Abstract
Introduction
The precise blood glucose (BG) profile of hemodialysis patients is unclear, as is the effectiveness of dipeptidyl peptidase-4 (DPP-4) inhibitors in hemodialysis patients with type 2 diabetes. Here, we used continuous glucose monitoring (CGM) to evaluate BG variability in these patients and to assess the efficacy of DPP-4 inhibitors, particularly during hemodialysis sessions and at nighttime (UMIN000012638).
Methods
We examined BG profiles using CGM in 31 maintenance hemodialysis patients with type 2 diabetes. Differences between patients with and without DPP-4 inhibitors (n = 15 and 16, respectively) were analyzed using a linear mixed-effects model to assess changes in glucose levels in 5-min intervals.
Results
The model revealed that DPP-4 inhibitor use was significantly associated with suppression of a rapid drop in glucose levels, both with and without adjustment for BG levels at the start of hemodialysis. Moreover, the model revealed that the two groups differed significantly in the pattern of changes in BG levels from 0:00 to 6:55 am. DPP-4 inhibitors suppressed the tendency for subsequent nocturnal hypoglycemia.
Conclusions
This prospective observational exploratory study showed that DPP-4 inhibitors could suppress BG variability during hemodialysis sessions as well as subsequent nocturnal changes in patients with type 2 diabetes.
Trial Registration
ClinicalTrials.gov identifier, UMIN000012638.
Electronic supplementary material
The online version of this article (10.1007/s13300-020-00928-5) contains supplementary material, which is available to authorized users.
Keywords: Blood glucose variability, Continuous glucose monitoring, DPP-4 inhibitors, Hemodialysis, Hypoglycemia
Key Summary Points
| Why carry out this study? |
| The precise blood glucose profile is unclear in patients with type 2 diabetes on maintenance hemodialysis, who are often reported to have asymptomatic hypoglycemia. |
| Few studies have examined in detail the effects of DPP-4 inhibitors in these patients. |
| Using continuous glucose monitoring (CGM), we investigated blood glucose variability and the efficacy of DPP-4 inhibitors in these patients. |
| What was learned from the study? |
| DPP-4 inhibitors can suppress blood glucose variability during hemodialysis sessions as well as subsequent nocturnal changes, and can prevent hypoglycemia in patients with type 2 diabetes. |
| A linear mixed-effects model is likely to be particularly useful for analyzing CGM data over time, such as during hemodialysis treatment or at nighttime. |
| Glycemic control with DPP-4 inhibitors may improve the prognosis of patients with diabetes on maintenance hemodialysis, and further studies are warranted to examine this hypothesis. |
Digital Features
This article is published with digital features to facilitate understanding of the article. To view digital features for this article, go to 10.6084/m9.figshare.12937694.
Introduction
The number of maintenance hemodialysis patients with diabetes has recently increased worldwide [1]. Previous studies revealed that poor glycemic control causes high cardiovascular morbidity and mortality in these patients, but also that intensive diabetes therapy has long-term beneficial effects on the risk of cardiovascular disease and all-cause death [2–5]. However, since the ACCORD study showed that severe hypoglycemia could increase the risk of cardiovascular death in participants with high underlying cardiovascular disease risk [6], hypoglycemia has become one of the most important considerations in diabetes treatment. Sun et al. found that 54 of 102 type 2 diabetes patients on hemodialysis had symptomatic hypoglycemia during a 3-month follow-up period [7]. However, asymptomatic hemodialysis-associated hypoglycemia might also be important in these patients. Patients with end-stage renal disease are at high risk of asymptomatic hypoglycemia [8], and asymptomatic hemodialysis-associated hypoglycemia has been reported in patients with type 2 diabetes [9–11].
Furthermore, high blood glucose (BG) variability in chronic glycemic control [standard deviation (SD) of glycated hemoglobin (HbA1c)] is associated with a high risk of hypoglycemia-related hospitalization [12]. BG fluctuations activate oxidative stress and have been associated with arteriosclerosis [13, 14]. The mean amplitude of glycemic exclusion (MAGE) is also used as an index of BG variability and has been reported to be associated with the presence and severity of coronary artery disease in patients with type 2 diabetes [15–17]. Hypoglycemia can also lead to vascular injury and is associated with an adverse prognosis in patients with diabetes and chronic kidney disease [18–21]. However, the precise BG profile and its variability in type 2 diabetes patients undergoing maintenance hemodialysis are unclear.
Continuous glucose monitoring (CGM) is useful for elucidating BG variability in diabetes patients by analyzing the amplitude and timing of glucose fluctuations [22]. CGM is now commonly used for monitoring BG levels in patients undergoing maintenance hemodialysis [9, 10, 23–32]. Nonetheless, there are only a few detailed reports on BG variability during hemodialysis therapy. Even though these studies showed a decrease in BG during hemodialysis [9, 29, 31, 32], BG variability in these patients was complex, and its details are still unknown. Thus, further study involving CGM is needed to understand BG variability in diabetes patients undergoing maintenance hemodialysis.
Dipeptidyl peptidase-4 (DPP-4) inhibitors enhance the therapeutic effects of glucagon-like peptide-1 (GLP-1) by blocking its rapid degradation. Therefore, by increasing the circulating levels of biologically active GLP-1, DPP-4 inhibitors can elevate insulin levels. Furthermore, GLP-1 reduces meal-stimulated glucagon levels and improves BG control in diabetic patients [33–36]. Some reports have suggested that DPP-4 inhibitors can potentially suppress BG variability in patients with type 2 diabetes [37, 38]. Moreover, DPP-4 inhibitors can be safely used even in type 2 diabetes patients undergoing maintenance hemodialysis, in conjunction with appropriate dose regulation [39, 40]. However, the effects of DPP-4 inhibitors on BG variability during hemodialysis and at nighttime in hemodialysis patients are unclear.
Accordingly, this study aimed to evaluate BG variability in type 2 diabetes patients undergoing maintenance hemodialysis by using CGM to assess the effectiveness of DPP-4 inhibitors, particularly during hemodialysis sessions and the subsequent nocturnal period.
Methods
Participants and Study Design
The primary outcome in this multicenter prospective observational exploratory study was BG variability in type 2 diabetes patients undergoing maintenance hemodialysis with or without a DPP-4 inhibitor [DPP-4 inhibitor (+) and DPP-4 inhibitor (−), respectively]. This variability was evaluated using the SD, MAGE, and a linear mixed-effects model. The frequency of hypoglycemia and the mean BG level were also evaluated. Participants were enrolled from November 2012 to March 2014 by T.I., M.H., and E.K. at Niigata University, Itoigawa General Hospital and Nagaoka Chuo Hospital, Niigata, Japan. The inclusion criteria were (1) age ≥ 20 years, (2) diagnosis of type 2 diabetes mellitus, (3) maintenance hemodialysis, and (4) the ability to provide informed consent. The exclusion criteria were (1) severe heart disease (New York Heart Association III or IV), (2) severe hepatic insufficiency, (3) apparent signs of current systemic infection or sepsis requiring active use of intravenous antibiotics, (4) perioperative status, (5) insulin therapy, (6) fasting, and (7) allergy to DPP-4 inhibitors. The reasons for hospitalization were shunt occlusions, pneumonia, and urinary tract infections. All participants were able to consume the prescribed meal, which had restricted calories, salt, and protein according to body weight, at 8:00 am, 12:00 pm, and 6:00 pm on the non-hemodialysis day and at 8:00 am, 2:00–3:00 pm after dialysis, and 6:00 pm on the day of hemodialysis.
This study was approved by the institutional review boards of Niigata University, Itoigawa General Hospital, and Nagaoka Chuo Hospital, and was performed in accordance with the principles embodied in the Declaration of Helsinki. All participants provided written informed consent. The Ethics Committee of the Niigata University School of Medicine approved the study (approval numbers: 2015-1476 and 2015-2119). The study was registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000012638).
Measurement of Glucose Profiles
Glucose levels were measured by CGM for 48–168 h. A CGM System Gold (Medtronic MiniMed, Northridge, CA, USA) was used for 7 patients and a CGM System iPro2 (Medtronic MiniMed) was used for 38 patients. Patients underwent 3–5 h of hemodialysis 3 days per week; the dialysate glucose concentration was 100 mg/dL or 150 mg/dL. The CGM system was calibrated by nurses four times a day by comparison with the capillary BG value using the Medisafe-Mini (Terumo, Tokyo, Japan), a self-monitoring BG device. CGM was used to evaluate glucose levels from 7:00 am on the day of hemodialysis for 24 h. Hypoglycemia was defined as a CGM reading of < 70 mg/dL. The CGM test was performed for at least 2 weeks after DPP-4 inhibitor therapy was started. Pre-hemodialysis venous blood samples were obtained at the beginning of the week, and routine biochemical parameters, including HbA1c, were measured.
Statistical Analysis
For continuous variables, we used an unpaired t test for parametric variables and the Mann–Whitney U test for nonparametric variables. Differences in proportions were evaluated using Fisher’s exact test. Relationships between two continuous variables were evaluated using Spearman’s rank correlation coefficient. Mean SD and MAGE were calculated to evaluate BG variability. We calculated MAGE as the average of the variability above 1 SD of the 24 h wave of BG variability from 7:00 am to the next morning on the hemodialysis and non-hemodialysis days. A linear mixed-effects model was used to evaluate the between-group and within-group differences in the slope of the BG levels. The slope represents the estimated coefficient for changes in BG levels per unit time calculated using the linear mixed-effects model. The model included the time since initiation of hemodialysis and the time at night, the DPP-4 inhibitor (+) and (−) groups, and the interaction between time and treatment. The approximate inference regarding fixed or covariate effects in linear mixed-effects models was determined using the Kenward–Roger approximation method for degrees of freedom. Given that the study was exploratory in nature, the sample size was calculated to detect a difference in slope of 0.1 mg/dL per minute during a hemodialysis session with 70% power at the 5% level of significance in the analysis using the linear mixed-effects model. From this calculation, the number of cases required in each group was 14. All tests were two-sided and P values of less than 0.05 were considered statistically significant. The statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and IBM SPSS Statistics 25 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Clinical Characteristics
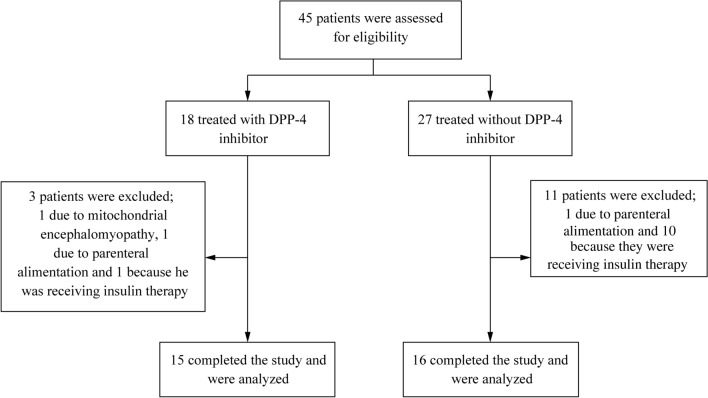
The recruitment process for participants is outlined in Fig. 1. Forty-five patients undergoing dialysis during the daytime were eligible for this study; 18 patients were treated with a DPP-4 inhibitor and 27 patients were not. Three patients were subsequently excluded from the DPP-4 inhibitor (+) group: 1 due to mitochondrial encephalomyopathy, 1 due to parenteral alimentation, and 1 because he was receiving insulin therapy. Eleven patients were also excluded from the DPP-4 inhibitor (−) group: 1 due to parenteral alimentation and 10 because they were receiving insulin therapy. Finally, we examined BG profiles using CGM in 31 patients with type 2 diabetes on maintenance hemodialysis.
Fig. 1.
Flow chart of patient enrollment
The clinical and biological characteristics of the participants are summarized in Table 1. There were no significant differences in age, sex, duration on hemodialysis, and duration of diabetes mellitus between the two groups. Fifteen patients were receiving a DPP-4 inhibitor: sitagliptin (12.5 mg daily), 1; vildagliptin (50 mg daily), 1; vildagliptin (100 mg daily), 2; alogliptin (6.25 mg daily), 4; linagliptin (5 mg daily), 4; teneligliptin (20 mg daily), 2; and teneligliptin (40 mg daily), 1. Moreover, 2 patients were receiving mitiglinide, and 1 patient was taking miglitol with a DPP-4 inhibitor. The patients in the DPP-4 inhibitor (−) group (n = 16) were not receiving any oral hypoglycemic agents or insulin therapy.
Table 1.
Patient demographics, clinical characteristics, and laboratory findings at baseline
| DPP-4 inhibitor (+) (n = 15) | DPP-4 inhibitor (−) (n = 16) | P value | |
|---|---|---|---|
| Age (years) | 58.3 ± 12.9 | 62.8 ± 11.9 | 0.323¶ |
| Sex (male: female) | 11:4 | 8:8 | 0.273ǂ |
| Height (cm) | 166.7 ± 11.8 | 161.2 ± 10.4 | 0.175¶ |
| Dry weight (kg) | 64.8 ± 16.4 | 60.3 ± 9.8 | 0.360¶ |
| Ideal body weight (kg) | 61.4 ± 8.6 | 57.4 ± 7.3 | 0.165¶ |
| Body mass index (kg/m2) | 23.2 ± 4.5 | 23.2 ± 2.8 | 0.999¶ |
| HD duration (months) | 32.0 (14.0, 132.0) | 13.0 (2.9, 68.4) | 0.281§ |
| Diabetes duration (years) | 24.0 (5.5, 30.8) | 9.0 (7.0, 24.0) | 0.270§ |
| Energy (kcal/kg/day) | 30.1 ± 2.4 | 29.3 ± 4.3 | 0.520¶ |
| HD time (3 h:4 h) | 1:14 | 2:14 | 1.000ǂ |
| Pre-HD body weight (kg) | 66.3 ± 17.0 | 61.8 ± 10.1 | 0.385¶ |
| Post-HD body weight (kg) | 64.7 ± 16.4 | 60.3 ± 9.7 | 0.382¶ |
| Water removal (kg) | 1.6 ± 1.1 | 1.5 ± 1.0 | 0.756¶ |
| Glucose concentration (100 mg/dL:150 mg/dL) | 13:2 | 14:2 | 1.000ǂ |
| Blood flow rate (mL/min) | 194.0 ± 30.9 | 185.6 ± 31.0 | 0.457¶ |
| Dialyzer membrane area (m2) | 1.6 (1.5, 1.8) | 1.6 (1.3, 1.8) | 0.800§ |
| HbA1c (%) | 6.2 ± 0.9 | 5.8 ± 0.7 | 0.172¶ |
| Hb (g/dL) | 10.3 ± 1.3 | 10.4 ± 1.4 | 0.864¶ |
| BUN (mg/dL) | 50.4 ± 23.0 | 51.0 ± 16.3 | 0.936¶ |
| Cr (mg/dL) | 8.9 ± 2.8 | 8.0 ± 3.0 | 0.377¶ |
| Alb (g/dL) | 3.5 ± 0.5 | 3.2 ± 0.6 | 0.086¶ |
| CRP (mg/dL) | 0.19 (0.11, 0.32) | 0.25 (0.05, 0.72) | 0.274¶ |
Normally distributed data are presented as the mean ± SD; non-normally distributed data are presented as medians and interquartile ranges
Alb albumin, BUN blood urea nitrogen, Cr creatinine, CRP C-reactive protein, Hb hemoglobin, HbA1c glycated hemoglobin, HD hemodialysis
¶P value calculated using the unpaired t test
§P value calculated using the Mann–Whitney U test
ǂP value calculated using Fisher’s exact test. Ideal body weight was calculated as height × height × 22, Energy amount of energy in the prescribed hospital meals, Water removal water removal during dialysis, Glucose concentration glucose concentration in dialysate
BG Variability on Hemodialysis and Nonhemodialysis Days
The average concentration and the SD of BG were not significantly different between hemodialysis and nonhemodialysis days [average BG concentration: 128.5 ± 19.0 mg/dL vs. 130.6 ± 18.7 mg/dL, respectively (P = 0.77); SD of BG: 29.9 ± 14.5 mg/dL vs. 24.0 ± 12.3 mg/dL, respectively (P = 0.05)]. However, MAGE was significantly higher on the hemodialysis day than on the nonhemodialysis day (76.7 ± 37.0 mg/dL vs. 60.5 ± 30.5 mg/dL, P = 0.04). No patient had symptomatic hypoglycemia.
Effects of DPP-4 Inhibitors on BG Variability and Hypoglycemia
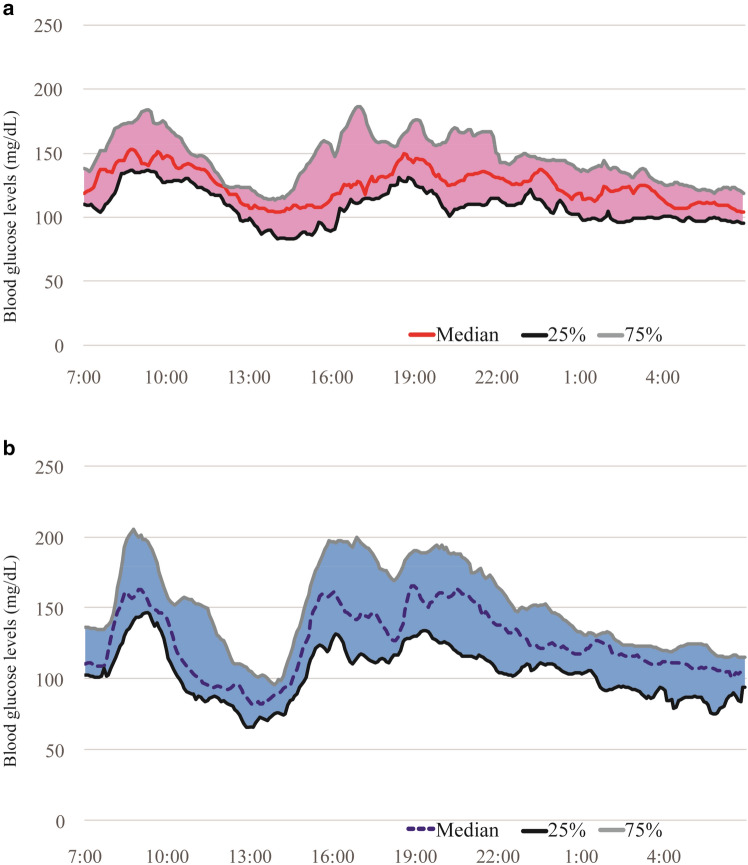
Next, we evaluated the efficacy of the DPP-4 inhibitors. There was no significant difference in mean glucose concentration between the DPP-4 inhibitor (+) and (−) groups, as shown in Figs. 1 and 2 [hemodialysis day: 128.7 ± 17.5 mg/dL vs. 128.4 ± 20.7 mg/dL, respectively (P = 0.96); nonhemodialysis day: 128.9 ± 20.5 mg/dL vs. 132.2 ± 17.6 mg/dL, respectively (P = 0.64)]. However, the magnitudes of the SD for BG and MAGE were markedly lower in the DPP-4 inhibitor (+) group than in the DPP-4 inhibitor (−) group [SD of BG: hemodialysis day, 24.3 ± 12.7 mg/dL vs. 35.1 ± 14.4 mg/dL (P = 0.04); nonhemodialysis day, 19.6 ± 12.2 mg/dL vs. 27.8 ± 11.5 mg/dL (P = 0.07); MAGE: hemodialysis day, 62.3 ± 32.1 mg/dL vs. 90.3 ± 37.1 mg/dL (P = 0.03); nonhemodialysis day, 48.6 ± 27.7 mg/dL vs. 70.9 ± 29.8 mg/dL (P = 0.04)] (Fig. 2).
Fig. 2.
Glucose levels on the day of hemodialysis in patients with or without a DPP-4 inhibitor. 24 h continuous glucose monitoring data on the day of hemodialysis are shown for patients a with or b without a DPP-4 inhibitor (n = 15 and n = 16, respectively). The curves show the median and 25th–75th percentiles
Next, we analyzed hypoglycemic events. Although no patients had symptomatic hypoglycemia, some patients showed nocturnal hypoglycemia on CGM, especially on the day of hemodialysis. There were no significant differences in the proportions of nocturnal hypoglycemic events from 0:00 am to 6:55 am between the DPP-4 inhibitor (+) and (−) groups [0 of 15 (0%) vs. 4 of 16 (25%), P = 0.058] (Table 2). The rates were similar on hemodialysis and nonhemodialysis days (data not shown).
Table 2.
Analysis of the contingency table for the effect of DPP-4 inhibitors and nocturnal glucose levels from 0:00 am to 6:55 am
| DPP-4 inhibitor | Total | ||
|---|---|---|---|
| (+) | (−) | ||
| BG < 70 mg/dL | 0 | 4 | 4 |
| BG ≥ 70 mg/dL | 15 | 12 | 27 |
| Total | 15 | 16 | 31 |
P value calculated using Fisher’s exact test was 0.058
BG blood glucose
BG Variability During Hemodialysis Sessions and at Nighttime in Analysis Using the Linear Mixed-Effects Model
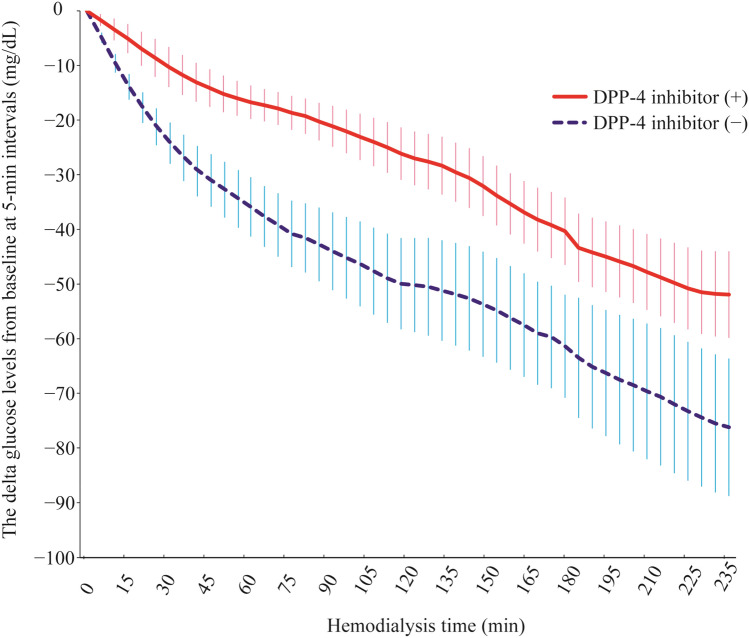
During hemodialysis (from time 0 to 235 min), the slope of the BG levels in the DPP-4 inhibitor (+) group was − 0.2 ± 0.01 mg/dL/min, whereas that in the DPP-4 inhibitor (−) group was − 0.3 ± 0.01 mg/dL/min. The absolute between-group difference in mean rate of change in BG levels during hemodialysis was 0.1 ± 0.01 mg/dL/min. Therefore, there was a significant difference in the slope of the BG levels between the two groups (P < 0.001, linear mixed-effects model; Table 3; Figs. 2, 3).
Table 3.
Linear mixed-effects model with glucose level (measured every 5 min from the start of HD to 235 min) as the dependent variable and DPP-4 inhibitor use and time as independent variables
| Source | Estimated coefficient | SE of coefficient | T value | P value |
|---|---|---|---|---|
| DPP-4 inhibitor | 12.2 | 8.4 | 1.5 | 0.155 |
| Time | − 0.3 | 0.0 | − 36.0 | < 0.001 |
| DPP-4 inhibitor × time | 0.1 | 0.0 | 6.0 | < 0.001 |
| Constant | − 15.0 | 5.8 | − 2.6 | 0.015 |
Time effect of time elapsed from the start of HD to 235 min, DPP-4 inhibitor × time interaction between DPP-4 inhibitor and time. The variables were coded as follows: time (in min): 0, 5, 10, …, 235. DPP-4 inhibitor group: 0, not receiving a DPP-4 inhibitor; 1, receiving a DPP-4 inhibitor
HD hemodialysis, SE standard error
Fig. 3.
Differences in blood glucose levels from baseline in 5-min intervals during each hemodialysis session in the DPP-4 inhibitor (+) and (−) groups. Error bars indicate 1 × standard error. Number of patients at risk: 31 (15 with a DPP-4 inhibitor and 16 without a DPP-4 inhibitor) between 0 and 175 min and 28 (14 with a DPP-4 inhibitor and 14 without a DPP-4 inhibitor) between 180 and 235 min
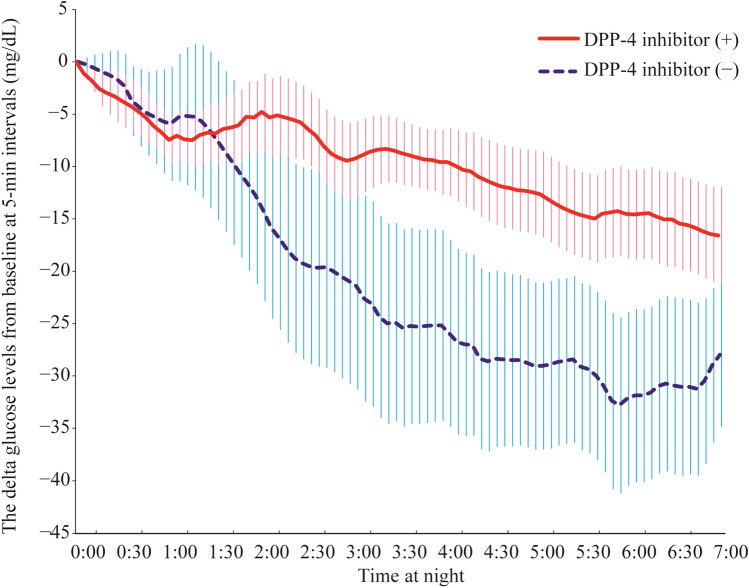
During the night (from 0:00 am to 6:55 am), the slope of the BG levels of the DPP-4 inhibitor (+) group was − 0.05 ± 0.002 mg/dL/min, whereas that of the DPP-4 inhibitor (−) group was − 0.1 ± 0.004 mg/dL/min. The absolute between-group difference in mean rate of change in BG levels during hemodialysis was 0.05 ± 0.004 mg/dL. There was thus a significant difference between the two groups in the slope of the BG levels (P < 0.001, linear mixed-effects model) (Table 4; Figs. 2, 4).
Table 4.
Linear mixed-effects model with glucose levels (measured every 5 min from 0:00 am to 6:55 am) as the dependent variable and DPP-4 inhibitor use and time as independent variables
| Source | Estimated coefficient | SE of coefficient | T value | P value |
|---|---|---|---|---|
| DPP-4 inhibitor | − 50.0 | 9.2 | − 5.5 | < 0.001 |
| Time | − 0.1 | 0.0 | − 26.7 | < 0.001 |
| DPP-4 inhibitor × time | 0.0 | 0.0 | 11.2 | < 0.001 |
| Constant | 80.3 | 6.4 | 12.6 | < 0.001 |
Time effect of time elapsed from 0:00 am to 6:55 am, DPP-4 inhibitor × time interaction between DPP-4 inhibitor and time. The variables were coded as follows: time (in minutes): 0, 5, 10, …, 235. DPP-4 inhibitor group: 0, not receiving a DPP-4 inhibitor; 1: receiving a DPP-4 inhibitor
HD hemodialysis, SE standard error
Fig. 4.
Differences in blood glucose levels from 0:00 am in 5 min intervals in the DPP-4 inhibitor (+) and (−) groups (0:00–6:55 am). Error bars indicate 1 × standard error. Number of patients at risk: 31 (15 with a DPP-4 inhibitor and 16 without a DPP-4 inhibitor)
Discussion
There is little information on BG variability in maintenance hemodialysis patients. Our study shows that maintenance hemodialysis patients with type 2 diabetes have high BG variability, as evidenced by the higher SD of BG, MAGE, and linear mixed-effects model coefficients. Also, our data from the linear mixed-effects model showed a rapid BG drop during hemodialysis and a tendency for hypoglycemia in the subsequent nighttime period in maintenance hemodialysis patients with type 2 diabetes. Although DPP-4 inhibitors have been shown to improve BG variability in patients with type 2 diabetes in many randomized controlled trials (RCTs) [41–49] (see the table in the Electronic supplementary material), few studies have reported the same effect in maintenance hemodialysis patients [50]. Our study illustrates the usefulness of DPP-4 inhibitors in these patients. Moreover, we showed that DPP-4 inhibitors ameliorated not only the 24 h BG variability but also the BG drop during hemodialysis and the tendency for nocturnal hypoglycemia in particular.
The ability of DPP-4 inhibitors to suppress BG variability in patients with type 2 diabetes has been shown in studies using CGM [51, 52]. Furthermore, several RCTs using CGM found that BG variability is suppressed more effectively by DPP-4 inhibitors than by other agents such as sulfonylureas [41, 42] and sodium glucose cotransporter 2 inhibitors [43–45] or by combination with insulin therapy [46–49]. The patients in these RCTs who showed suppressed BG variability with DPP-4 inhibitors had a mean age of less than 60 years [42–44, 46, 48, 49], had HbA1c > 7% [41–44, 46–49], were drug naïve [41, 49], or had used metformin only [41–43, 46]. There have also been some reports on the usefulness of DPP-4 inhibitors in patients on hemodialysis [50], but none has shown obvious suppression of BG variability. In this prospective observational exploratory study, analysis using a linear mixed-effects model showed that DPP-4 inhibitors could suppress BG variability in patients with type 2 diabetes. However, it is not known whether DPP-4 inhibitors are more effective at suppressing BG variability than any other drugs, even in patients on hemodialysis. This is an issue to be resolved in the future. Several reports have examined the efficacy of DPP-4 inhibitors for suppressing BG variability not only during the day and after a meal but also at night [53, 54]. However, until now, there has been no clear evidence that DPP-4 has a nocturnal effect. Using a linear mixed-effects model, we have shown that DPP-4 inhibitors could potentially suppress BG variability during hemodialysis sessions as well as subsequent nocturnal changes. Whether this effect is a feature of DPP-4 inhibitors in general or specific to patients on dialysis needs further investigation.
One of the essential endpoints of our study was the slope of the BG levels measured in mg/dL/min throughout hemodialysis and at night. This slope was calculated using CGM data obtained at baseline and every 5 min during the treatment phase. The difference in slope between the DPP-4 inhibitor (+) and (−) groups was evaluated with a linear mixed-effects model. CGM data usually show a periodic waveform. However, the changes between time points can be approximated using the slope of a linear mixed-effects model. Linear mixed-effects methods are used to fit the model to repeated-measures data such as those of CGM [55], in which measurements are obtained repeatedly over time or under different conditions. The model comprises repeated effects, fixed or covariate effects, and random effects, and the interactions among combinations of these as predictor variables. In general, many study designs generate longitudinal or repeated-measures data sets. These designs are applied in a variety of settings throughout the medical, biological, and physical sciences. Repeated-measures data obtained using such a design often involve missing values, but the linear mixed-effects model can nonetheless estimate the parameters without ad hoc imputation of the missing values. Therefore, linear mixed-effects models provide researchers with powerful and flexible analytic tools for these types of data. They are considered especially useful for analysis of CGM data over time, such as during hemodialysis or at nighttime.
A comparison of our maintenance hemodialysis patients treated with and without DPP-4 inhibitors revealed that the average BG was similar but that the SD of BG and MAGE were significantly lower in the DPP-4 inhibitor (+) group. Some studies have shown that DPP-4 inhibitors, which can increase insulin secretion but reduce prandial glucagon, improve the BG wave [51, 56]. The reduction in prandial glucagon is believed to be the most important mechanism through which DPP-4 inhibitors improve the BG wave [35]. The present study showed that DPP-4 inhibitors are as effective in dialysis patients as in type 2 diabetes patients not undergoing hemodialysis [41, 44, 48]. The mechanisms are unclear, and more detailed studies are needed, including those that analyze the fluctuation in glucagon.
The average BG, MAGE, and SD of BG were not significantly different between nonhemodialysis and hemodialysis days. However, a significant BG drop was seen during hemodialysis on the hemodialysis day. Asymptomatic hypoglycemia has been reported in hemodialysis patients during hemodialysis [25, 57, 58]. It has long been thought that the diffusion of glucose from blood to dialysate causes this hypoglycemia, and that the use of glucose-containing dialysate can prevent such hypoglycemia [11, 57, 59]. On the other hand, some patients experience a lower BG than the dialysate glucose concentration [58], and hypoglycemia is not completely preventable by glucose-containing dialysate [59]. In addition to chronic undernutrition [60, 61], a delay in lunchtime due to hemodialysis extends the fasting time, causing a loss of glycogen in maintenance hemodialysis patients and increasing the likelihood of developing hypoglycemia. Other mechanisms for hypoglycemia in maintenance hemodialysis patients have been reported. Takahashi et al. stated that excessive consumption of glucose is the result of an accelerated anaerobic metabolism, and causes hypoglycemia during hemodialysis in these patients [62].
Additionally, diabetic patients have less protection against a rapid fall in BG. Counter-regulation of the autonomic nervous system and the hormonal response to hypoglycemia are impaired by diabetic complications in patients with long-term diabetes [11, 63, 64]. These conditions may impair the body’s ability to control BG and cause a loss of response to increasing BG in the hypoglycemic state. In the present CGM study, there were only a few cases of hypoglycemia during or after hemodialysis, but almost all patients experienced a rapid fall in BG during hemodialysis. Compared with the DPP-4 inhibitor (−) group, the rate of decrease in BG during hemodialysis was reduced in the DPP-4 inhibitor (+) group. Our study thus suggests that DPP-4 inhibitors can prevent a rapid BG drop during hemodialysis. The mechanisms are unclear, but DPP-4 inhibitors may be able to improve the fluctuation in BG during hemodialysis in a similar manner to their ability to ameliorate the circadian variation of BG [51, 56, 65]. In terms of other factors, glucagon is probably associated with an improvement in BG fluctuation during hemodialysis in the same way as during nocturnal BG control. A previous study revealed that vildagliptin, a DPP-4 inhibitor, reduces postprandial glucagon levels and improves hyperglycemia in patients with type 2 diabetes [35]. Also, DPP-4 inhibitors improve the ability of both β-cells and α-cells to sense and appropriately respond to hypoglycemia [66]. Thus, DPP-4 inhibitors may be able to improve the response to hypoglycemia and prevent a rapid BG drop during hemodialysis in diabetic hemodialysis patients.
In our study, patients in the DPP-4 inhibitor (+) group seldom had BG < 70 mg/dL during the night. Diabetic patients frequently experience hypoglycemia due to a reduced autonomic nerve response, hormonal antagonism [63], and glucagon secretion in response to hypoglycemia [64]. Previous work also showed the potential ability of DPP-4 inhibitors to improve not only hyperglycemia but also hypoglycemia [37, 38]. Excessive glucagon secretion was seen in diabetes patients as well as a lack of glucagon responsiveness to hypoglycemia. When constant oversecretion of glucagon is inhibited by DPP-4 inhibitors [35], glucagon shows its normal response to a BG fall and may be able to prevent hypoglycemia [66]. Hypoglycemia in hemodialysis patients can occur even when they have never been diagnosed with diabetes or have not been exposed to hypoglycemic agents [25, 67]. Chronic kidney disease or hemodialysis can probably cause hypoglycemia per se [7, 8, 11, 18, 62]. Glycogenesis is as vital in the kidney as it is in the liver [68], especially under hypoglycemic conditions or during fasting [68–74]. When BG falls, glucagon and catecholamine secretion boosts glycogenesis in the kidney and increases BG levels in healthy people [75]. This mechanism is lost in maintenance hemodialysis patients, which is why they easily develop hypoglycemia under nocturnal fasting conditions.
Moreover, maintenance hemodialysis patients can often be undernourished due to protein loss during hemodialysis, and they often consume much smaller meals than recommended. Thus, not only are chronic malnutrition and lack of kidney glycogenesis important reasons for hypoglycemia in maintenance hemodialysis patients [61], but energy loss during hemodialysis and reduced energy storage can also explain nocturnal hypoglycemia on hemodialysis days [76]. Previous work showed that DPP-4 inhibitors improve hyperglycemia without inducing hypoglycemia during Ramadan, which has a long fasting time [77]. This efficacy of DPP-4 inhibitors during Ramadan may be somewhat similar to their efficacy during nocturnal fasting in maintenance hemodialysis patients.
There are some limitations to this study. Firstly, this study had a small number of patients. Moreover, the subjects were hospitalized patients, who are evidently in a different setting from outpatients. Secondly, this was not a RCT, so there could be confounding. Thirdly, in this study, five types of DPP-4 inhibitors were prescribed for 15 patients. However, Craddy et al. [78] recently reported that DPP-4 inhibitors have equivalent effects across the entire class in terms of essential efficacy and safety outcomes. Fourthly, although we surmise that glucagon plays a key role in hypoglycemia episodes and the prevention of hypoglycemia by DPP-4 inhibitors, we did not measure glucagon. This should be the next step. In the future, the use of only one type of DPP-4 inhibitor in a larger RCT is warranted.
Conclusion
In summary, this study used a linear mixed-effects model to show that DPP-4 inhibitors can suppress BG variability during hemodialysis sessions and subsequent nocturnal changes in patients with type 2 diabetes. DPP-4 inhibitors may be able to ameliorate the BG fluctuation and prevent hypoglycemia, thereby improving the prognosis of maintenance hemodialysis patients with diabetes. Further detailed examinations are needed in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
No funding or sponsorship was received for this study. The journal’s Rapid Service Fee was funded by Niigata University.
Editorial and Other Assistance
The authors thank Ms. Maiko Daisaka at Niigata University for providing technical assistance and ThinkSCIENCE Inc. (Tokyo, Japan) for providing language editing services. This assistance was funded by Niigata University.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Contributors T. Ishikawa-Tanaka and M. Hosojima were responsible for the conception and design of the study. T. Ishikawa-Tanaka was the chief investigator and also responsible for the data analysis. Contributors M. Hosojima, N. Kitamura, and T. Tanaka were responsible for the data analysis. Contributors M. Hosojima, H. Kabasawa, R. Kaseda, Yasukawa, Y. Yata, E. Kono, T. Takata, and N. Iino were responsible for the data acquisition. Contributors T. Ishikawa-Tanaka, M. Hosojima, H. Kabasawa, R. Kaseda, S. Kuwahara, N. Iino, Y. Suzuki, A. Saito, and I. Narita were responsible for the interpretation. T. Ishikawa-Tanaka and M. Hosojima were responsible for drafting. All authors contributed to the writing of the final manuscript.
Prior Presentations
The data included in this manuscript were presented, in part, at the American Diabetes Association 77th Scientific Sessions in 2017 in San Diego, CA, USA.
Disclosures
Tomomi Ishikawa-Tanaka has received lecture fees from Daiichi Sankyo Co., Eli Lilly Japan K.K., Kyowa Hakko Kirin Co., Ltd., MSD K.K., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd. Michihiro Hosojima and Akihiko Saito have received lecture fees from Daiichi Sankyo Co., Ltd., Eli Lilly Japan K.K., Kowa Company, Ltd., Kyowa Hakko Kirin Co., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd. Hideyuki Kabasawa has received lecture fees from Eli Lilly Japan K.K., Kyowa Hakko Kirin Co., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., and Ono Pharmaceutical Co., Ltd. Ryohei Kaseda has received lecture fees from Daiichi Sankyo Co., Ltd., Kyowa Hakko Kirin Co., Ltd., MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Sanwa Kagaku Kenkyusyo Co., Ltd., and Takeda Pharmaceutical Co., Ltd. Ryota Yasukawa has received lecture fees from Kyowa Hakko Kirin Co. and Mitsubishi Tanabe Pharma Corporation. S.K. has received a lecture fee from MSD K.K. Emiko Kono has received a lecture fee from Kyowa Hakko Kirin Co. Noriaki Iino has received lecture fees from Daiichi Sankyo Co., Ltd., Kyowa Hakko Kirin Co., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusyo Co., Ltd., and Takeda Pharmaceutical Co., Ltd. Yoshiki Suzuki has received lecture fees from Daiichi Sankyo Co., Ltd., Kowa Company, Ltd, Kyowa Hakko Kirin Co., Ltd., and Mitsubishi Tanabe Pharma Corporation. Ichiei Narita has received lecture fees from Kowa Company, Ltd. and Kyowa Hakko Kirin Co. Michihiro Hosojima and Akihiko Saito have received donations for research from Daiichi Sankyo Co., Ltd., Kowa Company, Ltd., Kyowa Hakko Kirin Co., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Sanwa Kagaku Kenkyusyo Co., Ltd., and Takeda Pharmaceutical Co., Ltd. Akihiko Saito has also received donations for research from Eli Lilly Japan K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., and Ono Pharmaceutical Co., Ltd. Akihiko Saito has received a collaborative research grant from Kyowa Hakko Kirin Co. Shoji Kuwahara has received donations for research from MSD K.K. Noriaki Iino has received donations for research from Kyowa Hakko Kirin Co., Ltd. and Takeda Pharmaceutical Co., Ltd. Ichiei Narita has received research support from Daiichi Sankyo Co., Ltd., Kyowa Hakko Kirin Co., Ltd., and Ono Pharmaceutical Co. Yusuke Yata, Takuma Takata, Takahiro Tanaka and Nobutaka Kitamura have nothing to disclose.
Compliance with Ethics Guidelines
The study was approved by the institutional review boards of Niigata University, Itoigawa General Hospital, and Nagaoka Chuo Hospital. All procedures were performed in accordance with the Helsinki Declaration of 1964 and its later amendments, and were in agreement with national regulations. The study was conceived as a retrospective data collection, and all patients provided written informed consent to reuse their clinical data for research purposes.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.12937694.
References
- 1.Thomas B, Wulf S, Bikbov B, et al. Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol. 2015;26:2621–2633. doi: 10.1681/ASN.2014101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morioka T, Emoto M, Tabata T, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care. 2001;24:909–913. doi: 10.2337/diacare.24.5.909. [DOI] [PubMed] [Google Scholar]
- 3.Shima K, Komatsu M, Kawahara K, et al. Stringent glycaemic control prolongs survival in diabetic patients with end-stage renal disease on haemodialysis. Nephrology (Carlton) 2010;15:632–638. doi: 10.1111/j.1440-1797.2010.01273.x. [DOI] [PubMed] [Google Scholar]
- 4.Hayashino Y, Fukuhara S, Akiba T, et al. Diabetes, glycaemic control and mortality risk in patients on haemodialysis: the Japan Dialysis Outcomes and Practice Pattern Study. Diabetologia. 2007;50:1170–1177. doi: 10.1007/s00125-007-0650-z. [DOI] [PubMed] [Google Scholar]
- 5.Ricks J, Molnar MZ, Kovesdy CP, et al. Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes. 2012;61:708–715. doi: 10.2337/db11-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun CY, Lee CC, Wu MS. Hypoglycemia in diabetic patients undergoing chronic hemodialysis. Ther Apher Dial. 2009;13:95–102. doi: 10.1111/j.1744-9987.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 8.Jackson MA, Holland MR, Nicholas J, et al. Occult hypoglycemia caused by hemodialysis. Clin Nephrol. 1999;51:242–247. [PubMed] [Google Scholar]
- 9.Jin YP, Su XF, Yin GP, et al. Blood glucose fluctuations in hemodialysis patients with end stage diabetic nephropathy. J Diabetes Complicat. 2015;29:395–399. doi: 10.1016/j.jdiacomp.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Jung HS, Kim HI, Kim MJ, et al. Analysis of hemodialysis-associated hypoglycemia in patients with type 2 diabetes using a continuous glucose monitoring system. Diabetes Technol Ther. 2010;12:801–807. doi: 10.1089/dia.2010.0067. [DOI] [PubMed] [Google Scholar]
- 11.Jackson MA, Holland MR, Nicholas J, et al. Hemodialysis-induced hypoglycemia in diabetic patients. Clin Nephrol. 2000;54:30–34. [PubMed] [Google Scholar]
- 12.Williams ME, Garg R, Wang W, et al. High hemoglobin A1c levels and glycemic variability increase risk of severe hypoglycemia in diabetic hemodialysis patients. Hemodial Int Symp Home Hemodial. 2014;18:423–32. [DOI] [PubMed]
- 13.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 14.Azuma K, Kawamori R, Toyofuku Y, et al. Repetitive fluctuations in blood glucose enhance monocyte adhesion to the endothelium of rat thoracic aorta. Arterioscler Thromb Vasc Biol. 2006;26:2275–2280. doi: 10.1161/01.ATV.0000239488.05069.03. [DOI] [PubMed] [Google Scholar]
- 15.Service FJ, Molnar GD, Rosevear JW, et al. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 16.Service FJ, O’Brien PC, Rizza RA. Measurements of glucose control. Diabetes Care. 1987;10:225–237. doi: 10.2337/diacare.10.2.225. [DOI] [PubMed] [Google Scholar]
- 17.Su G, Mi S, Tao H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:19. doi: 10.1186/1475-2840-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1121–1127. doi: 10.2215/CJN.00800209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright RJ, Newby DE, Stirling D, et al. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care. 2010;33:1591–1597. doi: 10.2337/dc10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desouza C, Salazar H, Cheong B, et al. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26:1485–1489. doi: 10.2337/diacare.26.5.1485. [DOI] [PubMed] [Google Scholar]
- 21.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 22.Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care. 2016;39:502–510. doi: 10.2337/dc15-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirani M, Berra C, Finazzi S, et al. Inter-day glycemic variability assessed by continuous glucose monitoring in insulin-treated type 2 diabetes patients on hemodialysis. Diabetes Technol Ther. 2010;12:749–753. doi: 10.1089/dia.2010.0052. [DOI] [PubMed] [Google Scholar]
- 24.Riveline JP, Teynie J, Belmouaz S, et al. Glycaemic control in type 2 diabetic patients on chronic haemodialysis: use of a continuous glucose monitoring system. Nephrol Dial Transpl. 2009;24:2866–2871. doi: 10.1093/ndt/gfp181. [DOI] [PubMed] [Google Scholar]
- 25.Kazempour-Ardebili S, Lecamwasam VL, Dassanyake T, et al. Assessing glycemic control in maintenance hemodialysis patients with type 2 diabetes. Diabetes Care. 2009;32:1137–1142. doi: 10.2337/dc08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.K/DOQI Workgroup K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:16–153. [PubMed] [Google Scholar]
- 27.Savu O, Elian V, Steriade O, et al. The impact of basal insulin analogues on glucose variability in patients with type 2 diabetes undergoing renal replacement therapy for end-stage renal disease. Int Urol Nephrol. 2016;48:265–270. doi: 10.1007/s11255-015-1175-x. [DOI] [PubMed] [Google Scholar]
- 28.Vigersky R, Shrivastav M. Role of continuous glucose monitoring for type 2 in diabetes management and research. J Diabetes Complicat. 2017;31:280–287. doi: 10.1016/j.jdiacomp.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Senda M, Ogawa S, Nako K, et al. The strong relation between post-hemodialysis blood methylglyoxal levels and post-hemodialysis blood glucose concentration rise. Clin Exp Nephrol. 2015;19:527–533. doi: 10.1007/s10157-014-1018-6. [DOI] [PubMed] [Google Scholar]
- 30.Joubert M, Fourmy C, Henri P, et al. Effectiveness of continuous glucose monitoring in dialysis patients with diabetes: the DIALYDIAB pilot study. Diabetes Res Clin Pract. 2015;107:348–354. doi: 10.1016/j.diabres.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Gai M, Merlo I, Dellepiane S, et al. Glycemic pattern in diabetic patients on hemodialysis: continuous glucose monitoring (CGM) analysis. Blood Purif. 2014;38:68–73. doi: 10.1159/000362863. [DOI] [PubMed] [Google Scholar]
- 32.Chantrel F, Sissoko H, Kepenekian L, et al. Influence of dialysis on the glucose profile in patients with diabetes: usefulness of continuous glucose monitoring. Horm Metab Res. 2014;46:810–813. doi: 10.1055/s-0034-1370963. [DOI] [PubMed] [Google Scholar]
- 33.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 34.Christensen M, Vedtofte L, Holst JJ, et al. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011;60:3103–3109. doi: 10.2337/db11-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahren B, Foley JE, Ferrannini E, et al. Changes in prandial glucagon levels after a 2-year treatment with vildagliptin or glimepiride in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care. 2010;33:730–732. doi: 10.2337/dc09-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunning BE, Foley JE, Ahren B. Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia. 2005;48:1700–1713. doi: 10.1007/s00125-005-1878-0. [DOI] [PubMed] [Google Scholar]
- 37.Fonseca V, Schweizer A, Albrecht D, et al. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148–1155. doi: 10.1007/s00125-007-0633-0. [DOI] [PubMed] [Google Scholar]
- 38.Li FF, Shen Y, Sun R, et al. Effects of vildagliptin add-on insulin therapy on nocturnal glycemic variations in uncontrolled type 2 diabetes. Diabetes Ther. 2017;8:1111–1122. doi: 10.1007/s13300-017-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abe M, Okada K. DPP-4 inhibitors in diabetic patients with chronic kidney disease and end-stage kidney disease on dialysis in clinical practice. Contrib Nephrol. 2015;185:98–115. doi: 10.1159/000380974. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura Y, Hasegawa H, Tsuji M, et al. Diabetes therapies in hemodialysis patients: dipeptidase-4 inhibitors. World J Diabetes. 2015;6:840–849. doi: 10.4239/wjd.v6.i6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki R, Eiki JI, Moritoyo T, et al. Effect of short-term treatment with sitagliptin or glibenclamide on daily glucose fluctuation in drug-naïve Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2018;20:2274–2281. doi: 10.1111/dom.13364. [DOI] [PubMed] [Google Scholar]
- 42.Kim G, Oh S, Jin SM, et al. The efficacy and safety of adding either vildagliptin or glimepiride to ongoing metformin therapy in patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2017;18:1179–1186. doi: 10.1080/14656566.2017.1353080. [DOI] [PubMed] [Google Scholar]
- 43.Fuchigami A, Shigiyama F, Kitazawa T, et al. Efficacy of dapagliflozin versus sitagliptin on cardiometabolic risk factors in Japanese patients with type 2 diabetes: a prospective, randomized study (DIVERSITY-CVR) Cardiovasc Diabetol. 2020;19:1. doi: 10.1186/s12933-019-0977-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwak SH, Hwang YC, Won JC, et al. Comparison of the effects of gemigliptin and dapagliflozin on glycaemic variability in type 2 diabetes: a randomized, open-label, active-controlled, 12-week study (STABLE II study) Diabetes Obes Metab. 2020;22:173–181. doi: 10.1111/dom.13882. [DOI] [PubMed] [Google Scholar]
- 45.Cho KY, Nomoto H, Nakamura A, et al. Favourable effect of the sodium-glucose co-transporter-2 inhibitor canagliflozin plus the dipeptidyl peptidase-4 inhibitor teneligliptin in combination on glycaemic fluctuation: an open-label, prospective, randomized, parallel-group comparison trial (the CALMER study) Diabetes Obes Metab. 2020;22:458–462. doi: 10.1111/dom.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyu FP, Huang BK, Su WJ, et al. Efficacy of vildagliptin added to continuous subcutaneous insulin infusion (CSII) in hospitalized patients with type 2 diabetes. Diabetes Ther. 2020;11:701–710. doi: 10.1007/s13300-020-00758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okajima F, Emoto N, Kato K, et al. Effect of glycemic control on chylomicron metabolism and correlation between postprandial metabolism of plasma glucose and chylomicron in patients with type 2 diabetes treated with basal-bolus insulin therapy with or without vildagliptin. J Atheroscler Thromb. 2017;24:157–168. doi: 10.5551/jat.32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li FF, Jiang LL, Yan RN, et al. Effects of saxagliptin add-on therapy to insulin on blood glycemic fluctuations in patients with type 2 diabetes: a randomized, control, open-labeled trial. Medicine (Baltimore) 2016;95:e5229. doi: 10.1097/MD.0000000000005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wan H, Zhao D, Shen J, et al. Comparison of the effects of continuous subcutaneous insulin infusion and add-on therapy with sitagliptin in patients with newly diagnosed type 2 diabetes mellitus. J Diabetes Res. 2016;2016:9849328. doi: 10.1155/2016/9849328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munch M, Meyer L, Hannedouche T, et al. Effect of adding vildagliptin to insulin in haemodialysed patients with type 2 diabetes: the VILDDIAL study, a randomized, multicentre, prospective study. Diabetes Obes Metab. 2020;22:978–987. doi: 10.1111/dom.13988. [DOI] [PubMed] [Google Scholar]
- 51.Mori Y, Taniguchi Y, Miyazaki S, et al. Effects of add-on treatment with sitagliptin on narrowing the range of glucose fluctuations in Japanese type 2 diabetes patients receiving insulin therapy. Diabetes Technol Ther. 2013;15:237–240. doi: 10.1089/dia.2012.0214. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka S, Suzuki K, Aoki C, et al. Add-on treatment with teneligliptin ameliorates glucose fluctuations and improves glycemic control index in Japanese patients with type 2 diabetes on insulin therapy. Diabetes Technol Ther. 2014;16:840–845. doi: 10.1089/dia.2014.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seo C, Sakamoto M, Nishimura R, et al. Comparison of glycemic variability in patients with type 2 diabetes given sitagliptin or voglibose: a continuous glucose monitoring-based pilot study. Diabetes Technol Ther. 2013;15:378–385. doi: 10.1089/dia.2012.0262. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi H, Nishimura R, Tsujino D, et al. Which is better, high-dose metformin monotherapy or low-dose metformin/linagliptin combination therapy, in improving glycemic variability in type 2 diabetes patients with insufficient glycemic control despite low-dose metformin monotherapy? A randomized, cross-over, continuous glucose monitoring-based pilot study. J Diabetes Investig. 2019;10:714–722. doi: 10.1111/jdi.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samukawa Y, Omiya H, Watase H, et al. Substantial effects of luseogliflozin revealed by analyzing responses to postprandial hyperglycemia: post hoc subanalyses of a randomized controlled study. Adv Ther. 2016;33:1215–1230. doi: 10.1007/s12325-016-0350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakamoto M, Nishimura R, Irako T, et al. Comparison of vildagliptin twice daily vs. sitagliptin once daily using continuous glucose monitoring (CGM): crossover pilot study (J-VICTORIA study) Cardiovasc Diabetol. 2012;11:92. doi: 10.1186/1475-2840-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burmeister JE, Campos JF, Miltersteiner DR. Effect of different levels of glucose in the dialysate on the risk of hypoglycaemia during hemodialysis in diabetic patients. J Bras Nefrol. 2012;34:323–7. [DOI] [PubMed]
- 58.Raimann JG, Kruse A, Thijssen S, et al. Metabolic effects of dialyzate glucose in chronic hemodialysis: results from a prospective, randomized crossover trial. Nephrol Dial Transpl. 2012;27:1559–1568. doi: 10.1093/ndt/gfr520. [DOI] [PubMed] [Google Scholar]
- 59.Burmeister JE, Scapini A, da Rosa Miltersteiner D, et al. Glucose-added dialysis fluid prevents asymptomatic hypoglycaemia in regular haemodialysis. Nephrol Dial Transpl. 2007;22:1184–1189. doi: 10.1093/ndt/gfl710. [DOI] [PubMed] [Google Scholar]
- 60.Chertow GM, Johansen KL, Lew N, et al. Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int. 2000;57:1176–1181. doi: 10.1046/j.1523-1755.2000.00945.x. [DOI] [PubMed] [Google Scholar]
- 61.Arem R. Hypoglycemia associated with renal failure. Endocrinol Metab Clin N Am. 1989;18:103–121. [PubMed] [Google Scholar]
- 62.Takahashi A, Kubota T, Shibahara N, et al. The mechanism of hypoglycemia caused by hemodialysis. Clin Nephrol. 2004;62:362–368. doi: 10.5414/cnp62362. [DOI] [PubMed] [Google Scholar]
- 63.Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51:724–733. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- 64.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350:2272–2279. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- 65.Mori Y, Taniguchi Y, Matsuura K, et al. Effects of sitagliptin on 24-h glycemic changes in Japanese patients with type 2 diabetes assessed using continuous glucose monitoring. Diabetes Technol Ther. 2011;13:699–703. doi: 10.1089/dia.2011.0025. [DOI] [PubMed] [Google Scholar]
- 66.Ahren B, Schweizer A, Dejager S, et al. Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:1236–1243. doi: 10.1210/jc.2008-2152. [DOI] [PubMed] [Google Scholar]
- 67.Sobngwi E, Ashuntantang G, Ndounia E, et al. Continuous interstitial glucose monitoring in non-diabetic subjects with end-stage renal disease undergoing maintenance haemodialysis. Diabetes Res Clin Pract. 2010;90:22–25. doi: 10.1016/j.diabres.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Battezzati A, Caumo A, Martino F, et al. Nonhepatic glucose production in humans. Am J Physiol Endocrinol Metab. 2004;286:E129–E135. doi: 10.1152/ajpendo.00486.2002. [DOI] [PubMed] [Google Scholar]
- 69.Stumvoll M. Glucose production by the human kidney—its importance has been underestimated. Nephrol Dial Transpl. 1998;13:2996–9. [DOI] [PubMed]
- 70.Meyer C, Stumvoll M, Dostou J, et al. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am J Physiol Endocrinol Metab. 2002;282:E428–E434. doi: 10.1152/ajpendo.00116.2001. [DOI] [PubMed] [Google Scholar]
- 71.Meyer C, Dostou JM, Welle SL, et al. Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am J Physiol Endocrinol Metab. 2002;282:E419–E427. doi: 10.1152/ajpendo.00032.2001. [DOI] [PubMed] [Google Scholar]
- 72.Meyer C, Woerle HJ, Dostou JM, et al. Abnormal renal, hepatic, and muscle glucose metabolism following glucose ingestion in type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287:E1049–E1056. doi: 10.1152/ajpendo.00041.2004. [DOI] [PubMed] [Google Scholar]
- 73.Ekberg K, Landau BR, Wajngot A, et al. Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes. 1999;48:292–298. doi: 10.2337/diabetes.48.2.292. [DOI] [PubMed] [Google Scholar]
- 74.Cersosimo E, Garlick P, Ferretti J. Renal substrate metabolism and gluconeogenesis during hypoglycemia in humans. Diabetes. 2000;49:1186–1193. doi: 10.2337/diabetes.49.7.1186. [DOI] [PubMed] [Google Scholar]
- 75.Cersosimo E, Garlick P, Ferretti J. Renal glucose production during insulin-induced hypoglycemia in humans. Diabetes. 1999;48:261–266. doi: 10.2337/diabetes.48.2.261. [DOI] [PubMed] [Google Scholar]
- 76.Gutierrez A, Bergstrom J, Alvestrand A. Hemodialysis-associated protein catabolism with and without glucose in the dialysis fluid. Kidney Int. 1994;46:814–822. doi: 10.1038/ki.1994.337. [DOI] [PubMed] [Google Scholar]
- 77.Schweizer A, Halimi S, Dejager S. Experience with DPP-4 inhibitors in the management of patients with type 2 diabetes fasting during Ramadan. Vasc Health Risk Manag. 2014;10:15–24. doi: 10.2147/VHRM.S54585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Craddy P, Palin HJ, Johnson KI. Comparative effectiveness of dipeptidylpeptidase-4 inhibitors in type 2 diabetes: a systematic review and mixed treatment comparison. Diabetes Ther. 2014;5:1–41. doi: 10.1007/s13300-014-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.