Abstract
Objective
Weight stigma is associated with poor dietary adherence, yet adherence is essential for weight loss and maintenance. This study aimed to determine differences in dietary adherence and perceived hunger between lean individuals and two groups of individuals with obesity.
Methods
In a 6‐week outpatient dietary intervention (23 males; aged 48 [SD 14] years), lean participants (n = 23; BMI 23 [SD 2] kg/m2) received a weight‐maintaining energy needs (WMEN) diet, and participants with obesity (BMI 36 [SD 7]) were randomized to either WMEN (n = 18) or a 35% calorie‐reduced (CR) diet (n = 19). All food was provided, and multiple adherence and hunger ratings were assessed daily and weekly on an outpatient basis and in person at twice‐weekly visits (e.g., 24‐hour recall, diaries).
Results
Weight decreased more in the group of CR individuals with obesity (β = −0.301 kg/wk, P = 0.02) compared with the group of lean individuals and the group of WMEN individuals with obesity. However, total percent adherence did not differ between groups (P = 0.60), and hunger scores did not change across groups over time (P = 0.08).
Conclusions
Results indicate that there are no differences in dietary adherence between lean individuals and individuals with obesity and adherence is not associated with adiposity or hunger. Thus, the belief that nonadherence (e.g., lack of willpower) is unique to obesity is untrue and may perpetuate weight bias and stigma.
Study Importance.
What is already known?
-
►
Weight stigma is associated with poor dietary adherence.
-
►
Dietary adherence is a critical aspect of successful weight loss, and greater dietary adherence has previously been associated with greater weight loss.
What does this study add?
-
►
Dietary adherence did not differ between lean individuals and individuals with obesity or between those with obesity on a weight‐maintaining versus a calorie‐reduced diet.
-
►
Hunger ratings also did not differ between lean individuals and both groups of individuals with obesity and were not associated with adherence.
How might these results change the direction of research of the focus of clinical practice?
-
►
The belief that nonadherence and lack of willpower are unique behaviors that characterize individuals with obesity is untrue and may perpetuate weight bias and stigma.
-
►
Designing models to measure and predict adherence that are not influenced by weight bias is crucial to the development of successful dietary interventions.
Introduction
Between 50% and 58% of adults with obesity in the United States are attempting to lose weight (1). Yet successful weight reduction and maintenance remain elusive for most dieters. The annual probability of achieving a 5% reduction in body weight is approximately one in eight, and among these individuals, > 50% at 2 years and > 75% at 5 years fail to maintain weight loss (2).
Adherence, defined as the “extent to which patients follow instructions given to them for prescribed treatments,” is essential for achieving successful health outcomes (3). Nonadherence is a primary treatment barrier for most medical conditions, and rates of nonadherence are substantially higher for lifestyle prescriptions and behaviorally demanding regimens (4).
Dietary adherence is critical for successful initial weight loss and long‐term weight maintenance (5), and it is the most likely explanation for the observed low efficacy of low‐calorie diets, even after considering metabolic adaptations to weight loss (6). Greater adherence to multiple treatment components results in greater weight loss (7, 8, 9, 10, 11, 12). However, factors that underlie adherence are not well understood, especially whether individuals with obesity manifest greater difficulty with dietary adherence compared with lean individuals. Previous literature has reported differences in cognitive function between lean individuals and individuals with obesity. Thus, it is possible that increased adiposity is associated with differences in decision‐making, which may affect adherence (13). Furthermore, in regard to calorie restriction, it is not known whether it is a dietary prescription itself (even when given to maintain weight) that affects adherence (13). To our knowledge, no study to date has examined dietary adherence in lean individuals or in comparison with individuals with obesity. The aim of the current study was to determine differences in dietary adherence between lean individuals and two groups of individuals with obesity assigned to different dietary prescriptions (weight‐maintaining energy needs [WMEN] diet vs. calorie‐reduced [CR] diet). We also investigated differences in perceived hunger across groups and whether adherence scores would be associated with weight loss in the CR group.
Methods
Participants
From May 2013 to March 2018, 100 nondiabetic individuals aged 18 to 70 years were recruited from the greater Phoenix, Arizona, metropolitan area via advertisement to participate in a 6‐week outpatient dietary intervention program. Screening, eligibility, and analyzed data are reported in the CONSORT (Consolidated Standards of Reporting Trials) diagram (Figure 1A) (ClinicalTrials.gov identifier: NCT01862796). A total of 100 individuals were screened and assessed for eligibility, 30 were determined ineligible, 9 did not complete the run‐in phase, and 61 were determined eligible and included in the study. All participants were healthy based on medical history, physical examinations, and laboratory tests; weight stable (± 2%) for the last 3 months; and had BMI < 25 kg/m2 (lean) or ≥ 30 kg/m2 (obesity). Of the 61, a total of 24 with BMI < 25 were included in the lean group while the remaining 37 participants were randomized to either a CR or WMEN diet. One of the twenty‐four participants in the lean group was excluded from all analyses because study staff were informed he had been taking medication outlined in the exclusion criteria. Prior to participation, participants were informed of the nature, purpose, and risks of the study and they provided written informed consent. The protocol (Figure 1B) was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases.
Figure 1.
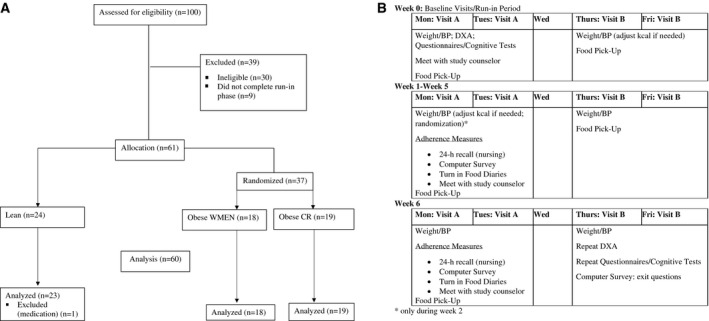
(A) CONSORT diagram. (B) Study protocol design.
Study design
Baseline visits
During the first baseline visit, participants completed the MacArthur Scale of Subjective Social Status (14) to assess subjective socioeconomic status, and body composition was determined by dual‐energy x‐ray absorptiometry (DPX‐L; Lunar Radiation, Madison, Wisconsin). WMEN were calculated for each participant based on the World Health Organization equation for weight, height, sex, and results from the Physical Activity Recall questionnaire (15, 16, 17). Participants met with a study counselor to review the WMEN dietary prescription (20%, 30%, and 50% of daily calories provided as protein, fat, and carbohydrate). All food items were provided by our metabolic kitchen, primarily as prepackaged meals and snacks for the participants to take home (Supporting Information Tables S1‐S2). They were given 4 days of food and dietary instructions to eat only the foods provided, consume no additional foods, maintain current levels of physical activity, and keep track on a self‐monitoring food record form. During the first week, and prior to randomization, participants were weighed at two outpatient baseline visits. If their weight changed by ± 2%, their WMEN was adjusted by 200 kcal accordingly.
Outpatient visits (6 weeks)
During the first outpatient visit, participants with obesity were stratified by sex and age using a block design and then randomized by an investigator who was not a part of the study to receive either a 35% underfeeding (CR) diet or continue their WMEN diet. Lean participants continued their WMEN diet. Participants and study staff were aware of group allocation following randomization. Participants were provided with 4 days of food, and the dietary instructions were repeated. Additionally, they were trained to use a smartphone system for momentary data collection. Weight and blood pressure were obtained at each visit, and meals were picked up two times per week. Participants met with the study counselor one time per week to collect food records and assess dietary adherence.
Adherence assessments at weekly in person visits
Following their weekly counselor meeting, a 24‐hour food recall was conducted by a different staff member (to avoid bias). Participants also completed a computerized survey in private, which consisted of a similar 24‐hour recall with prompts and additional questions to assess hunger levels and liking of the provided food.
Subjective hunger ratings
Hunger was assessed in three different ways during the in‐person visit: (1) the counselor asked participants to rate their overall level of hunger over the past week on a 5‐point Likert scale (1 = low hunger, 5 = high hunger); during the computer survey, participants were asked to rate (2) current hunger levels and (3) hunger levels over the past week compared with usual (before starting the study) based on a 5‐point Likert scale (1 = not at all hungry; much less hungry compared with usual, 5 = extremely hungry; much hungrier compared with usual). Pearson correlation coefficients revealed that the three hunger measures were highly correlated and therefore a single hunger score was created by taking the average of the three hunger score measures at each of the six visits for a total of six average hunger score ratings for each participant.
Outpatient adherence assessments
Further adherence assessments outside of the in‐person visits were measured as follows:
A member of the study staff called participants one time per week at random to conduct a 24‐hour food recall via phone.
Ecological momentary assessment (EMA) using a smartphone data collection system called ReTAINE (https://retaine.org/) was used to reflect repeated, real‐time (momentary) assessment in the participants’ natural environment (18). Signal contingent recording occurred at semirandom times two times per day: once between 8 am and 3 pm and once between 3 pm and 9 pm. When signaled, participants were asked, “Since the last time you were signaled, have you eaten anything?”, “If yes, did you eat the study food provided to you?”, “If no, which food didn’t you eat?”, “Did you eat anything else (in addition to the food provided)?”, and “If yes, what did you eat?”.
Postintervention visit
During the final visit of the 6‐week outpatient study, participants repeated laboratory tests, DXA, and behavioral questionnaires and provided a final adherence measurement via computer survey.
Scoring adherence
Adherence was the primary outcome of interest and was coded as a binary variable: 0 points if nonadherent and 1 point if adherent. Assessments included attendance at each of the two weekly appointments (12 possible points over the course of study) and being on time (± 15 minutes) for the once‐weekly counselor appointment (6 points possible). Four additional measures were assessed one time per week, including (1) food diaries, (2) 24‐hour recall in‐person interview, (3) computer survey, and (4) 24‐hour outpatient telephone recall. Adherence points for each of these assessments were awarded for (1) completing the assessment, (2) eating all food provided, and (3) not eating any additional foods, totaling 18 possible points over the course of the study.
EMA recordings
There were two signaled assessments per day, and adherence points were awarded for (1) completing the EMA, (2) eating all the food provided, and (3) not eating any additional foods, totaling 6 possible points per day. EMA weekly scores were divided by 7 to obtain average daily scores, allowing participants to earn 36 total possible points over the course of the study.
Total adherence score
The scores of all variables were summed to calculate a total adherence score, which was then divided by 126 (total points possible) to derive a total percent adherence score for each participant. A more detailed description and example of the scoring algorithm can be found in the online Supporting Material and Supporting Information Table S3.
Statistical analysis
Power calculations performed prior to starting the study determined that 60 participants (20 per group) had greater than 90% power with an α of 0.05 to detect a clinically meaningful, pairwise difference (mean [SD]) of 3.5 (6.0) adherence points per week between the groups. The total number of points earned per week was 21; we assumed that the group of CR individuals with obesity would be 62% adherent (equivalent to 13 adherence points/wk) (19). All statistical data analyses were preplanned and performed using SAS Enterprise Guide (version 7.1; SAS Institute Inc., Cary, North Carolina) and SPSS Statistics (version 25; IBM Corp., Armonk, New York). The α was set at 0.05, and two‐sided P values were reported. Normally distributed data are presented as means (SDs) or with 95% CIs, while skewed data are presented as medians with interquartile ranges. Differences in baseline continuous measures between groups were evaluated using one‐way ANCOVA to adjust for age, whereas group differences in categorical variables were analyzed using the χ2 test. Paired t tests were used to assess differences in cholesterol and triglycerides at baseline and the end of the study within each group. To address multiple comparisons between the three groups, Tukey post hoc analyses were performed. Associations among baseline variables, adherence measures, and subjective hunger scores were assessed using Pearson (r) or Spearman (ρ) correlation coefficients as appropriate. Differences in total percent adherence by group status (lean and individuals with obesity, WMEN and CR) were assessed using one‐way ANOVA.
A principal component analysis (PCA) (20) was applied to the entire set of 17 adherence variables to identify groups of correlated variables, to reduce dimensionality of data, and to produce overall adherence scores for each participant by properly weighing each single variable. The number of significant principal components (PCs) used to calculate the individual overall adherence scores (in standardized units) was determined by the scree test criterion of eigenvalues versus rank and further confirmed statistically by parallel analysis (21). The varimax rotation (22) was applied to improve the clinical interpretation of PCs. For each adherence variable included in the PCA, the Pearson correlation coefficient (PCA loading) was used to quantify the contribution of that adherence variable to each PC. Secondary outcomes were assessed using mixed models to analyze repeated measures of weight and hunger over time using a first‐order autoregressive covariance structure.
Results
Participant baseline characteristics are described in Table 1. The lean group consisted of 23 individuals, 18 individuals with obesity were randomized to the WMEN diet, and 19 individuals with obesity were randomized to the CR diet. Despite recruitment stratification by BMI, there was overlap in percent body fat (PFAT) between lean individuals and individuals with obesity (Figure 2). However, BMI and PFAT were significantly lower in the lean group compared with both groups with obesity (P < 0.001) and were not significantly different between the CR group and the WMEN group (P > 0.05). There were no significant differences between baseline and the final study visit on measures of cholesterol or triglycerides within any of the groups (P > 0.05).
Table 1.
Participant demographics
| Variable | All | Lean | Individuals with obesity, WMEN | Individuals with obesity, CR |
|---|---|---|---|---|
| n | 60 | 23 | 18 | 19 |
| Race/ethnicity | 5AA, 1A, 29C, | 3AA, 1A, 17C | 2AA, 3C, 5H, | 9C, 2H, 7NA, |
| 8H, 16NA, 1P | 1H, 1NA | 8NA | 1P | |
| Sex | 23m, 37f | 9m, 14f | 5m, 13f | 9m, 10f |
| Age (y) | 48.3 (14.1) | 49.7 (12.5) | 49.8 (13.1) | 45.3 (16.8) |
| Education (y) | 14.0 (2.4) | 14.7 (2.0) | 13.7 (2.9) | 13.4 (2.0) |
| SSS (US) | 5.7 (2.2) | 5.4 (1.8) | 5.7 (2.2) | 6.2 (2.7) |
| Weight (kg) ** | 86.1 (27.3) | 63.9 (8.0) | 88.1 (15.3) | 111.0 (29.1) |
| Height (cm) * | 166.5 (9.9) | 166.9 (8.6) | 161.7 (9.4) | 170.5 (10.4) |
| BMI (kg/m2) ** | 30.9 (8.5) | 22.9 (1.8) | 33.5 (4.0) | 38.0 (8.7) |
| PFAT (%) ** | 33.4 (9.5) | 25.0 (8.4) | 37.8 (5.5) | 39.4 (5.6) |
| FM (kg) ** | 30.0 (15.6) | 15.7 (5.5) | 33.0 (5.9) | 44.3 (15.5) |
| FFM (kg) ** | 55.9 (14.7) | 47.7 (8.9) | 55.1 (12.9) | 66.7 (15.7) |
| Waist (in) ** | 39.1 (8.0) | 31.6 (2.8) | 41.1 (3.7) | 46.3 (7.5) |
| WMEN (kcal) * | 2,550.5 (619.0) | 2,330.4 (421.5) | 2,538.9 (543.6) | 2,827.9 (785.1) |
Race/ethnicity, sex, and n presented as frequencies, while other variables presented as means (SDs).
P < 0.05.
P < 0.01 from ANOVA tests between groups.
A, Asian; AA, African American; C, Caucasian; CR, calorie reduced; f, female; FFM, fat‐free mass; FM, fat mass; H, Hispanic; m, male; NA, Native American; P, Pacific Islander; PFAT, percent body fat; SSS, subjective socioeconomic status (US); Waist, waist circumference; WMEN, weight‐maintaining energy needs.
Figure 2.
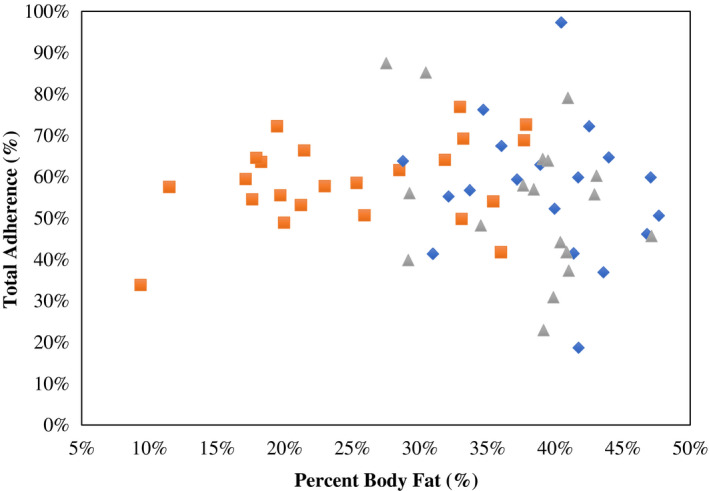
Association between baseline percent body fat (PFAT) and total adherence scores (%); Pearson correlation coefficient (r) is reported along with its significance. Lean group is represented by orange squares, individuals with obesity calorie reduced (CR) by blue diamonds, and individuals with obesity weight‐maintaining energy needs (WMEN) by gray triangles. [Color figure can be viewed at wileyonlinelibrary.com]
Adherence scores
Total percent adherence scores did not differ by sex, level of education, or subjective socioeconomic status (all P > 0.10). Adherence scores were positively associated with age (r = 0.32, P = 0.01) but not with PFAT in the entire sample (r = −0.10, P = 0.46) or within each group (all P > 0.05; Figure 2). Total percent adherence scores were not different between the groups (mean adherence score, lean: 59% [95% CI: 53%‐65%]; WMEN: 54% [95% CI: 47%‐61%]; CR: 57% [95% CI: 50%‐64%]; P = 0.54; Figure 3 and Supporting Information Table S5) even after adjustment for age. Results were similar even after segregating the data by World Health Organization PFAT criteria (23).
Figure 3.
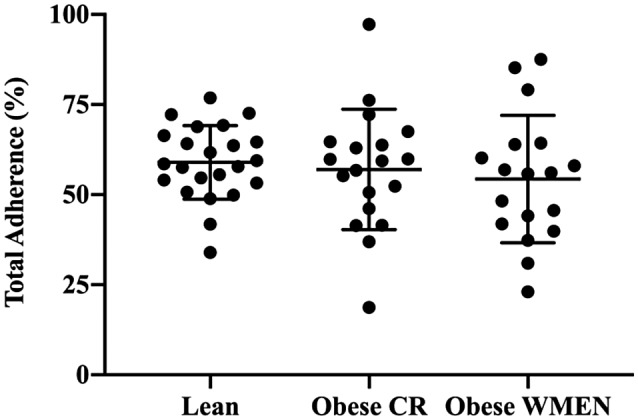
Total adherence scores (%) between the three groups, lean = 59%, individuals with obesity weight‐maintaining energy needs (WMEN) = 54%, and individuals with obesity calorie reduced (CR) = 57%, were not significantly different, P = 0.60. Error bars represent the means with 95% confidence intervals.
PCA
The scree plot and parallel analysis identified three PCs as significant, together explaining 56% of the total variance in the adherence variables submitted to PCA (Supporting Information Table S4). Adherence variables clustered into three components: the first component (explained variance = 30%) was characterized by the following questions regarding both the EMA and 24‐hour phone recall: “participant completed EMA or 24‐hour phone recall,” “ate all study food,” and “did not eat other food.” The second component (explained variance = 14%) clustered around the following questions regarding the food diary and 24‐hour in‐person interview/computer survey: “ate all food” and “did not eat other food.” Lastly, the third component (explained variance = 12%) clustered around attendance and questions regarding participants’ completion of the food diary/24‐hour in‐person interview and computer survey. Similarly to the results obtained from analyses of percent adherence scores, there were no group differences on the three PCA adherence factors (all P > 0.05).
Body weight change
After controlling for baseline weight, age, and sex, the CR group had a significant decline in body weight over the 6 weeks (β = −0.3 kg/wk, 95% CI: −0.55 to −0.05, P = 0.02) compared with lean individuals and the WMEN group (P > 0.05; Figure 4A). The average weight change after 6 weeks was as follows: lean, 0.11 kg (95% CI: −0.03 to 0.24) WMEN, −0.65 kg (95% CI: −0.88 to −0.42); and CR, −1.46 kg (95% CI: −1.77 to −1.15). We did not observe any effect of adherence score on weight change over the 6 weeks, both expressed as the absolute weight change and percent weight change (P > 0.05). Results were similar after stratification by group (all three P > 0.05). Similarly, there was no effect for any of the three PCA adherence factors on rate of weight change over the 6 weeks (P > 0.05).
Figure 4.
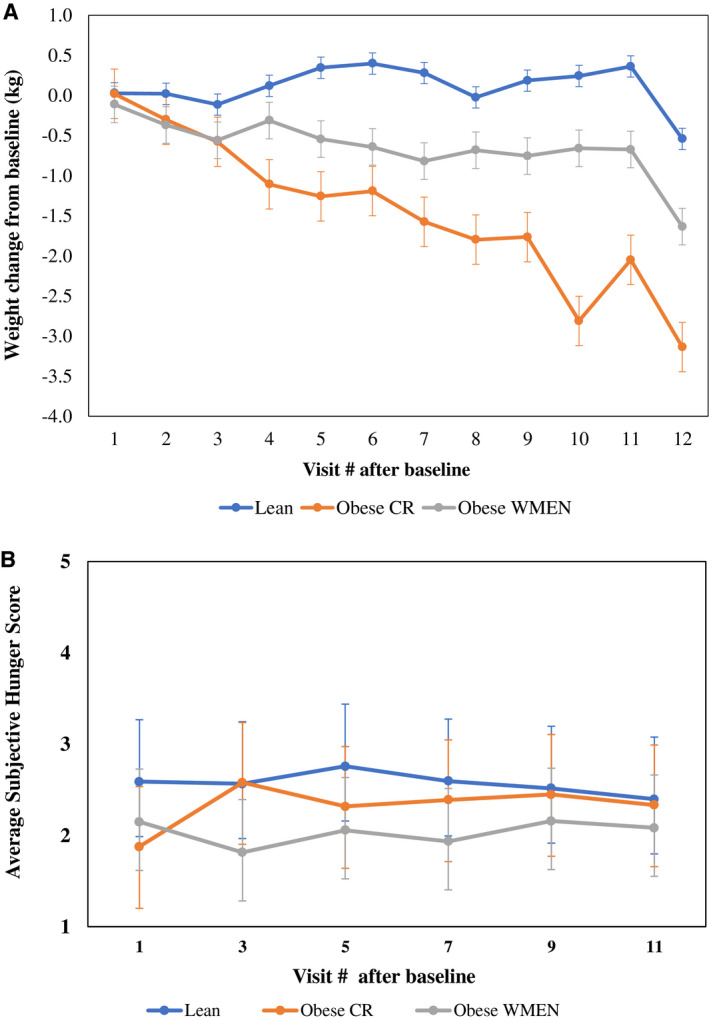
(A) Absolute weight change trajectories (visit weight minus baseline weight) show that the individuals with obesity calorie reduced (CR) lost significantly more weight over the 6 weeks compared with the individuals with obesity weight‐maintaining energy needs (WMEN) and the lean group (P = 0.02). (B) Average subjective hunger score trajectories were not significantly different between groups (P = 0.08). Error bars represent 95% confidence intervals of the means. Mixed models were adjusted for age, time (days), sex, and baseline weight (weight loss model only) using a first‐order autoregressive covariance structure. [Color figure can be viewed at wileyonlinelibrary.com]
Subjective hunger ratings
In‐person hunger ratings were associated with both computer hunger scores: hunger over the past week (ρ = 0.63, P < 0.0001) and hunger ratings at the time of the computer survey (ρ = 0.45, P = 0.0003). Similarly, the computer hunger ratings were correlated with each other (ρ = 0.53, P < 0.0001), indicating that all three of these assessments were measuring the same “hunger” construct. Overall, the lean group had significantly higher hunger scores compared with the WMEN group (P = 0.02), but hunger scores did not change over the 6 weeks (P = 0.08; Figure 4B), and hunger score trajectories were not significantly different between the three groups. Results were similar when the three hunger scores were analyzed separately (P > 0.05). Moreover, hunger scores were not associated with adherence scores in the entire cohort (ρ = −0.06, P = 0.64; Supporting Information Figure S1) or within each group (P > 0.05).
Adverse events
There were no adverse events or unintended effects in any study participants.
Discussion
In the present study, we found no differences in dietary adherence among lean individuals and two groups of individuals with obesity assigned different dietary prescriptions (WMEN vs. CR). Using PCA, we identified three independent factors of adherence, which also did not differ between groups, bolstering our null finding with the aggregate adherence score. In addition, hunger ratings were similar across all groups. Most importantly, adherence scores were not associated with hunger ratings in any group or with the amount of weight lost in the CR group.
A strength of the current study is that adherence was measured by combining multiple assessment components. Previous studies have generally focused on only one component of adherence such as self‐monitoring (10, 11, 24), attendance (9), or attrition (25, 26, 27) whereas we examined a total of 17 adherence variables, including attendance, food diaries, 24‐hour in‐person food recalls, EMA, 24‐hour computer food recalls, and 24‐hour phone food recalls. Moreover, because adherence is a multidimensional construct and because we assessed adherence in multiple ways, we were able to perform PCA identifying three independent factors, which can be broadly grouped as (1) outpatient/phone adherence, (2) in‐person adherence, and (3) attendance adherence. Although the results indicated that there were no significant differences between the three groups on total adherence (percentage) or the three independent factors of adherence, the PCA results support the notion that adherence is multidimensional. Furthermore, we demonstrated that the first PCA component, outpatient/phone adherence, accounted for the largest portion of variance in adherence scores. Thus, future studies should address this by weighting the assessment of these items to more accurately capture adherence.
Although adherence did not differ between the three groups, the CR group lost weight, as expected. Within this group, weight loss was not associated with level of adherence, contrary to other studies (7, 24). While interindividual levels of adherence may be important within a group prescribed calorie restriction (7, 9, 24), our findings indicated that it does not appear to be adiposity or calorie restriction per se that increases nonadherence. The evidence for this lies, in part, in the lack of difference in the hunger scores by group across time, indicating that nonadherence to modest calorie restriction was independent of subjective hunger. Thus, the nonadherence so commonly observed in weight reduction programs is not a “hunger” problem but is likely attributable to other factors that are also observed across various medical conditions. For example, poorer problem‐solving skills have been shown to mediate the relationship between treatment adherence and weight loss (28, 29), while neuroticism was the second strongest predictor of nonadherence to continuous positive airway pressure treatment for obstructive sleep apnea (30). Higher levels of neuroticism, lower levels of conscientiousness (31), and poor performance on an everyday problem‐solving questionnaire (32) have been associated with lower levels of medication adherence. Individuals with obesity who were considered frequent dieters (e.g., diet resistant) underreported their food intake by nearly 30% more than a comparison group with obesity who were not diet resistant (33).
This study has several limitations. First, this was a 6‐week study, enabling us to assess only short‐term outcomes. It is possible that one group may have become less adherent over time, which has been demonstrated in longer studies (7, 34). Second, all our dietary adherence measures were participant‐driven assessments. Lastly, the sample size was small, specifically for the CR group, which decreased our power to detect a relationship between adherence factors and weight loss. This study also has several strengths, including the novel use of multiple measurements completed during both outpatient and inpatient visits in combination with EMA, which measured daily adherence in real time. We had a high retention rate (87%) and provided volunteers with prepackaged meals and exact menus. Combined, these methods provided added accuracy for the overall measure of adherence.
Conclusion
In our study, we did not observe differences in dietary adherence between lean individuals and those with obesity who were provided a WMEN or CR diet. Although we observed weight loss in the CR group, overall hunger ratings did not differ by group and they were not associated with amount of weight lost. These findings have implications for reducing weight bias and social stigma often faced by individuals with obesity. The misperception that nonadherence (e.g., lack of willpower) is uniquely characteristic of individuals with obesity is untrue and it could be prejudicial. Rather, dietary adherence is similar among lean individuals, is not associated with increased adiposity, and is not associated with calorie reduction or hunger. Developing models to measure and predict adherence that are not influenced by weight bias is an important next step and is critical to the establishment of successful dietary interventions.
Funding agencies
This study was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Disclosure
The authors declared no conflict of interest.
Author contributions
EJS conducted research, analyzed data, and wrote the paper. SBV and CV provided advice and developed the dietary menus and protocols. BLM was responsible for patient care. PP and EJS analyzed data. SE provided significant advice and consultation regarding the implementation of the ecological momentary assessment protocol. MEG and JK designed the study protocol. MEG had study oversight and primary responsibility for the paper’s final content. All authors read and approved the final manuscript.
Clinical trial registration
ClinicalTrials.gov identifier NCT01862796.
Supporting information
Supplementary Material
Acknowledgments
The authors thank the volunteers who participated in the studies. They also thank the clinical staff of the Phoenix Epidemiology and Clinical Research Branch for conducting the examinations. The deidentified data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
References
- 1. Kruger J, Galuska DA, Serdula MK, Jones DA. Attempting to lose weight: specific practices among U.S. adults. Am J Prev Med 2004;26:402‐406. [DOI] [PubMed] [Google Scholar]
- 2. Fildes A, Charlton J, Rudisill C, Littlejohns P, Prevost AT, Gulliford MC. Probability of an obese person attaining normal body weight: cohort study using electronic health records. Am J Public Health 2015;105:e54‐e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2008:Cd000011. doi: 10.1002/14651858.CD000011.pub3 [DOI] [PubMed] [Google Scholar]
- 4. Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA 2002;288:2880‐2883. [DOI] [PubMed] [Google Scholar]
- 5. Lemstra M, Bird Y, Nwankwo C, Rogers M, Moraros J. Weight loss intervention adherence and factors promoting adherence: a meta‐analysis. Patient Prefer Adherence 2016;10:1547‐1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heymsfield SB, Harp JB, Reitman ML, et al. Why do obese patients not lose more weight when treated with low‐calorie diets? A mechanistic perspective. Am J Clin Nutr 2007;85:346‐354. [DOI] [PubMed] [Google Scholar]
- 7. Acharya SD, Elci OU, Sereika SM, et al. Adherence to a behavioral weight loss treatment program enhances weight loss and improvements in biomarkers. Patient Prefer Adherence 2009;3:151‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laitner MH, Minski SA, Perri MG. The role of self‐monitoring in the maintenance of weight loss success. Eat Behav 2016;21:193‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Compher CW, Hanlon A, Kang Y, Elkin L, Williams NN. Attendance at clinical visits predicts weight loss after gastric bypass surgery. Obes Surg 2012;22:927‐934. [DOI] [PubMed] [Google Scholar]
- 10. Krukowski RA, Harvey‐Berino J, Bursac Z, Ashikaga T, West DS. Patterns of success: online self‐monitoring in a web‐based behavioral weight control program. Health Psychol 2013;32:164‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peterson ND, Middleton KR, Nackers LM, Medina KE, Milsom VA, Perri MG. Dietary self‐monitoring and long‐term success with weight management. Obesity (Silver Spring) 2014;22:1962‐1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fitzpatrick SL, Bandeen‐Roche K, Stevens VJ, et al. Examining behavioral processes through which lifestyle interventions promote weight loss: results from PREMIER. Obesity (Silver Spring) 2014;22:1002‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davis C, Levitan RD, Muglia P, Bewell C, Kennedy JL. Decision‐making deficits and overeating: a risk model for obesity. Obes Res 2004;12:929‐935. [DOI] [PubMed] [Google Scholar]
- 14. Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol 2000;19:586‐592. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization . Energy and protein requirements: report of a joint FAO/WHO/UNU Expert Consultation. WHO;1985. [PubMed]
- 16. Garrel DR, Jobin N, de Jonge LH. Should we still use the Harris and Benedict equations? Nutr Clin Pract 1996;11:99‐103. [DOI] [PubMed] [Google Scholar]
- 17. Wilson PW, Paffenbarger RS Jr, Morris JN, Havlik RJ. Assessment methods for physical activity and physical fitness in population studies: report of a NHLBI workshop. Am Heart J 1986;111:1177‐1192. [DOI] [PubMed] [Google Scholar]
- 18. Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavorial medicine. Ann Behav Med 1994;16:199‐202. [Google Scholar]
- 19. Williamson DA, Anton SD, Han H, et al. Adherence is a multi‐dimensional construct in the POUNDS LOST trial. J Behav Med 2010;33:35‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jolliffe T. Principal Component Analysis. 2nd ed. Springer‐Verlag; 2002. [Google Scholar]
- 21. Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika 1965;30:179‐185. [DOI] [PubMed] [Google Scholar]
- 22. Kaiser HF. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958;23:187‐200. [Google Scholar]
- 23. Snitker S. Use of body fatness cutoff points. Mayo Clinic Proc 2010;85:1057. doi: 10.4065/mcp.2010.0583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burke LE, Conroy MB, Sereika SM, et al. The effect of electronic self‐monitoring on weight loss and dietary intake: a randomized behavioral weight loss trial. Obesity (Silver Spring) 2011;19:338‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Handjieva‐Darlenska T, Holst C, Grau K, et al. Clinical correlates of weight loss and attrition during a 10‐week dietary intervention study: results from the NUGENOB project. Obes Facts 2012;5:928‐936. [DOI] [PubMed] [Google Scholar]
- 26. Dalle Grave R, Calugi S, Compare A, et al. Weight loss expectations and attrition in treatment‐seeking obese women. Obes Facts 2015;8:311‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hadziabdic MO, Mucalo I, Hrabac P, Matic T, Rahelic D, Bozikov V. Factors predictive of drop‐out and weight loss success in weight management of obese patients. J Hum Nutr Diet 2015;28(suppl 2):24‐32. [DOI] [PubMed] [Google Scholar]
- 28. Murawski ME, Milsom VA, Ross KM, et al. Problem solving, treatment adherence, and weight‐loss outcome among women participating in lifestyle treatment for obesity. Eat Behav 2009;10:146‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem‐solving therapy in the long‐term management of obesity. J Consult Clin Psychol 2001;69:722‐726. [PubMed] [Google Scholar]
- 30. Moran AM, Everhart DE, Davis CE, Wuensch KL, Lee DO, Demaree HA. Personality correlates of adherence with continuous positive airway pressure (CPAP). Sleep Breath 2011;15:687‐694. [DOI] [PubMed] [Google Scholar]
- 31. Jerant A, Chapman B, Duberstein P, Robbins J, Franks P. Personality and medication non‐adherence among older adults enrolled in a six‐year trial. Br J Health Psychol 2011;16:151‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gelb SR, Shapiro RJ, Thornton WJ. Predicting medication adherence and employment status following kidney transplant: the relative utility of traditional and everyday cognitive approaches. Neuropsychology 2010;24:514‐526. [DOI] [PubMed] [Google Scholar]
- 33. Lichtman SW, Pisarska K, Berman ER, et al. Discrepancy between self‐reported and actual caloric intake and exercise in obese subjects. N Engl J Med 1992;327:1893‐1898. [DOI] [PubMed] [Google Scholar]
- 34. Zheng Y, Burke LE, Danford CA, Ewing LJ, Terry MA, Sereika SM. Patterns of self‐weighing behavior and weight change in a weight loss trial. Int J Obes (Lond) 2016;40:1392‐1396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


