulation, 133, 447–454. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2017). Mplus: Statistical Analysis with Latent Variables: User’s Guide (Version 8). Los Angeles, CA: Authors. [Google Scholar]
- North KE, Graff M, Adair LS, et al. (2010). Genetic epidemiology of BMI and body mass change from adolescence to young adulthood. Obesity, 18, 1474–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuru-Jeter AM, Thorpe RJ, & Fuller-Thompson E (2011). Black-White differences in self-reported disability outcomes in the U.S.: Early childhood to older adulthood. Public Health Reports, 126(6), 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Miller GE (2016). Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biological Psychiatry, 80(1), 23–32. doi: 10.1016/j.biopsych.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, & Flegal KM (2014). Prevalence of childhood and adult obesity in the United States, 2011–2012. Journal of the American Medical Association. 311(8), 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ et al. (2005). A potential decline in life expectancy in the United States in the 21st century. New England Journal of Medicine, 352, 1138–1145. [DOI] [PubMed] [Google Scholar]
- Osborn M, & Widom CS (2019). Do documented records and retrospective reports of childhood maltreatment similarly predict chronic inflammation? Psychological Medicine. doi: 10.1017/S0033291719002575 [DOI] [PubMed] [Google Scholar]
- Phelan JC, & Link BG (2005). Controlling disease and creating disparities: A fundamental cause perspective. Journals of Gerontology Series B-Psychological Sciences and Social Sciences, 60, 27–33. [DOI] [PubMed] [Google Scholar]
- Plomin R, Haworth CM, & Davis OSP (2009). Common disorders are quantitative traits. Nature Review Genetics, 10(12), 872–878. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, et al. (2006). Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics, 38, 904–909. [DOI] [PubMed] [Google Scholar]
- Radford L, Corral S, Bradley C, & Fisher HL (2013). The prevalence and impact of child maltreatment and other types of victimization in the UK: findings from a population survey of caregivers, children and young people and young adults. Child Abuse & Neglect, 37, 801–813. [DOI] [PubMed] [Google Scholar]
- Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, Hogan S, Ramrakha S, Poulton R, & Danese A (2016). Lest we forget: Comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology and Psychiatry, 57 (10), 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende LF, Rodrigues Lopes M, Rey-López JP, Matsudo VKR, Luiz O (2014) Sedentary behavior and health outcomes: An overview of systematic reviews. PLoS ONE, 9(8), e105620. 10.1371/journal.pone.0105620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm EB, Stampfer MJ, Giovannucci E, et al. (1995). Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. American Journal of Epidemiology,141(12),1117–1127. [DOI] [PubMed] [Google Scholar]
- Rissanen A, Heliövaara M, Knekt P, Reunanen A Aromaa A, & Maatela J (1990). Risk of disability and mortality due to overweight in a Finnish population. The BMJ, 301(6756), 835–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders-Phillips K (2009). Racial discrimination: A continuum of violence exposure for children of color. Clinical Child and Family Psychology Review, 12, 174–195. [DOI] [PubMed] [Google Scholar]
- Sankar P, Cho MK, Condit CM, et al. (2004). Genetic research and health disparities. Journal of the American Medical Association, 291(24), 2985–2989. doi: 10.1001/jama.291.24.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl L & Hoffmann G (2015). Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension Score, and health outcomes: A systematic review and meta-analysis of cohort studies. Journal of the Academy of Nutrition and Dietetics, 115(5), 780–800.e5. [DOI] [PubMed] [Google Scholar]
- Shonkoff J, Boyce T, & McEwen B (2009). Neuroscience, molecular biology, and the childhood roots of health disparities. Journal of the American Medical Association, 301 (21), 2252–2259 [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, & Bales SN (2011). Science does not speak for itself: Translating child development research for the public and its policymakers. Child Development, 82, 17–32. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, McGuinn L, … & Wood DL (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129, 232–246. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Jelenkovic A, Sund R, Yokoyama Y, Hur Y-M, Cozen W, …., Kaprio J (2017). Differences in genetic and environmental variation in adult BMI by sex, age, time period, and region: An individual-based pooled analyses of 40 twin cohorts. American Journal of Clinical Nutrition, 106, 457–466. doi: 10.3945/ajcn.117.153643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei MK, Beach SRH, Barr AB, Simons LG, Gibbons FX, & Philibert RA (2018). Discrimination, segregation, and chronic inflammation: Testing the weathering explanation for the poor health of Black Americans. Developmental Psychology, 54, 1993–2006. doi: 10.1037/dev0000511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei MK, Beach SR, Barr AB, Cutrona CE, Gibbons FX, & Philibert RA (2017). An index of the ratio of inflammatory to antiviral cell types mediates the effects of social adversity and age on chronic illness. Social Science & Medicine, 185, 158–165. [DOI] [PubMed] [Google Scholar]
- Slopen N, Williams DR, Seedat S, Moomal H, Herman A, & Stein DJ (2010). Adversities in childhood and adult psychopathology in the South Africa Stress and Health Study: Associations with first-onset DSM-IV disorders. Social Science & Medicine. 71(10), 1847–18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solís CB, Kelly-Irving M, Fantin R, Darnaudéry M, Torrisani J, Lang T, & Delpierre C (2015). Adverse childhood experiences and physiological wear-and-tear in midlife: Findings from the 1958 British birth cohort. PNAS, 112(7), E738–E746. 10.1073/pnas.1417325112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovio U, Mook-Kanamori DO, Warrington NM, Lawrence R, Briollais L, Palmer CN … Timpson NJ (2011). Association between common variation at the FTO locus and changes in body mass index from infancy to late childhood: the complex nature of genetic association through growth and development. PLoS Genetics, 7(2), e1001307. doi: 10.1371/journal.pgen.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, et al. (2010). Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nature Genetics, 42(11), 937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanikova I, Baker EH, Simoni ZR, Zhu A, Rutland SB, Sims M, & Wilkinson LL (2017). The role of perceived discrimination in obesity among African Americans. American Journal of Preventive Medicine, 52(1S1), S77–S85. doi: 10.1016/j.amepre.2016.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, Koenen KC, Boyton-Jarrett R, Chan PS, Clark CJ, Danese A, Faith MS, Goldstein BI, Hayman LL, Isasi CR, Pratt CA, Slopen N, Sumner JA, Turer A, Turer CB, & Zachariah JP (2018). Childhood and adolescent adversity and cardiometabolic outcomes: A scientific statement from the American Heart Association. Circulation, 137, e15–e28. doi: 10.1161/CIR.0000000000000536Circulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Griffith JW, & Mineka S (2010). Over general autobiographical memory as a predictor of the course of depression: A meta-analysis. Behaviour Research and Therapy, 48, 614–625. doi: 10.1016/j.brat.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo T, Chrinstian H, & Rathmann W (2010). Impact of early psychosocial factors (Childhood socioeconomic factors and adversities) on future risk of type 2 diabetes, metabolic disturbances, and obesity: A systematic review. BMC Public Health, 10, 525–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D, Edelsberg J, Colditz GA, Bird AP, & Oster G (1999). Lifetime health and economic consequences of obesity. Archives of Internal Medicine, 159, 2177–2183. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Thomas CS, & Brown TH (2016). Childhood adversity and adult health: evaluating intervening mechanisms. Social Science and Medicine, 156, 114e124. [DOI] [PubMed] [Google Scholar]
- van Vreeswijk MF & de Wilde EJ (2004). Autobiographical memory specificity, psychopathology, depressed mood and the use of the Autobiographical Memory Test: A meta-analysis. Behaviour Research and Therapy, 42, 731–743. doi: 10.1016/S0005-7967(03)00194-3 [DOI] [PubMed] [Google Scholar]
- Visscher TLS & Seidell JC (2001). The public health impact of obesity. Annual Review of Public Health, 22, 355–375. [DOI] [PubMed] [Google Scholar]
- Wall MM, Mason SM, Liu J, Olfson M, Neumark-Sztainer D, & Blanco C (2019). Childhood psychosocial challenges and risk for obesity in U.S. men and women. Translational Psychiatry, 9, 16–28. doi: 10.1038/s41398-018-0341-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickrama KA, Lee TK, & O’Neal CW (2015). Stressful life experiences in adolescence and cardiometabolic risk factors in young adulthood. Journal of Adolescent Health, 56, 456–63. [DOI] [PubMed] [Google Scholar]
- Williams DR (2012). Miles to go before we sleep: Racial inequities in health. Journal of Health and Social Behavior, 53(3), 279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JMG, Barnhofer T, Crane C, Herman D, Raes F, Watkins E, & Dalgleish T (2007). Autobiographical memory specificity and emotional disorder. Psychological Bulletin, 133(1), 122–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik GL, Graff M, Nishimura KK, Tao R, Haessler J, Gignoux CR…. & Carlson CS (2019). Genetic analyses of diverse populations improves discovery for complex traits. Nature, 570, 514–518. doi: 10.1038/s41586-019-1310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Whelton PK, Xi B, Krousel-Wood M, Bazzano L, He J, Chen W, & Li S (2019). Rate of change in body mass index at different ages during childhood and adult obesity risk. Pediatric Obesity, 14 (7), e12513. doi: 10.1111/ijpo.12513 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.