Summary:
With the explosion of digital media and technologies, commentators have become increasingly vocal about the role that an ‘attention economy’ plays in our lives.1 The rise of today’s digital culture coincides with longstanding scientific questions about why humans sometimes remember and sometimes forget, and why some individuals remember better than others.2–6 We examined whether spontaneous attention lapses –– in the moment7–12, across individuals13–15, and as a function of everyday media multitasking16–19 –– negatively relate to remembering. EEG+pupillometry measures of attention20–21 were recorded as 80 young adults performed a goal-directed episodic encoding and retrieval task22. Trait-level sustained attention was further quantified via task-based23 and questionnaire measures24–25. Leveraging trial-to-trial retrieval data, we show that tonic lapses of attention in the moment prior to remembering, assayed by posterior alpha power and pupil diameter, related to reductions in neural signals of goal coding and memory, along with behavioral forgetting. Independent measures of trait-level attention lapsing mediated the relationship between neural assays of lapsing and memory performance, and between media multitasking and memory. Attention lapses partially account for why we remember or forget in the moment, and why some individuals remember better than others. Heavier media multitasking is associated with a propensity to suffer attention lapses and forgetting.
Fluctuations in spontaneous states of preparatory attention might help to account for three puzzles of neuroscience and behavioral science: Why do humans sometimes remember and sometimes forget? Why do some cognitively healthy individuals remember better than others? And, why does memory vary as a function of engagement with the modern media landscape? To examine links between attention, goal coding, and episodic remembering within individuals, and how they correlate with individual differences and media multitasking, participants completed a goal-directed episodic memory task with EEG+pupillometry (ED Fig1) and a trait-level battery.
We first leveraged retrieval data to probe whether and how lapses of attention in the moment before remembering relate to neural signals of goal coding and memory, and behavioral forgetting. Pre-stimulus tonic increases in posterior alpha power from EEG, an expression of release from top-down inhibitory control, and pre-stimulus tonic decreases in pupil diameter from pupillometry, an expression of hypoarousal linked to a locus coeruleus circuit of noradrenaline, are associated with attention lapsing and reduced accuracy on working memory, perceptual discrimination, and vigilance tasks, and thus could extend to episodic remembering.7–12,20–21 Little is known about the role that spontaneous fluctuations in attention play in the representation of retrieval goals and cues, engaged post-encoding5, that govern attempts to remember the past. To assay spontaneous lapses, tonic posterior alpha power and pupillometry were extracted from the 1s preceding the onset of each trial’s retrieval goal cue (‘pre-goal’) and object probe (‘pre-probe’). To measure the strength of goal coding on three retrieval tasks (conceptual source recognition, perceptual source recognition, and novelty detection), goal-cue locked ERPs were extracted from an a priori midfrontal cluster shown26–27 to track goal processing. To measure neural signals of recollection- and familiarity-based memory, object-probe locked ERPs were extracted from a priori left posterior and left midfrontal clusters, canonical sites of Parietal Old/New and FN400 mnemonic components28 (ED Fig1b). All assays were z-scored within run to account for potential time-on-task effects across runs.
Spontaneous changes in attention, just prior to retrieval goal-cue onset, related to subsequent remembering of studied items (Fig1). Specifically, lapses early in a trial –– marked by pre-goal increases in posterior alpha power and decreases in pupil diameter –– related to a greater likelihood of memory failure (misses) vs. remembering (hits) across the three goal-state conditions (alpha: b=−0.46, z(6822)=−3.61, p<.001; pupil: b=0.36, z(7197)=2.18, p=.03). In contrast, later in a trial, pre-retrieval probe alpha and pupil did not relate to misses vs. hits (correct rejections vs. false alarms were also unaffected; SI).
Figure 1.
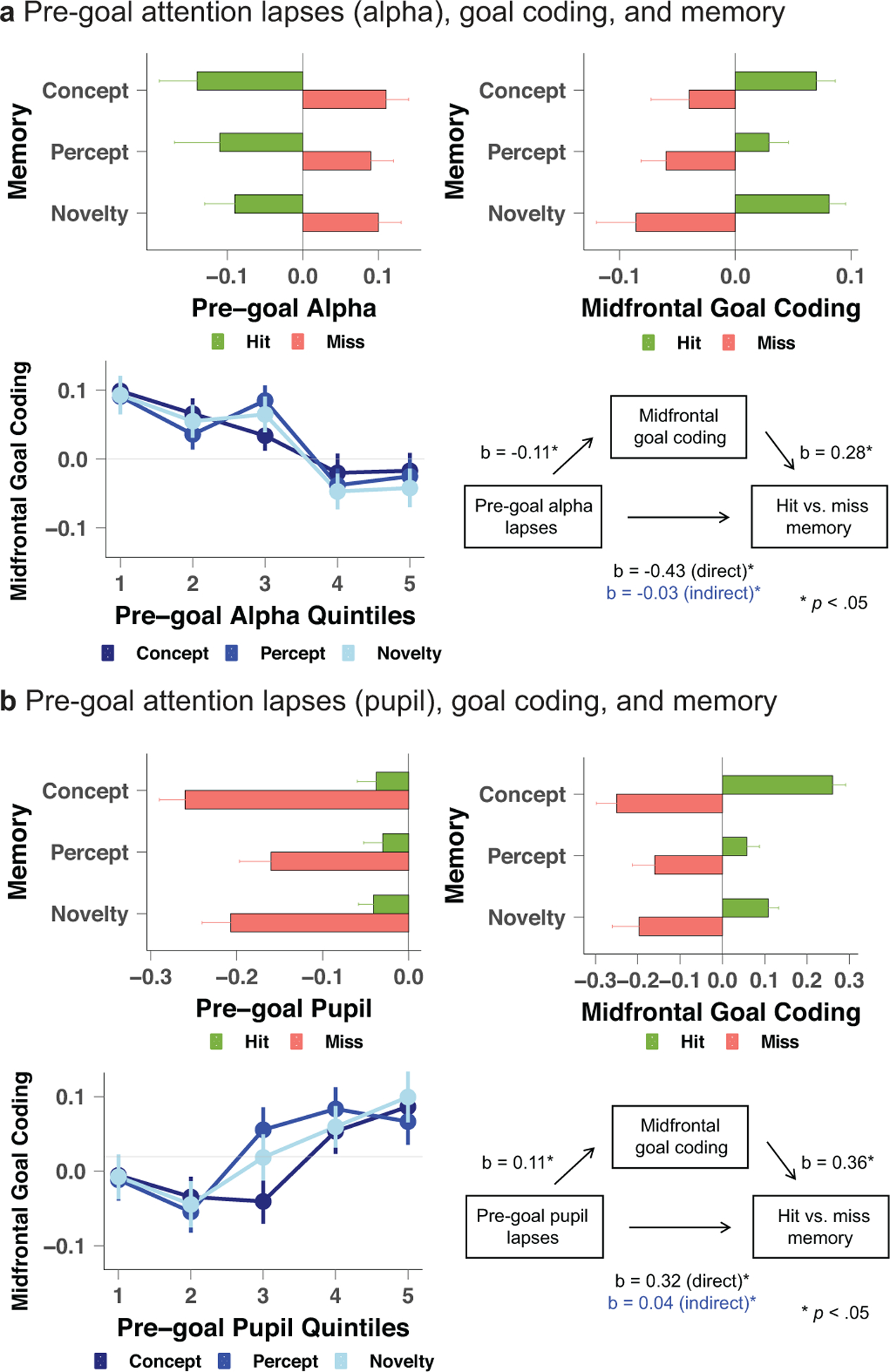
Pre-goal attention lapses relate to forgetting (misses) vs. remembering (hits) across three retrieval goal conditions, and this relationship is partially mediated by goal-coding strength via midfrontal ERP cluster. a, b, Attention lapses from alpha (a) and pupil (b) assays. Mean unweighted standardized betas are shown in graphs with x- and y-axes, and quintiles for lapsing-goal coding (error bars represent 1 SEM); statistics included interaction term for retrieval goal, and treated lapsing-goal coding continuously (weighted standardized betas) in trial-level mixed models. Numeric axis units with decimals are z-scores. The trial-wise mediation models reflect mean weighted standardized betas, with two-sided p-values (alpha indirect effect: p=.042 and direct effect: p=.017; pupil indirect effect: p=.041 and direct effect: p=.008). Z and t statistical tests, where applicable, with no multiple comparison adjustment. N=75 human subjects from 1 independent experiment.
Why do pre-goal lapses of attention relate to forgetting vs. remembering? One possibility is that lapsing just prior to processing a retrieval goal results in a reduction in the strength of subsequent goal coding, which then influences retrieval. Consistent with this hypothesis, lapsing just prior to goal cuing was significantly related to a reduction in the subsequent goal-cue elicited midfrontal ERP signal (alpha: b=−0.11, t(6819)=−2.29, p=.02; pupil: b=0.11, t(7194)=2.09, p=.04). Importantly, this reduction in goal-state coding in turn significantly related to misses vs. hits across the three goal-state conditions (alpha: b=0.28, z(6823)=2.23, p=.03; pupil: b=0.36, z(7197)=2.11, p=.04; note: effect sizes (bs) between goal coding and memory are not identical by modality due to trial-level modality-specific artifacts; Methods). Trial-wise mediation analyses revealed that the relationship between pre-goal lapsing and later forgetting was partially explained by the strength of goal coding, and this was the case both when attention was assayed via pre-goal alpha power (Fig1a; indirect effect b=−0.03 (95%CI=−0.05,−0.01), direct effect b=−0.43, total effect b=−0.46) and pupil diameter (Fig1b; indirect effect b=0.04 (95%CI=0.02,0.07), direct effect b=0.32, total effect b=0.36). The total, indirect, and direct effects were significant in each mediation (ps<.05; Methods). These outcomes indicate that moment-to-moment lapses prior to goal cuing relate to concomitant reductions in goal coding that influence source recollection and novelty detection, and also have significant direct effects on memory.
Beyond memory behavior, we leveraged EEG to measure neural markers of retrieval and their relation to lapsing. Consistent with prior work6,28, we first confirmed that, irrespective of attentional state, canonical Parietal Old/New (ED Fig2a) and FN400 (ED Fig2c) neural signals were observed (SI and ED Figs3–4 for additional results). We next examined whether pre-goal lapses related to the magnitude of the retrieval-probe elicited Parietal Old/New and FN400 neural signals. Focusing on source recollection signals in the conceptual and perceptual retrieval tasks, we observed a significant interaction between trial-level pre-goal lapsing and the magnitude of the Parietal Old/New effect (500–600ms post-probe) when remembering (hits) vs. forgetting (misses) (alpha: b=−0.14, t(4041)=−2.16, p=.03; pupil: b=0.17, t(4341)=2.26, p=.02) (ED Fig2b). Pre-goal lapses were significantly related to the strength of Parietal signal on miss trials (alpha: b=−0.14, t(1521)=−2.52, p=.01; pupil: b=0.15, t(1721)=2.41, p=.02) but not hit trials (alpha: b<.001, t(2470)=−0.09, p=.89; pupil: b=−0.01, t(2620)=−0.37, p=.71). That is, moment-to-moment increases in alpha power and decreases in pupil diameter related to reductions in Parietal Old/New signal for source recollection failures (misses), suggesting that fluctuations in attention relate to responses to sub-threshold recollection items, driving forgetting.
Focusing on neural memory signals in the novelty detection task, a significant interaction was also exhibited between trial-level pre-goal lapsing and the magnitude of the FN400 component (400–500ms post-probe) when correctly endorsing new items (hits) vs. misses (alpha: b=0.17, t(2771)=2.18, p=.03; pupil: b=−0.23, t(2846)=−2.17, p=.03) (ED Fig2d). Pre-goal lapses were significantly related to the strength of FN400 signal on misses (alpha: b=0.11, t(974)=2.03, p=.04; pupil: b=−0.22, t(896)=−2.51, p=.01) but not hits (alpha: b=−0.07, t(1797)=−1.41, p=.16; pupil: b=0.02, t(1947)=0.45, p=.65). That is, moment-to-moment increases in alpha power and decreases in pupil diameter related to increasing positivity in FN400 signal for misses, driving signal to appear more like that of an incorrect old rather than correct new item. These results indicate that attentional fluctuations relate to neural responses that underpin familiarity-based memory.
Along with the relationship between moment-to-moment lapses and subsequent multimodal signals of memory failures, we assessed whether trait-level differences in the propensity to lapse could help explain why some individuals are more likely to forget than others (Fig2). Emerging evidence indicates that trait-level differences in sustained attention relate to differences in working memory13, and thus might extend to long-term memory processes. We first examined how trait-level task-based metrics of preparatory lapsing relate to memory, computing subject-level lapse markers –– mean alpha power and pupil diameter variability averaged over all pre-goal retrieval epochs –– and behavioral markers of retrieval success –– memory discriminability (d’) for each goal-state condition. Higher trait-level alpha power in the absence of external distraction has been associated with release from inhibition, higher pupil variability has been associated with more off-task thoughts, and higher d’ denotes higher memory accuracy.7,10 We found that increases in trait-level mean alpha power and pupil variability were significantly negatively related to individual differences in d’ (Fig2a and SI). In addition, we found that individual differences in pre-goal lapses of attention at encoding, assayed from mean alpha power and pupil variability, were significantly related to individual differences in pre-goal lapses at retrieval and to memory ability (ED Fig5 and SI). Importantly, when controlling for differences in attention at encoding, there remains a significant relationship between individual-differences in pre-goal attention at retrieval and remembering (SI).
Figure 2.

Trait-level differences in sustained attention help to explain why individuals are more prone to remembering or forgetting. a, b, Greater lapsing is related to worse d’ on the memory tasks (a) and attention on the gradCPT (b). c, Worse attention on the gradCPT is related to worse memory d’. Raw scores are plotted for graphs with x- and y-axes; statistics included z-scores with Pearson correlations. d, Formal mediation models with mean standardized betas, and two-sided p-values (alpha indirect effect: p=.003; pupil indirect effect: p=.018). N=75 for alpha data and N=80 for all other data with human subjects from 1 independent experiment; hence the effect sizes between commission error and d’ are not identical for alpha and pupil models (Methods). Z and t statistical tests, where applicable, with no multiple comparison adjustment. gradCPT=gradual-onset continuous performance task.
Following the experiment, participants also completed an independent task-based assessment of sustained attention (the gradual-onset continuous performance task, gradCPT23). Two of its metrics –– commission error rate (CER; responses to no-go trials) and RT variability (RTV; coefficient of variation on correct responses to go trials) –– are reliable indicators of trait-level lapsing.23 In our sample, trait-level lapsing, as assayed by mean alpha power and pupil variability on the memory task, significantly correlated with CER on the gradCPT (alpha: r=.48, p<.001; pupil: r=.26, p=.009), but not RTV (alpha: r=.18, p=.065; pupil: r=.12, p=.14) (Fig2b). Critically, CER and RTV on the gradCPT were negatively related to d’ on the conceptual (CER: r=−.36, p<.001; RTV: r=−.21, p=.03), perceptual (CER: r=−.39, p<.001; RTV: r=−.26, p=.009), and novelty retrieval tasks (CER: r=−.42, p<.001; RTV: r=−.30, p=.003) (Fig2c). Thus, trait-level differences in sustained attention are related to individual differences in forgetting.
Trait-level mediation analyses further revealed that the relationship between individual differences in lapsing and memory was explained by differences in sustained attention as indexed via CER on the gradCPT (indirect effect with alpha b=−0.18 (95%CI=−0.33,−0.05), direct effect b=−0.13, total effect b=−0.31; indirect effect with pupil b=−0.10 (95%CI=−0.22,−0.02), direct effect b=−0.19, total effect b=−0.29) (Fig2d). The total and indirect effects were significant in each mediation (ps<.02; Methods). These results indicate that trait differences in sustained attention may explain pre-goal lapsing and memory ability. Confirmatory factor analysis with a trait-level ‘attention’ factor (pupil variability and mean alpha power from the memory task, and CER and RTV from the gradCPT) and a ‘memory’ factor (d’ from each retrieval task) also indicated a significant model fit (χ2(21)=185.68, p<.001) and a significant negative relationship between inattention and memory (covariance=−0.52, z=−4.99, p<.001).
Given observations that everyday media multitasking (MMT) is negatively associated with episodic memory performance18, we leveraged the present multimodal approach to also test whether elevated forgetting in heavier media multitaskers is related to a higher propensity to suffer lapses prior to goal-directed behavior. Data on the relationship between lab-based assays of cognition (specifically, attention and memory) and real-world MMT behavior16 –– that is the degree to which an individual engages with multiple media in a given media consumption hour (e.g., watching television while texting)24 –– are provocative, in part because heavier MMT is associated with reduced working memory and episodic memory even when single tasking, possibly because of its positive relationship with failures of sustained attention17 and increased mind wandering16,25. Given that engagement with concurrent streams of media is pervasive in everyday life, there is a need to pinpoint the mechanism or mechanisms that underlie trait-level relationships between MMT and memory. Participants completed an individual differences battery that consisted of several self-report questionnaires, including a modified Media Multitasking Inventory, where a higher score indicates heavier MMT and a lower score lighter MMT. We first observed that participants who self-reported heavier MMT showed significantly lower d’ on the conceptual (r=−.32, p=.002), perceptual (r=−.28, p=.007), and novelty retrieval tasks (r=−.32, p=.002) (Fig3a). Second, heavier MMT was significantly related to higher CER (r=.31, p=.003) and RTV (r=.30, p=.003) on the gradCPT (Fig3c), and higher mean pre-goal alpha power (r=.21, p=.036) and pupil variability (r=.23, p=.019) during the memory tasks (Fig3b). The same patterns of findings were exhibited with an extreme groups approach (SI). Finally, a test of trait-level mediation revealed that the relationship between MMT and memory was partially explained by differences in sustained attention as assayed by CER from the gradCPT (Fig3d; indirect effect b=−0.11, 95%CI=−0.23,−0.02, direct effect b=−0.24, total effect b=−0.35), with the total, indirect, and direct effects showing significance (ps<.02; Methods).
Figure 3.

Trait-level differences in sustained attention partially explain the negative relationship between media multitasking and memory. a, b, Heavier MMT is related to worse d’ (a) and greater lapsing during retrieval (b). c, Heavier MMT is related to higher commission error and RT variability during the gradCPT. Raw scores are plotted for graphs with x- and y-axes; statistics included z-scored assays with Pearson correlations. d, Formal mediation model with mean standardized betas, and two-sided p-values (indirect effect: p=.005 and direct effect: p=.024). N=75 for alpha data and N=80 for all other data with human subjects from 1 independent experiment. Z and t statistical tests, where applicable, with no multiple comparison adjustment. MMT=Media multitasking, gradCPT=gradual-onset continuous performance task.
Exploratory factor analysis (principal components) further revealed that MMT loaded on a ‘sustained attention’ factor extracted from other relevant questionnaires –– including spontaneous mind wandering, ADHD, and BIS-11 attentional impulsivity. Moreover, we note that MMT was the only self-report measure that was significantly related to all memory and attention metrics (SI). Our task-based and self-report measures indicate that lapsing is one plausible explanation for why heavier MMT is related to poorer episodic memory.
To retrieve a memory, a number of neurocognitive processes dynamically interact. Various sources of forgetting at retrieval have been studied, including cue availability, mnemonic interference, and memory weakening.4 Here, we document that when pre-goal lapses of attention occur during retrieval, the strength of goal coding is reduced and forgetting is a price paid. The trial-level relationships were observed between lapsing, goal cuing, and hit vs. miss memory decisions but not correct rejection vs. false alarm memory decisions in the three retrieval tasks. This novel observation suggests that effects of attention interact with the congruency between one’s mnemonic goal and the retrieval cue, perhaps modulating mnemonic evidence as it begins to emerge. In addition, the trial-wise mediations and ERP analyses indicate that pre-goal lapses of attention at retrieval have robust direct effects on mnemonic behavior and neural signals above and beyond those attributed to goal cue processing. An interesting possibility is that attention modulates the processing of contextual cues, or one’s retrieval mode, that is leveraged in the moment to remember, which is ripe for direct assessment. Translating basic science findings to real-world behaviors, we further show that heavier MMT is associated with worse episodic memory, in part, because of a greater propensity to suffer more frequent or disruptive lapses of attention.
These results highlight how multimodal approaches can advance understanding of the role of attention in memory at both the trial- and trait-levels. The independent biological and behavioral metrics converge on the role of attention in partially accounting for mnemonic and MMT differences (for consideration of working memory, see SI). Another strength is that effects of attention at retrieval are not due to effects of attention at encoding, nor to variable perceptual encoding of goal cues at retrieval (see SI).
Future work focused on longitudinal assays that can inform causality29 in terms of whether differences in MMT produce differences in attention (or vice versa) will be important. Adopting complementary multimodal approaches to quantify attention and goal-state coding30 also holds promise for building models of how interactions between attention, goal-states, contextual cue processing, and memory explain why we sometimes remember and sometimes forget, and why some individuals remember better than others.
Methods
Participants
Eighty participants were enrolled in the study (49 female; Mage=21.7yrs, SD=1.48, range=18–26). Participants were recruited from online advertisements at Stanford University and the surrounding community, were right-handed, and had normal or corrected-to-normal vision, no history of neurological or psychological impairment, and no current medication. All participants provided written informed consent and were compensated $60 ($15/hr), in accordance with procedures approved by Stanford’s Institutional Review Board. Data from five participants were excluded from trial-level analyses due to insufficient trial numbers (<10 hits or <10 misses in each retrieval goal condition) stemming from technical artifacts and/or task performance. Data from five participants were excluded from trait-level analyses involving alpha power due to technical artifacts. Thus, 75 participants were retained for all trial-level analytics and trait-level alpha analytics, and 80 participants were retained for all other trait-level analytics.
Experimental design
Participants completed a 4-hr session: set-up and instructions (.5hrs), goal-directed encoding (1hr), goal-directed retrieval (1.5hrs), and the individual differences battery (1hr). Only the memory tasks included EEG+pupillometry. The memory tasks ––design, goal states, and common object pictures –– were adapted from previous research22 indicating behavioral and neural impacts of goal-state cuing on encoding and retrieval. The individual differences battery included questionnaires and a task-based sustained attention assay that were also adapted for present purposes.23–24,31–35 The present set of hypotheses relate to attention, goal-states, and episodic retrieval; encoding-related hypotheses and data will be reported elsewhere.
Goal-directed encoding and retrieval task
All tasks were run using the Psychophysics Toolbox in MATLAB.36 For each trial, the background screen was white, and the fixation, goal cue, or object appeared centrally. All fixations, goal cues, and objects were luminance- and chrominance-controlled and matched using the SHINE toolbox in MATLAB to ensure low-level visual properties did not impact oscillatory or pupillary assays.37
Encoding.
Participants viewed 168 objects twice across 6 incidental encoding runs (8m11s per run) of 56 trials each (once in runs 1–3, and runs 4–6). On each trial, a goal cue (Pleasant/Unpleasant? or Bigger/Smaller?) appeared for 1.60s, followed by a 0.10s inter-stimulus interval (ISI), followed by an object for 2.80s, followed by a 4s inter-trial interval (ITI). Participants performed object classification, based on a conceptual goal (is the object semantically pleasant or unpleasant) or a perceptual goal (is the object bigger or smaller in size on the screen, irrespective of real-world size), responding as quickly and as accurately as possible. Participants made each judgment with one of two button presses (pleasant or unpleasant; bigger or smaller) using their right index or middle finger (button-press mappings counterbalanced across participants). There were 8 practice trials to ensure comprehension.
In each encoding run, 28 objects were classified on the conceptual goal dimension (Pleasant/Unpleasant?) and 28 on the perceptual dimension (Bigger/Smaller?), yielding 84 conceptual object trials and 84 perceptual trials across runs. Prior to the experiment, each object was rated by 100 Amazon Mechanical Turk workers as being either more semantically pleasant or unpleasant; in the experiment, each object appeared either bigger (450×450 pixels) or smaller (150×150 pixels) in size on the screen (counterbalanced across participants). Thus, objects were crossed in a 2×2 design, with 14 objects from each goal crossing (pleasant/bigger, pleasant/smaller, unpleasant/bigger, unpleasant/smaller) appearing in a random order per run (with the constraint that a particular goal –– e.g., Pleasant/Unpleasant? –– could not appear more than 4 times consecutively). Each participant received a random assignment of goal-object pairings.
Retrieval.
After a 10m delay, participants performed the critical retrieval phase. At retrieval, they viewed 252 objects –– 168 studied and 84 novel –– across 12 goal-directed retrieval runs (7m15s per run) of 21 trials each. On each trial, participants made a yes/no memory judgment on an individual medium-sized object (300×300 pixels) after viewing one of three retrieval goal cues (Pleasant/Unpleasant Before? or Bigger/Smaller Before? or New Item?). Participants were instructed that some of the objects would be old and some new. The retrieval goal cue was preceded by an 8s ITI and then appeared for 2s, followed by an 8s ISI, followed by an object for 2s. An epoch of 8s was adopted for each ITI and ISI based on previous work demonstrating that attention lapses are more likely to be induced via 8s vs. 2s fixed intervals.38 Participants made each retrieval judgment as quickly and as accurately as possible, making one of two button presses (yes or no) with their right index or middle finger (button-press mapping was counterbalanced). There were 8 practice trials to ensure comprehension.
In each retrieval run, 7 objects were tested via the conceptual goal (Pleasant/Unpleasant Before?), 7 via the perceptual goal (Bigger/Smaller Before?), and 7 via novelty detection (New Item?), yielding 84 unique conceptual judgment trials, 84 unique perceptual trials, and 84 unique novelty trials across runs. To ensure adequate trial numbers: (a) for each of the source retrieval goals (conceptual, perceptual), 32 objects had been encoded via pleasant/unpleasant orienting, 32 had been encoded via bigger/smaller orienting, and 20 were new; and (b) for novelty detection, 20 objects had been encoded via pleasant/unpleasant, 20 via bigger/smaller, and 44 were new. Assignment of old and new objects to the three retrieval conditions was random by subject. The goal-object pairings were presented in a random order per run (with the additional constraint that a particular retrieval goal could not appear more than 3 times consecutively). Each participant received a random assortment of goal-object pairings.
Behaviorally, accuracy on the two source memory judgments requires object recognition and recollection of how the object was processed at encoding –– so as to differentiate conceptually vs. perceptually encoded objects, and to respond ‘yes’ or ‘no’ as a function of the cued goal; accurate novelty detection can be based on weak item memory strength and/or the absence of recollection.
Individual differences battery
After the encoding and retrieval task, participants filled out nine self-report questionnaires (self-paced) with paper-and-pencil assessment and the Qualtrics Survey platform (Qualtrics, Provo, Utah, USA), and performed the gradCPT in the following order:
Media Multitasking Inventory (MMI; modified from24).
The MMI yields an estimate of the number of media engaged in a typical media consumption hour; a higher score denotes heavier MMT. Part 1 assesses the number of hours per week typically spent doing each of nine activities: reading, homework (other than reading), watching videos/movies/ TV, listening to music/radio/audiobooks/other, playing video games, browsing the internet, texting/using social media/instant messaging, talking on the phone/video chatting, and other computer activities. In Part 2, participants indicate for each activity how often they simultaneously engage in each of the other activities on a 4-point Likert scale. In this modified MMI, participants rate each media pairing once.
Adult ADHD Self-Report Scale (ADHD;31).
A six-item questionnaire (Part A) assessing ADHD symptoms.
Barratt Impulsiveness Scale-11 (BIS-11;32).
A questionnaire with three 11-item subcomponents assessing non-planning, motor impulsivity, and attentional impulsivity.
Video Game Usage (VGU;33).
A questionnaire assessing the extent of playing five types of video games in the past 12 months.
Attentional Control Distractibility and Shifting (AC-D and AC-S;34).
An assay of everyday attentional control (or inattention), with two 4-item subsections assessing distractibility and shifting.
Mind Wandering Deliberate and Spontaneous (MW-D and MW-S;34).
A four-item questionnaire for each subtype, assessing everyday mind wandering.
Attention-Related Cognitive Errors Scale (ARCES;35).
A 12-item questionnaire assessing the frequency of cognitive errors in everyday situations that are attributed to attention lapsing.
Memory Failures Scale (MFS;35).
A 12-item questionnaire assessing the frequency of minor memory lapses in everyday situations.
Mindful Attention Awareness Scale-Lapses Only (MAAS-LO;35).
A 12-item questionnaire assessing everyday attention lapsing.
gradCPT.23
In this 10-minute task-based assay of sustained attention, participants viewed a stream of city (90% of trials) and mountain (10% of trials) scene images (497 trials total), and were to press the space bar to cities and withhold responding to mountains. The scenes were round, grayscaled images of 10 cities and 10 mountains that repeated across the task. Images appeared individually and centrally on a computer screen, gradually onset over 0.80s, paused for 0.40s when fully cohered (thus, each trial was 1.20s), and then offset. The task utilized linear pixel-by-pixel interpolation with a non-repeating scene rule (i.e., the same scene could not successively repeat). Participants were instructed to respond as accurately as possible; a response deadline was implicit in the task but not explicitly referenced.
EEG+pupillometry data acquisition
During encoding and retrieval, EEG and pupillary data were recorded concurrently in an electric- and sound-proof chamber to minimize artifacts. Real-time EEG, eyetracking, behavioral data, and stimulus display were monitored from an outside bay. EEG data were recorded with a 128-channel HydroCell Sensor Net (Electrical Geodesics, Eugene, Oregon, USA) at a sampling rate of 1000Hz through a Netstation 300 amplifier with 24-bit resolution/sample and Netstation 5.4 software. Impedance was set to <50 kV and checked approximately every 20m. Pupillary data from the right eye were recorded via an Eyelink 1000 Eye Tracker system (SR Research, Ottawa, Canada) at a sampling rate of 1000Hz. Participants were seated 60cm from the eyetracker and stimulus monitor with a chin mount in the chamber. Eyetracking calibration and validation steps were completed every ~20m with impedance checks. Trial-level EEG, pupillary, and behavioral data (response and response time) were synced via custom MATLAB code with event message tags.
Data analyses
The R environment (version 3.3.3) and SPSS (version 26) were leveraged for data preprocessing, statistics, and visualization. The following R packages were primarily utilized with in-house scripts: openxlsx, tidyverse, dplyr, lme4, lmerTest, mediation, lavaan, ggplot2, ggpubr, and eyetrackingR. The exception was EEG data preprocessing, for which MATLAB with an EEGLAB interface39 was utilized with in-house scripts.
Memory behavioral data analyses
Behavioral analyses focused on metrics of memory retrieval accuracy at the trial and trait levels. For trial-level retrieval, each ‘yes’ or ‘no’ response was classified as a hit, miss, correct rejection to old or new item, or false alarm to old or new item, depending on the retrieval goal condition. In conceptual goal cuing, responding ‘yes’ to an old pleasant/unpleasant encoded object was classified as a hit (‘no’=a miss), while responding ‘no’ to an old bigger/smaller encoded object was classified as a correct rejection to old item (‘yes’=false alarm to old item), and responding ‘no’ to a new object was classified as a correct rejection to new item (‘yes’=false alarm to new item). The same logic applies for perceptual goal cuing. In novelty detection, responding ‘yes’ to a new object was classified as a hit (‘no’=miss), while responding ‘no’ to an old pleasant/unpleasant or bigger/smaller object was classified as a correct rejection to old item (‘yes’=false alarm to old item). For trait-level retrieval, we adopted classic signal detection functions40 to compute d’ (Zhit–Zfalsealarm) for each goal-state condition; source false alarms and novelty false alarms were included when computing the conceptual and perceptual condition d’s (see ED Table 1 for false alarm rates by memory type), and novelty false alarms were included when computing the novelty detection condition d’.
A repeated-measures ANOVA was run to examine differences in memory performance as a function of retrieval goal condition with the dependent variable being d’. Significance was set at p<.05.
Trial-level EEG+pupillometry data analyses
A number of preprocessing steps were implemented on raw EEG and pupillary data for the analyses targeting moment-to-moment lapsing. For EEG, the 1000Hz data were decimated to 100Hz, and bandpass filtered to 0.1–30Hz using zero-phase Hanning windows. We then used the BLINKER automated method41 to identify blink artifacts, and visually inspected each trial of each participant’s data to identify bad electrode channels also due to artifacts (e.g., ocular). Data were average referenced and filtered for alpha power (8–12Hz) via Hilbert Transform. To assay trial-level spontaneous tonic lapses pre-goal and pre-retrieval, epochs were a priori set at 1s pre-goal to 0s goal, and 1s pre-retrieval probe to 0s probe. Mean alpha power from each epoch was extracted and computed from an a priori posterior cluster of electrodes (channels 62, 67, 71, 72, and 75–77; ED Fig1b in main text) previously associated7 with lapsing. For pupil diameter, the 1000Hz data were decimated to 100Hz. Note that real-time Eye Linker functions during acquisition remove blinks, off-center fixations, and eyetracker malfunctions from the data output, minimizing preprocessing. Additional preprocessing was done for saccades over 10 degrees. Again, to assay trial-level spontaneous tonic lapses pre-goal and pre-retrieval, epochs were a priori set at 1s pre-goal to 0s goal, and 1s pre-retrieval probe to 0s probe. Mean pupil diameter was computed from each epoch, given prior evidence that this metric captures tonic lapsing trial-to-trial.10–11,21 Rather than applying linear interpolation, we removed from analyses epochs that did not have full data (e.g., data missing from blinks) to ensure perceptual encoding of stimuli on a trial-to-trial basis. Noisy trial-level epochs were then removed within run (±3.5standard deviations (SDs) from mean alpha power or pupil diameter, respectively), and the remaining trial-level epochs were z-scored within run, rather than across runs, to account for time-on-task effects.
A number of preprocessing steps were also implemented on raw EEG data to assay goal coding at the trial level. After implementing the above described sampling rate, bandpass filter, and artifact identification and rejection methods, we average referenced and filtered the data for ERP signal. Epochs were set at −0.20s pre-goal to 2s post-goal onset to assay goal coding during the goal cuing window. Mean ERPs from these epochs were extracted from an a priori midfrontal cluster of electrodes (numbered 5–7, 11–13, 106, and 112; ED Fig1b in main text) previously associated with goal processing.26–27 Only epochs with full data were analyzed to ensure perceptual encoding of stimuli. Baseline correction was implemented at the trial level by subtracting the 0.20s tonic mean from the 2s phasic/goal cue-locked mean. Noisy trial-level epochs were removed within run (±3.5SDs from mean ERP), and the remaining trial-level epochs were z-scored within run.
After preprocessing, trialwise logistic mixed effects models, with a restricted maximum likelihood (REML) approach, quantified the relationship between moment-to-moment lapsing and memory accuracy. Two main logistic models quantified pre-goal lapsing on hits vs. misses: one model with a continuous fixed effect of pre-goal alpha power and a categorical fixed effect of hit (1) vs. miss (0), and another model with a continuous fixed effect of pre-goal pupil diameter. In addition, two linear mixed effects models quantified the relationship between pre-goal lapsing and the ERP goal coding metric at the trial level: one model with a continuous fixed effect of pre-goal alpha power and a continuous fixed effect of goal coding, and another with a continuous fixed effect of pre-goal pupil diameter. Finally, logistic mixed effects models quantified the relationship between goal coding and memory accuracy at the trial level: one model with a continuous fixed effect of goal coding and a categorical fixed effect of hit (1) vs. miss (0) for the alpha analysis and another for the pupil analysis. In all cases, each model included a random effect of subject, and an interaction term with retrieval goal (conceptual vs. perceptual vs. novelty goal cuing); the linear mixed effects models were restricted to hit and miss trials to match the other models. Significance was tested using the p-value of each individual beta for each effect in the respective model (p<.05).
Given significant paths between pre-goal lapsing and memory accuracy, pre-goal lapsing and goal coding, and goal coding and memory, two formal trialwise mediation models were implemented, one with the alpha power assay of lapsing and one with the pupil diameter assay of lapsing. The indirect path from pre-goal lapsing to goal coding (a) and goal coding to memory (b) was computed, as was the direct path from pre-goal lapsing to memory (c’). The indirect path or mediation was computed as the product of a*b, with a resulting 95% confidence interval for each indirect path from 10,000 bootstrapped samples. The total, direct, and indirect effects were also significance tested with p<.05.
While these primary analyses addressed our first core question, we repeated the same approach but replaced pre-goal lapsing with pre-retrieval probe lapsing, and hit vs. miss memory with correct rejection vs. false alarm memory. As reported in SI, there were no significant results that stemmed from these analyses.
Trial-level Parietal Old/New and FN400 ERP data analyses
A number of preprocessing steps were implemented on raw EEG data for the analyses targeting trial-level canonical neural signals of recollection- (Parietal Old/New) and familiarity-based (FN400) memory. After implementing the sampling rate, bandpass filter, and artifact identification and removal steps (see above), we average referenced and filtered the data for ERP signal. Epochs were set to −0.20s pre-retrieval probe to 1s post-probe onset. For the Parietal Old/New component, mean ERPs from these epochs were extracted and computed from an a priori left posterior cluster of electrodes (numbered 42, 47, 52–54, 61; note that 52 is P3; ED Fig1b in main text) canonically demonstrating recollection-based memory signal.28 For the FN400 component, mean ERPs from these epochs were extracted and computed from an a priori left midfrontal cluster of electrodes (numbered 19, 20, 23, 24, 27, 28, 33, 34; note that 24 is F3; ED Fig1b in main text) canonically demonstrating familiarity-based memory signal.28 Only epochs with full data were analyzed to ensure perceptual encoding of stimuli. Data were segmented into 0.10s (100ms) bins based on prior research28 noting that a Parietal Old/New component typically onsets at ~400ms, peaks at ~500ms, and offsets at ~800ms (all post-probe), and a FN400 component typically onsets at ~300ms, peaks at ~400ms, and offsets at ~500ms (all post-probe). Baseline correction was implemented at the trial level by subtracting the 0.20s tonic mean from each of the ten 0.10s mean phasic/stimulus-locked bins. Noisy trial-level epochs were removed within run (±3.5SDs from mean ERP for Parietal Old/New or FN400, respectively), and the remaining trial-level epochs were z-scored within run (by component).
Analyses first focused on replicating canonical signals of recollection- and familiarity-based memory in the three goal-state conditions, irrespective of lapsing. For Parietal Old/New in the left posterior cluster, we contrasted ERP signal on (a) source hits to old pleasant/unpleasant or bigger/smaller objects relative to (b) correct rejections to new objects in conceptual and perceptual retrieval tasks, respectively. For FN400 in the left midfrontal cluster, we contrasted ERP signal on (a) correct rejections to old pleasant/unpleasant or bigger/smaller objects relative to (b) hits to new objects in novelty detection (note: the terminology here is in relation to the retrieval task goal, such that new items called ‘new’ are termed hits). Following prior research, a series of repeated-measures ANOVAs and posthoc F tests were adopted. For Parietal Old/New, the model was a 2 (retrieval type: source hit vs. correct rejection to new object) × 2 (retrieval goal: conceptual vs. perceptual goal cuing) × 10 (time bin: e.g., 0–0.10s) with the dependent variable being ERP signal. For FN400, the model was a 2 (retrieval type: correct rejection to old object vs. hit to new object) × 10 (time bin: e.g., 0–0.10s) in the novelty detection task with the dependent variable being ERP signal. Significance was set at p<.05 for the ANOVAs and posthoc tests. Because z-scoring ERP signal within run and time-binning in 100ms intervals can smooth the data, reducing smaller temporal effects often observed in grand-average ERPs, for completeness we also plot these ERPs using 10ms time-bin intervals (ED Fig4). As a complementary analysis to ensure specificity of findings28, we extracted ERP signal from right posterior (numbered 78, 79, 86, 92, 93, 98; ED Fig1b in main text for localization) and right midfrontal (numbered 3, 4, 116–118, 122–124; ED Fig1b in main text for localization) clusters adopting the same preprocessing and analytic steps. We then included lateralization (left vs. right) as an additional factor in the repeated-measures ANOVAs for Parietal Old/New and FN400, respectively. Significance was set at p<.05 for the ANOVAs and posthoc tests, and the interaction results by lateralization are reported in SI.
Next, trialwise linear mixed effects models, with an REML approach, quantified the relationship between moment-to-moment lapsing and these canonical neural memory signals. We focused on hit vs. miss memory in conceptual and perceptual goal cuing and novelty detection given the relationships observed between trial-to-trial lapsing, goal coding, and memory accuracy in response to these types of trials. For Parietal Old/New, two main models assessed the relationship between pre-goal lapsing and ERP signal during the 500–600ms post-probe window for remembered vs. forgotten trials in conceptual and perceptual retrieval tasks: one model with a continuous fixed effect of pre-goal alpha power and a continuous fixed effect of ERP signal, and another model with a continuous fixed effect of pre-goal pupil diameter. For FN400, two main models assessed the relationship between pre-goal lapsing and ERP signal during the 400–500ms post-probe window for correctly endorsed new items (hits) vs. miss trials in novelty detection: one model with a continuous fixed effect of pre-goal alpha power and a continuous fixed effect of ERP signal, and another model with a continuous fixed effect of pre-goal pupil diameter. Each model included a random effect of subject, and an interaction term with retrieval accuracy (hit vs. miss). The Parietal Old/New models included an additional interaction term with retrieval goal (conceptual vs. perceptual cuing). The post-probe windows were selected based on previously reported signal peaks for the Parietal Old/New and FN400 effects.28 Significance was tested using the p-value of each individual beta for each effect in the respective model (p<.05). As a complementary analysis28, we also included an interaction term for lateralization (left vs. right) in each model, and the interaction results by lateralization are reported in SI.
Trial-level phasic Pupil Old/New analysis
While we addressed our second core question regarding relationships between trial-to-trial lapsing and neural signals of remembering from canonical ERP components, we also leveraged prior findings42–44 documenting differences in phasic pupillary signal post-retrieval probe as a function of memory to provide a secondary internal check on memory performance. Akin to an FN400 effect, higher peak pupil diameter is typically exhibited for correctly identified old vs. new items post-retrieval probe in old/new recognition memory, a phenomenon referred to as a Pupil Old/New effect42–44. We focused on phasic pupillary signal post-retrieval probe in correctly rejected old objects vs. hits to new objects (i.e., old vs. new) in the novelty detection retrieval condition to examine evidence of a Pupil Old/New effect. We did not examine tonic lapses on phasic pupil diameter post-retrieval probe given research demonstrating strong anti-correlations between tonic and phasic pupil diameter that could lead to overinterpretation of findings.45
After implementing the eyetracking preprocessing steps (see above), phasic pupil diameter was extracted for each trial using a −0.2 pre-retrieval probe to 1s post- probe epoch. Only epochs with full data were analyzed, and data were segmented into 0.10s time bins. As is typical in phasic pupillary work42–45, we then extracted a peak (i.e., max) pupil diameter value from each of the 10 post-probe time bins for each trial. Baseline correction was implemented at the trial level by subtracting the 0.20s tonic mean from each of the ten 0.10s peak phasic/stimulus-locked bins. Noisy trial-level epochs were then removed within run (±3.5SDs from phasic pupil), and the remaining trial-level epochs were z-scored within run to account for time-on-task effects.
To examine evidence of a Pupil Old/New effect (ED Fig6), we ran a repeated-measures ANOVA with a 2 (retrieval type: correct rejection to old object vs. hit to new object) × 10 (time bin: e.g., 0–0.10s) model within novelty detection cuing, with the dependent variable being phasic pupillary signal. Significance was set at p<.05 for the ANOVAs and posthoc tests.
Trait-level analyses
To answer our third core question, we first examined the relationship between trait-level lapsing and d’ on the memory tasks. Prior research suggests that trait-level increases in tonic alpha power and variability in tonic pupil diameter are related to trait-level inattention. An increase in mean alpha power in the absence of external distraction is thought to reflect reduced suppression (i.e., release from inhibition), and an increase in pupil variability is related to increases in mind wandering and decrements in psychomotor vigilance.7,10 To quantify these trait-level metrics, we computed each participant’s mean alpha power and pupil diameter variability across the 1s pre-goal epochs during memory retrieval. Only full epochs were used. For pupil variability, we computed a coefficient of variation (CoV): SD across each participant’s pre-goal epochs divided by the mean across the epochs, multiplied by 100. To standardize the key metrics, we z-scored the mean alpha power, pupil variability, and d’ in each retrieval goal condition across participants. We then ran one-tailed Pearson correlations between mean alpha power and each d’, as well as pupil variability and each d’, setting significance to p<.05. One-tailed correlations were used for all trait-level analytics given our a priori hypotheses about directionality between lapsing and memory, and lapsing, MMT, and memory.
To more directly assess relationships between trait-level lapsing and memory, we also leveraged assays of lapsing from a canonical sustained attention task, the gradCPT23,46. We computed two assays of lapsing from the task: (a) commission error rate (CER), the proportion of responses to ‘no-go’ mountain trials over the total number of ‘no-go’ mountain trials, and (b) reaction time variability (RTV) for responses to ‘go’ city trials, using a CoV metric (SD of RT on city trials divided by the mean RT, multiplied by 100). Following previous work with the gradCPT23,46, we also computed canonical assays of vigilance (mean RT over five 2m windows) and omission error rate (proportion of no responses on ‘go’ city trials divided by total number of ‘go’ trials), which we report in SI. We z-scored all gradCPT metrics across participants and ran one-tailed Pearson correlations: (a) between mean alpha power or pupil variability from the memory task and CER or RTV from the gradCPT, and (b) between d’ from each retrieval task and CER or RTV from the gradCPT.
Given significant paths between lapsing and memory, lapsing and CER on the gradCPT, and CER on the gradCPT and memory, two formal subject-level mediation models were implemented on the z-scored metrics to examine whether the relationship between lapsing and memory on the memory task was partially explained by trait-level differences in sustained attention, as indexed by the gradCPT. Note that we computed a common metric of memory by averaging across the d’s from the three retrieval goal conditions; the same findings were exhibited with each d’ metric separately. One mediation model examined the indirect path from lapsing (mean alpha power) to gradCPT (a) and gradCPT to memory (b), as well as the direct path from lapsing to memory (c’). The other model examined the same paths but replaced mean alpha power with pupil variability. Each indirect path or mediation was computed as the product of a*b, with a resulting 95% confidence interval for each indirect path from 10,000 bootstrapped samples. The total, direct, and indirect effects were also significance tested with p<.05.
As a final step, a confirmatory factor analysis tested for a trait-level relationship between sustained attention and memory. The ‘attention’ latent variable consisted of z-scored pre-goal lapsing from the memory task (mean alpha power and pupil variability) along with z-scored lapsing from the gradCPT (CER and RTV). The ‘memory’ latent variable consisted of z-scored d’ for each retrieval goal condition from the memory task (conceptual, perceptual, and novelty detection). Model fit and the covariance between components were set at significance p<.05. We treat this result as preliminary given that a higher sample size is sometimes recommended for confirmatory factor analysis.
To answer our fourth core question about trait-level cognitive differences related to MMT, we first assessed the relationship between MMT and memory. We computed a MMI score for each participant, using the standard formula24, and then z-scored across participants for standardization. For the main continuous approach, Pearson correlations quantified the relationship between MMT and each d’ from the memory tasks. Given prior research18, we also incorporated an extreme groups approach where the lowest 25% of MMI scorers were treated as light media multitaskers (LMMs) and the top 25% of MMI scorers were treated as heavy media multitaskers (HMMs); we note that this analysis was treated as secondary given the sample size (20/group). A 2 (MMT: light vs. heavy) × 3 (retrieval goal: conceptual vs. perceptual vs. novelty) mixed-factorial ANOVA was run with d’ as the dependent variable, and significance set at p<.05.
We then adopted the same continuous and extreme groups approaches to assess the relationship between MMT and attention. In terms of attention, we examined z-scored gradCPT performance (CER and RTV) and lapsing on the memory task (mean alpha power and pupil variability).
Based on significant paths between MMT and memory, MMT and lapsing, and lapsing and memory, we leveraged four subject-level mediation models to examine whether the relationship between MMT and memory is partially explained by trait-level differences in sustained attention. An average d’ across the 3 retrieval measures was used; again, the same findings were exhibited when examining each d’ separately. The mediation models tested the indirect path from MMT to each gradCPT and attention lapsing metric (CER, RTV, mean alpha power, or pupil variability; a) and from each gradCPT and lapsing metric to memory (b), as well as the direct path from MMT to memory (c’). Each indirect path or mediation was computed as the product of a*b, with a resulting 95% confidence interval for each indirect path from 10,000 bootstrapped samples. The total, direct, and indirect effects were also significance tested with p<.05.
The above described primary analyses addressed our fourth core question. We also took three additional steps for completeness. First, we used canonical formulas to compute a trait-level score for each self-report questionnaire and implemented an exploratory factor analysis (EFA) to quantify how MMT relates to these other constructs. The mean across items was used for ADHD, AC-D and AC-S, and MW-D and MW-S, and the sum across items was used for BIS-11 subcomponents (which included reverse-scoring of appropriate items), ARCES, MFS, and MAAS-LO. Each questionnaire assay was z-scored across participants. A categorical frequency of video game playing (>5hrs/wk of action video games) was used for VGU; note that we did not perform further analyses on this questionnaire because under 10% of the sample endorsed video game playing. For the EFA, we implemented a principal components analysis (PCA) that extracted latent factors from the questionnaire scores and maximized the loading of each score on one factor and minimized its loading on the other factors. To account for collinearity and sampling distribution adequacy, we qualitatively examined correlations among questionnaire scores and quantitatively assessed the determinant, KMO and Bartlett Test statistics, and the communality of each questionnaire score. We then examined factor output with a Scree plot before and after extraction, and after varimax rotation, and selected a three-factor model given that three factors showed eigenvalues >1.0. We then assessed which questionnaire scores with a communality above 0.5 loaded on each of the three extracted factors. While we adopted typical EFA/PCA steps and parameters, our analysis should be treated as preliminary given the sample size. Subsequently, Pearson correlations between the other questionnaire scores on the one hand and the gradCPT and memory metrics on the other were examined to assess whether additional variance not captured by MMT might explain task-based attention and memory assays. Significance was set at p<.05. As a final step, we verified that the same findings for trait-level analytics were exhibited when we computed lapsing assays from our memory task that incorporated mean alpha power and pupil variability across (a) pre-retrieval probe epochs alone, and (b) both pre-goal and pre-retrieval probe epochs.
Extended Data
Extended Data Figure 1.

Experimental design. a, Schematic of goal-directed memory task with EEG+pupillometry epochs. b, Schematic of electrode clusters from which alpha or ERP signal was extracted for respective analyses; electrode clusters are illustrated on a 128-channel net. Pupil diameter from the right eye (upper right) was recorded concurrently via an eyetracking system. L=left, R=right.
Extended Data Figure 2.

Pre-goal attention lapses relate to canonical neural signals of recollection- and familiarity-based memory as assayed by grand-average left-lateralized Parietal Old/New and FN400 ERP effects, respectively. a, Evidence of peak Parietal Old/New signal (indicated by black arrow) in the 500–600ms post-probe window as a function of memory outcome in conceptual and perceptual source retrieval trials. b, Trial-level interaction between pre-goal attention lapses and Parietal Old/New signal on remembered (source hits) vs. forgotten (misses) trials. c, Evidence of peak FN400 signal (indicated by black arrow) in the 400–500ms post-probe window as a function of memory outcome in novelty detection trials. d, Trial-level interaction between pre-goal attention lapses and FN400 signal on correctly endorsed new items (hits) vs. misses. For visualization, quintiles are shown for the relationship between pre-goal lapsing and ERP signal; statistics included an interaction term for retrieval goal state (for Parietal Old/New) and treated pre-goal lapsing and ERP signal continuously in trial-level mixed models. Y-axis units are z-scores. Error bars represent 1 standard error of the mean. Note that z-scoring within run and time-binning in 0.1s (100ms) intervals reduces smaller temporal effects that are sometimes exhibited in grand-average ERP plots (for visualization of grand-average ERP plots downsampled to 0.01s intervals (10ms), see ED Fig4). CR=correct rejection; FA=false alarm. N=75.
Extended Data Figure 3.

Evidence of mean peak Parietal Old/New signal (indicated by black arrow) in the 500–600ms post-probe window as a function of memory outcome in source retrieval trials. a, b, Data are split by conceptual (a) and perceptual (b) source trials. CRold=correct rejection to old item; FAold=false alarm to old item. For conceptual cuing, hits and misses are to conceptually studied items and CRs and FAs are to perceptually studied items. For perceptual cuing, hits and misses are to perceptually studied items and CRs and FAs are to conceptually studied items. N=75.
Extended Data Figure 4.

Grand-average left-lateralized ERPs revealing recollection-based Parietal Old/New and familiarity-based FN400 memory effects, downsampled to 10ms time-bin intervals. a, b, The same profile of findings is observed as with the 100ms time-bins (see main text), such that evidence of peak Parietal Old/New signal (indicated by black arrow) is exhibited 500–600ms post-probe onset as a function of memory outcome in conceptual and perceptual source retrieval trials (a) and evidence of peak FN400 signal (indicated by black arrow) is exhibited 400–500ms post-probe onset as a function of memory outcome in novelty detection trials (b). Y-axis units are within-run z-scores. CR=correct rejection; FA=false alarm. N=75.
Extended Data Figure 5.
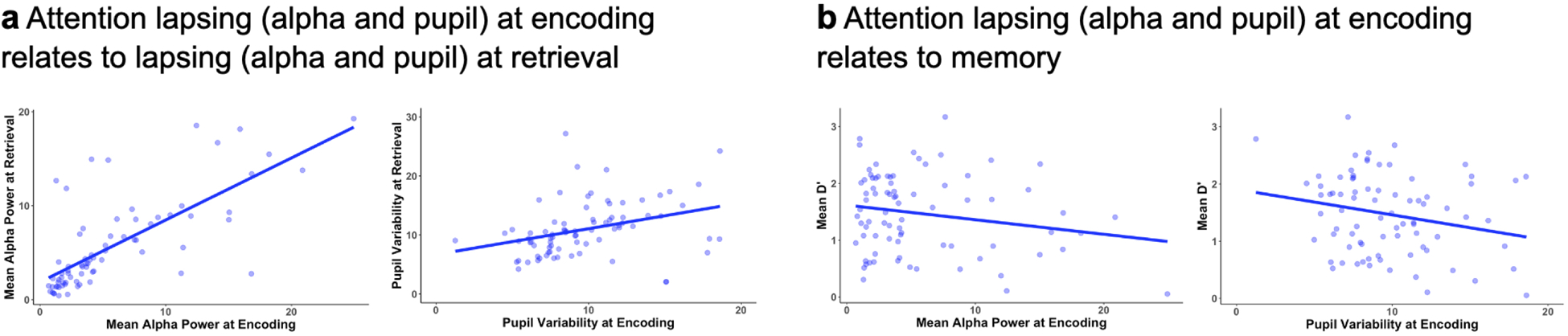
Trait-level differences in sustained attention at encoding help to explain why individuals are more prone to remembering or forgetting. a, b, Greater pre-goal attention lapsing at encoding is related to greater pre-goal attention lapsing at retrieval (a) and lower d’ on the memory task (b). For visualization, raw scores are plotted; statistics included z-scored assays with Pearson correlations. N=75 for alpha retrieval data and N=80 for all other data. Importantly, as reported in SI, these trait differences in attention at encoding do not fully explain the relationship between trait differences in attention at retrieval and memory ability.
Extended Data Figure 6.

Evidence of a phasic Pupil Old/New effect in novelty detection trials 300–500ms post-probe, particularly between correctly rejected old objects vs. hits to new objects. The mean peak difference is at 400ms post-probe (indicated by black arrow). X-axis units are 100ms time-bin intervals, and Y-axis units are within-run z-scores. CR=correct rejection; FA=false alarm. N=75.
Extended Data Figure 7.
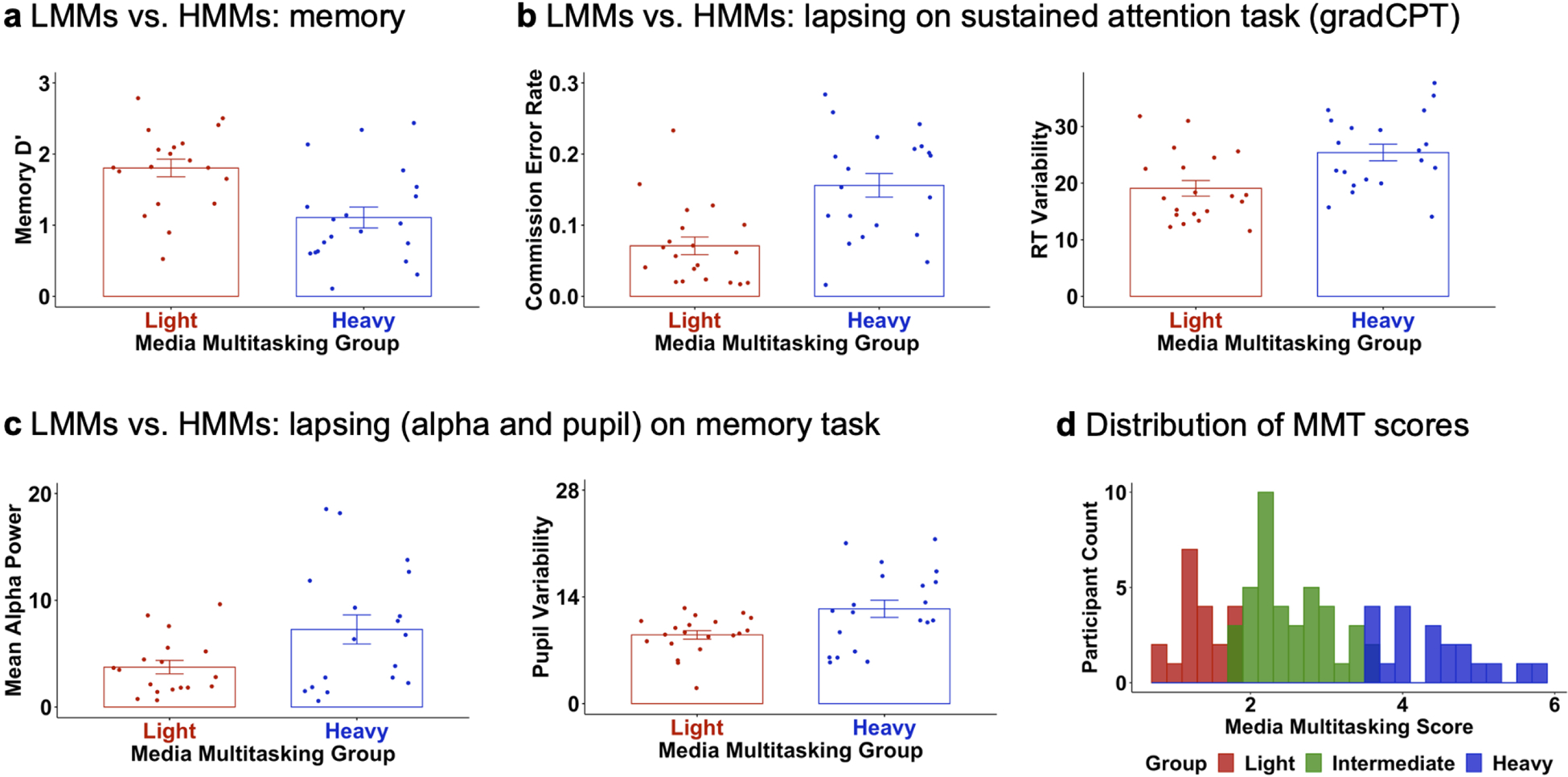
Key results from extreme groups analyses of multitasking, memory, and sustained attention for light and heavy media multitaskers. a, b, c, Heavy media multitaskers exhibited lower d’ on the memory tasks (a), more attention lapses on the gradCPT (b), and more evidence of attention lapsing (assayed by mean alpha power and pupil variability) on the memory task (c), relative to light media multitaskers. Error bars represent one standard error of the mean, and mean metrics are shown. N=18 light and N=18 heavy media multitaskers for alpha data, and N=20 light and N=20 heavy media multitaskers for all other data. d, Histogram of scores (N=80) on the Media Multitasking Inventory, illustrated via the bottom 25% of scores (light media multitaskers), the middle 50% of scores (intermediate media multitaskers), and the top 25% of scores (heavy media multitaskers). LMM=Light media multitasker, HMM=Heavier media multitasker, MMT=Media multitasking.
Extended Data Table 1.
Mean (and SE) for each type (i.e., rate) of memory outcome for each goal cue condition at retrieval. Note that miss rate for each condition is the complement of hit rate (1-hit rate), and false alarm rate to old items or new items is the complement of respective correct rejection rate (1-correct rejection rate). All analyses in the manuscript were computed over d’s (main text and SI), rather than mnemonic outcome rates, because the d’ metric accounts for response bias. CR=correct rejection.
| Conceptual cuing | Perceptual cuing | Novelty cuing | |
|---|---|---|---|
| Hit rate | 0.63 (0.02) | 0.60 (0.02) | 0.69 (0.02) |
| CRold rate | 0.77 (0.02) | 0.80 (0.02) | 0.80 (0.02) |
| CRnew rate | 0.88 (0.02) | 0.91 (0.01) | - |
Supplementary Material
Acknowledgments
We thank Alex Gonzalez and Jonathan Qi for assistance with various aspects of the study. This research was supported by the National Institute of Mental Health (R56MH111672 to ADW) and the National Institute on Aging (R01AG065255 to ADW; F32AG059341 to KPM). The content is solely the views of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests
None declared.
Additional information
Supplementary Information (SI) is available for this paper. Correspondence and requests for materials should be addressed to ADW or KPM. Reprints and permissions information is available at www.nature.com/reprints.
Data availability
Data that support the findings of this study are publicly available via Open Science Framework: https://osf.io/zj7tb.47 Identifier is zj7tb. Data used in the preparation of this manuscript are also publicly available from the National Institute of Mental Health (NIMH) Data Archive (NDA) with DOI: 10.15154/1519022.48 The source data underlying all figures are provided as a Source Data file.
Code availability
Analytic code that support the findings of this study are publicly available via Open Science Framework: https://osf.io/zj7tb. Identifier is zj7tb.
References
- 1.Harris T “Optimizing for engagement: Understanding the use of persuasive technology on internet platforms” (US Senate Testimony on behalf of Center for Humane Technology; June 25, 2019; http://humanetech.com/wp-content/uploads/2019/06/Testimony-Background-Tristan-Harris_CHT.pdf). [Google Scholar]
- 2.Baddeley A, Lewis V, Eldridge M & Thomson N Attention and retrieval from long-term memory. J Exp Psychol Gen. 113, 518–540 (1984). [Google Scholar]
- 3.Craik FI, Govoni R, Naveh-Benjamin M & Anderson ND The effects of divided attention on encoding and retrieval processes in human memory. J Exp Psychol Gen. 125, 159–180 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Anderson MC & Spellman BA On the status of inhibitory mechanisms in cognition: Memory retrieval as a model case. Psychol Rev. 102, 68–100 (1995). [DOI] [PubMed] [Google Scholar]
- 5.Evans LH & Herron JE Pre-retrieval event-related potentials predict source memory during task switching. Neuroimage. 194, 174–181 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curran T Effects of attention and confidence on the hypothesized ERP correlates of recollection and familiarity. Neuropsychologia. 42, 1088–1106 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Klimesch W Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 16, 606–617 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanslmayr S, Staudigl T & Fellner M Oscillatory power decreases and long-term memory: The information via desynchronization hypothesis. Front Hum Neurosci. 6, 74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin CY, Borst JP & van Vugt MK, Predicting task-general mind-wandering with EEG. Cogn Affect Behav Neurosci. 19, 1059–1073 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unsworth N & Robison MK The importance of arousal for variation in working memory capacity and attention control: A latent variable pupillometry study. J Exp Psychol Learn Mem Cogn. 43, 1962–1987 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Unsworth N & Robison MK Pupillary correlates of lapses of sustained attention. Cogn Affect Behav Neurosci. 16, 601–615 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Konishi M, Brown K, Battaglini L & Smallwood J When attention wanders: Pupillometric signatures of fluctuations in external attention. Cognition. 168, 16–26 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Fortenbaugh FC, DeGutis J & Esterman M Recent theoretical, neural, and clinical advances in sustained attention research. Ann N Y Acad Sci. 1396, 70–91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unsworth N Individual differences in long-term memory. Psychol Bull. 145, 79–139 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Kahana MJ, Aggarwal EV & Phan TD The variability puzzle in human memory. J Exp Psychol Learn Mem Cogn. 44, 1857–1863 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uncapher MR & Wagner AD Minds and brains of media multitaskers: Current findings and future directions. Proc. Natl. Acad. Sci. U.S.A 115, 9889–9896 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ralph BCW, Thomson DR, Seli P, Carriere JS & Smilek D Media multitasking and behavioral measures of sustained attention. Atten Percept Psychophys. 77, 390–401 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Uncapher MR, Thieu M & Wagner AD Media multitasking and memory: Differences in working memory and long-term memory. Psychon Bull Rev. 23, 483–490 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumgartner SE, van der Schuur WA, Lemmens JS & te Poel F The relationship between media multitasking and attention problems in adolescents: Results of two longitudinal studies. Hum Commun Res. 44, 3–30 (2018). [Google Scholar]
- 20.MacDonald JSP, Mathan S & Yeung N Trial-by-trial variations in subjective attentional state are reflected in ongoing prestimulus EEG alpha oscillations. Front Psychol. 2, 82 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unsworth N & Robison MK Tracking arousal state and mind wandering with pupillometry. Cogn Affect Behav Neurosci. 18, 638–664 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Dobbins IG & Wagner AD Domain-general and domain-specific prefrontal mechanisms for recollecting events and detecting novelty. Cereb. Cortex 29, 150–166 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Esterman M, Noonan SL, Rosenberg M & DeGutis J In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cereb. Cortex 23, 2712–2723 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Ophir E, Nass C & Wagner AD Cognitive control in media multitaskers. Proc. Natl. Acad. Sci. U.S.A 106, 15583–15587 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ralph BCW, Thomson DR, Cheyne JA & Smilek D Media multitasking and failures of attention in everyday life. Psychol Res. 78, 661–669 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Herron JE & Evans LH Preparation breeds success: Brain activity predicts remembering. Cortex. 106, 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forstmann BU, Ridderinkhof KR, Kaiser J & Bledowski C At your own peril: An ERP study of voluntary task set selection processes in the medial frontal cortex. Cogn Affect Behav Neurosci. 7, 286–296 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Rugg MD & Curran T Event-related potentials and recognition memory. Trends Cogn Sci. 15, 467–474 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Ra CK, et al. Association of digital media use with subsequent symptoms of attention-deficit/hyperactivity disorder among adolescents. JAMA. 320, 255–263 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanslmayr S, et al. The relationship between brain oscillations and BOLD signal during memory formation: A combined EEG-fMRI study. J Neurosci. 31, 15674–15680 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessler RC, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): A short screening scale for use in the general population. Psychol Med. 33, 476–489 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Patton JH, Stanford MS & Barratt ES Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 51, 768–774 (1995). [DOI] [PubMed] [Google Scholar]
- 33.Green CS & Bavelier D Action-video-game experience alters the spatial resolution of vision. Psych Sci. 18, 88–94 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carriere JSA, Seli P & Smilek D Wandering in both mind and body: Individual differences in mind wandering and inattention predict fidgeting. Can J Exp Psychol. 67, 19–31 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Carriere JSA, Cheyne JA & Smilek D Everyday attention lapses and memory failures: The affective consequences of mindlessness. Consciousness Cogn. 17, 835–847 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Brainard DH The Psychophysics Toolbox. Spat Vis. 10, 433–436 (1997). [PubMed] [Google Scholar]
- 37.Willenbockel V, et al. Controlling low-level image properties: The SHINE toolbox. Behav Res Methods. 42, 671–684 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Unsworth N, Robison MK & Miller AL Pupillary correlates of fluctuations in sustained attention. J Cogn Neurosci. 30, 1241–1253 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Delorme A & Makeig S EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 134, 9–21 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Green DM & Swets JA Signal Detection Theory and Psychophysics (Wiley Press: New York, NY: 1966). [Google Scholar]
- 41.Kleifges K, Bigdely-Shamlo N, Kerick SE & Robbins KA BLINKER: Automated extraction of ocular indices from EEG enabling large-scale analysis. Front Neurosci. 11, 12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldinger SD & Papesh MH Pupil dilation reflects the creation and retrieval of memories. Curr Dir Psychol Sci. 21, 90–95 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otero SC, Weekes BS & Hutton SB Pupil size changes during recognition memory. Psychophysiology. 48, 1346–1353 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Vo ML-H, et al. The coupling of emotion and cognition in the eye: Introducing the pupil old/new effect. Psychophysiology. 45, 139–140 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Hong L, Walz JM & Sajda P Your eyes give you away: Prestimulus changes in pupil diameter correlate with poststimulus task-related EEG dynamics. PLoS One. 9, e91321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg M, Noonan S, DeGutis J & Esterman M Sustaining visual attention in the face of distraction: A novel gradual-onset continuous performance task (gradCPT). Atten Percept Psychophys. 75, 426–439 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Madore KP Dataset and analytic code for Memory failure predicted by attention lapsing and media multitasking. Retrieved from https://osf.io/zj7tb (2020). [DOI] [PMC free article] [PubMed]
- 48.Madore KP (2020). Dataset for Memory failure predicted by attention lapsing and media multitasking. Retrieved from National institute of Mental Health Data Archive 10.15154/1519022 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drew T, McCollough AW, Horowitz TS & Vogel EK Attentional enhancement during multiple-object tracking. Psychon Bull Rev. 16, 411–417 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norcia AM, Appelbaum LG, Ales JM, Cottereau BR, & Rossion B The steady-state visual evoked potential in vision research: A review. J Vis. 15, 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hauk O, Davis MH, Ford M, Pulvermuller F, & Marslen-Wilson WD The time course of visual word recognition as revealed by linear regression analysis of ERP data. NeuroImage. 30, 1383–1400 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


