Abstract
Purpose
To evaluate the efficacy of testosterone supplementation for improving aromatase inhibitor musculoskeletal symptoms (AIMSS).
Methods
Postmenopausal women experiencing moderate-to-severe arthralgias while taking adjuvant aromatase inhibitors for breast cancer were enrolled in this trial. Initially, patients were randomly allocated to receive either a subcutaneous testosterone pellet versus a placebo pellet. Due to slow accrual, the protocol was modified such that additional participants were randomized to receive either a topical testosterone gel, or a placebo gel. Changes in patientreported joint pain were compared between patients receiving testosterone and those receiving placebo using a two-sample t-test. Changes in hot flashes and other vasomotor symptoms were also analyzed. Further analyses were conducted to evaluate whether 27 single nucleotide polymorphisms (SNPs) in 14 genes previously associated with AIMSS were associated with testosterone supplementation benefit.
Results
While 64% of patients reported an improvement in joint pain at 3 months, there were no significant differences in average pain or joint stiffness at 3 or 6 months between testosterone and placebo arms. Patients receiving testosterone did report improvements in strength, lack of energy, urinary frequency, and stress incontinence (p<0.05). The subset of patients receiving subcutaneous testosterone also experienced improvements in hot flashes and mood swings. An inherited variant (rs7984870 CC genotype) in TNFSF11 was more likely to be associated with improvements in hot flashes in patients receiving testosterone.
Conclusion
The doses of testosterone supplementation used in this study did not significantly improve AIMSS.
Keywords: aromatase inhibitor musculoskeletal symptoms, testosterone, toxicity of endocrine therapy, hot flashes
INTRODUCTION
Aromatase inhibitors, effective agents for treating breast cancer [1, 2], can cause musculoskeletal symptoms (AIMSS) in up to 50% and vasomotor symptoms, including hot flashes and night sweats, in 20–60% of women [2–4] taking them, limiting treatment adherence and affecting life quality [5, 6]. There is a critical need to find new strategies to mitigate these side effects.
Patients with AIMSS typically experience symmetrical joint pains, often affecting hands, wrists and knees [5]. AIMSS generally start within 1–2 months after aromatase inhibitor initiation and peak at approximately 6 months [6].
Genetic differences may be associated with the likelihood of acquiring AIMSS and vasomotor symptoms. Initial genetic studies on patients with AIMSS reported that single nucleotide polymorphisms (SNPs) involved in drug metabolism and transport, including CYP19A1 (aromatase gene), ABCB1 (ATP-binding cassette sub-family B member 1-transporter gene) and ESR1 (estrogen receptor 1 gene), were associated with AIMSS [7–9]. More recently, a genomewide association study (GWAS) identified four linked SNPs in the TCL1A (T-Cell Leukemia/Lymphoma 1A) gene to be significantly associated with AIMSS [10]. SNPs in genes involved in bone regeneration and remodeling, namely TNFRSF11B (tumor necrosis factor [TNF] receptor superfamily member 11B, OPG) and TNFSF11 (TNF superfamily member 11, RANKL) have also been studied in association with AIMSS [11]. SNPs in hormone metabolizing genes, such as CYP1A1 and CYP19A1, have previously been associated with the likelihood of acquiring vasomotor symptoms [12–14], but a recent GWAS has identified SNPs in TACR3 (tachykinin receptor gene) to be significantly associated with vasomotor symptoms [12, 15]. Multicenter, randomized controlled trials have reported that antidepressants, such as venlafaxine, lessen vasomotor symptoms in breast cancer survivors [16]. However, at the time that the current trial protocol was written, there were no definitive randomized trial data reporting effective treatments for alleviating AIMSS. Estrogen replacement therapy has been shown to improve arthralgias and joint health in postmenopausal women [17], likely because hormonal changes associated with menopause contribute to the development of arthralgias [18]. Estrogen is thought to have pain-modulating effects through opioid pain fibers, and is important in maintaining a healthy synovium, which expresses estrogen receptors [19, 20]. Joint cartilage turnover and subsequent damage may accelerate in the absence of estrogen [21, 22]. Moreover, the balance between androgens and estrogen, mediated by the aromatase enzyme, appears to be pivotal in maintaining joint health [23]. Androgens, especially testosterone and dihydrotestosterone, appear to be important in countering the pro-inflammatory cytokines leading to joint pain and damage [23–26]. One cohort study reported that postmenopausal women with higher dehydroepiandrosterone-sulfate (DHEAS) concentrations, in particular, experienced fewer AIMSS [27]. As such, it has been hypothesized that testosterone supplementation may relieve AIMSS.
Testosterone supplementation for the treatment of AIMSS was suggested to be effective in a small, double-blind, phase II clinical trial [28], wherein ninety women on adjuvant anastrozole for breast cancer were randomized to one of three arms: placebo, 40 mg of oral testosterone undecanoate (TU), or 80 mg of TU. The higher dose of TU was associated with a significant improvement in pain scores without significant side effects. Pain reduction at 3 months was observed in all three arms of the study: 35% with placebo, 43% with testosterone 40 mg (p=0.06), and 70% with testosterone 80 mg (p=0.04). Testosterone levels stabilized within a physiologic range at 3 months. Importantly, participants receiving testosterone supplementation did not experience an elevation in estradiol levels. A subsequent study evaluated symptoms of hormonal depletion in women treated with combination subcutaneous pellets of testosterone 120 mg with anastrozole 8 mg. The combination implant provides continuous release of bioavailable testosterone as well as simultaneous release of low dose anastrozole, in an effort to prevent local aromatization of the testosterone. Women reported improved symptoms including arthralgias and hot flashes; testosterone concentrations were within a therapeutic range, and estradiol concentrations were not significantly increased [28, 29]. The results from these trials led to the conduct of the present trial, A221102, by the Alliance for Clinical Trials in Oncology (Alliance), to determine whether testosterone supplementation reduced AIMSS. Patient reports of vasomotor symptoms were also evaluated, as there were rumors, in the last century, that androgens decreased hot flashes in women.
METHODS
Eligibility Criteria
Postmenopausal women with estrogen and progesterone receptor-positive (>26% or Allred score ≥5, for both) primary breast cancers experiencing moderate-to-severe arthralgias (rated ≥5 in a 10-point scale, with higher scores reflecting greater pain) attributed to anastrozole or letrozole were trial eligible. Patients were required to have been receiving anastrozole or letrozole for ≥21 days prior to registration and to have plans to continue it throughout the duration of the study. Additional eligibility criteria included: body mass index (BMI) between 18 and 35 kg/m2; Eastern Cooperative Oncology Group (ECOG) performance status 0–2; and adequate laboratory parameters including hemoglobin, white blood cells, platelets, creatinine and AST.
Patients were not allowed on trial if they had residual or recurrent cancer, glucose intolerance, coronary artery disease or venous thromboembolism; were receiving any estrogen therapy, cyclosporine, anticoagulants, insulin, vitamin D doses > 4,000 IU/day, prolonged (>2 weeks) systemic corticosteroid treatment, concurrent chemotherapy or radiation therapy; or were receiving any other investigational agent.
Study Design and Oversight
The protocol was approved per US federal guidelines and patients provided IRB-approved informed written consent. Patients were randomly assigned at a 1:1 ratio to receive testosterone or placebo using the Pocock and Simon dynamic randomization procedure. Stratification factors included baseline pain score (5–6 versus 7–10) and age (<50 years versus 50–60 years versus > 60 years). The trial was monitored by the Alliance Data and Safety Monitoring Committee.
Interventions
At study entry, history, physical examination and laboratory tests were obtained. Study participants were asked to complete a Hot Flash Diary [30] daily for seven days prior to study treatment, and then daily for two months. Additional baseline and 6 follow-up monthly questionnaires were administered to study participants to assess several quality of life measures, including: 1) AI-induced arthralgia and associated joint symptoms [Modified Brief Pain Inventory for Aromatase Inhibitor Arthralgia (BPI-AIA) [31]. 2) mood [Profile of mood states (POMS)], [32] 3) libido [Menopause Specific Quality of Life Questionnaire (MENQOL)], [33] and 4) hot flashes [Hot Flash Diary and Hot Flash Related Daily Interference Scale (HFRDIS)] [30]. Potential testosterone-associated toxicities were evaluated using a symptom experience questionnaire. Adverse events were assessed at baseline and once monthly until the end of the study, 6 months after enrollment.
After baseline questionnaire completion, the initial participants were randomly allocated to receive two surgically implanted pellets containing either a combination of testosterone 120 mg and anastrozole 8 mg or a placebo. Treatment assignments were blinded to both the patient and medical professional. As such, after patient randomization, registration personnel assigned the patient a pellet from the Alliance Research Base Pharmacy, which was marked with a deidentified kit number and the label of “testosterone OR placebo.” Pellets were to be implanted at two time points: at the end of the first week on-study (following completion of the hot flash baseline week ascertainment), and 3 months later.
With this design, study accrual was slow, which was attributed to the need for a minor surgical procedure with the subcutaneous pellet preparation. In response, the protocol was amended on January 15, 2016, to change the route of delivery from subcutaneous pellet implants to a topical application of a gel containing either testosterone 10.4 mg (without anastrozole), or placebo. The gel was applied to the skin once daily for 6 months utilizing an AccuPen Dispensing Device for accuracy of dosing. After completion of the 6-month active trial period, patients could choose to continue being followed for an additional 6-month observation period.
Pharmacogenetic studies
All patients on study had DNA samples genotyped for 27 SNPs in 14 genes which have been associated with aromatase inhibitors and testosterone biotransformation including ABCB1, CYP11A1, CYP17A1, CYP19A1, CYP27B1, ESR1, ESR2, MAP4K4, TCL1A, TNFRSF11B (OPG), TNFSF11 (RANKL), TRAM2-ASI, TUBB1, and VDR. Replicates of the samples (n=10) and 3 hapmap CEU samples in triplicates (n=9) served as controls for genotyping. The genotyping was performed on the sequenom platform in the Genotyping Core of the Mayo Clinic Medical Genome Facility (MGF).
Analysis
Patient baseline characteristics were summarized by mean (standard deviation) or median (range) for continuous variables and frequency (percentage) for categorical variables.
Primary Endpoint
The primary endpoint of the study was the intra-patient change in joint pain at 3 months from baseline, as measured by the participant’s average pain on a scale from 0–10 (with 10 reflecting the worst amount of pain) on the modified BPI-AIA (item #3). If a patient was missing their baseline BPI-AIA pain score, their on-study pain score was used, instead. If the patient was missing their month 3 pain score, that pain score was imputed using their last reported value. The two-sample, two-sided t-test with unequal variances was applied for comparison of the changes of joint pain at 3 months between testosterone and placebo arms. Based on results from the ART 2 trial, the standard deviations for change from baseline were estimated as 2.9 for the placebo arm and 1.9 for the testosterone arm. An absolute difference of joint pain between placebo and testosterone arms of 1.0 point was considered to be a clinically meaningful effect size. Based on the primary analysis, using a two-sample t-test, it was estimated that a total sample size of 194 patients (97 per arm) would provide 80% power to detect the effect size at the 5% significance level. This sample size was inflated to a total of 224 patients (112 patients per arm) to account for 15% non-evaluable patients due to ineligibility, cancel or major violations.
In addition, item #3 of the BPI-AIA was also utilized to evaluate three additional points. 1) The proportion of women with improvement in joint pain, measured by a reduction in pain of at least 10% at 3 months compared to baseline, measured by the Fisher’s exact test; 2) The intra-patient change in joint pain at 6 months from baseline (measured by the two sample t-test, as above); and 3) the intra-patient changes in joint pain at each month from baseline, measured by the repeated-measures analysis of variance (RM-ANOVA) model.
Secondary Endpoints
The secondary endpoints were pre-specified, and were evaluated by additional items on the BPI-AIA, including the monthly change from baseline of worst pain, least pain, current pain, stiffness, and interference in activities. All of these analyses were completed using the RM-ANOVA model. Furthermore, the changes in hot flashes from baseline to the first two months were evaluated by the Hot Flash Diary. The area-under-the-curve of hot flash score and frequency were compared by two-sample t-tests between testosterone and placebo arms. The monthly change of libido and menopause-specific quality of life were measured by the MENQOL and POMS monthly. The RM-ANOVA model was used to compare monthly changes from baseline for these measures.
The safety and tolerability of testosterone were measured using the CTCAE 4.0 criteria, in addition to questionnaires inquiring about self-reports of alopecia, acne, and hirsutism. Descriptive statistics and statistical plots, including frequency and histogram, were used to summarize safety and tolerability data.
Genotype and allele frequencies were calculated, and then SNP frequencies were compared to that of MA.27 control samples, a prior study which included women on aromatase inhibitors, some of whom did and some of whom did not experience any AIMSS [10]. Two-sample, two-sided t-tests were used for this comparison. Fisher’s exact tests were used to assess the associations between genetic markers and having any improvement on the testosterone arm. Adjustments for multiple testing were done using the method of Benjamini-Hochberg [34].
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. All analyses were based on the study database frozen on 1/23/2018. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson, following Alliance policies.
RESULTS
Baseline characteristics
This study accrued 227 patients between September 10, 2013 and November 29, 2017 (55 patients prior to the January 15, 2016 amendment; 172 thereafter) from 71 sites. Nineteen patients canceled (Figure 1). Baseline patient characteristics (Table 1) were relatively well balanced between arms.
Figure 1.
CONSORT diagram.
Table 1.
Baseline Patient Characteristics
| Placebo (N=104) | Testosterone (N=104) | |
|---|---|---|
| Age years [mean (SD)] | 60.1 (9.7) | 59.9 (8.7) |
|
Race
White |
94 (90%) |
100 (96%) |
| Black or African American | 4 (4%) | 2 (2%) |
| Asian | 3 (3%) | 2 (2%) |
| Not reported or unknown | 3 (3%) | 0 (0%) |
|
Ethnicity
Hispanic |
7 (7%) |
9 (9%) |
| Non-Hispanic | 96 (92%) | 93 (89%) |
| Not reported or unknown | 1 (1%) | 2 (1%) |
|
AI Duration
<6 months |
27 (26%) |
14 (14%) |
| 6–12 months | 24 (23%) | 26 (25%) |
| >12 months Missing |
52 (50%) 1 | 63 (61%) 1 |
|
Baseline weight [mean (SD)] Weight in Kg |
71.3 (12.2) |
75.5 (12.8) |
| BMI1 | 26.8 (4.2) | 28.2 (4.2) |
|
ECOG PS2
0 |
81 (78%) |
81 (78%) |
| 1 | 23 (22%) | 20 (19%) |
| 2 | 0 (0%) | 3 (3%) |
| Baseline Average Pain Score [mean (SD)] | 5.5 (1.8) | 5.4 (1.7) |
Body Mass Index 2
Eastern Cooperative Oncology Group Performance Status
Baseline symptomatology and quality of life measures at study entry were balanced between the two arms, with the exception of patients assigned to placebo being more significantly bothered by breast tenderness, dissatisfaction with their personal life, feeling depressed or anxious, and by changes in skin appearance, texture or tone (Supplementary Table S2). The majority of participants (79%) had been on an AI for 6 or more months at study entry, with no substantial differences in AI duration between the study arms.
Patients remained on study an average of 23.2 weeks (range 2.0–45.9 weeks; includes additional observation period) with no differences between the testosterone and placebo arms (mean 24.0 and 22.4 weeks, respectively, p=0.19).
Average pain over time
Per the BPI-AIA questionnaire, there were no significant differences between the two arms in the average joint pain at 3 months compared to baseline (Figure 2. Pain scores were reduced by a mean (95% confidence interval (CI)) of 2.0 (1.5,2.5) points on testosterone compared to a mean reduction of 1.9 (1.3,2.4) points on placebo (p=0.50). Sixty-four percent of patients reported an improvement of at least one point in BPI-AIA average pain at 3 months, but this was not significantly different between arms. There was also no difference between the study arms in BPI average pain from baseline to month 6: an average (±SD) pain decrease of −1.9±2.2 was reported in the testosterone arm compared −2.2±2.7 in the placebo arm (p=0.67). Patients on testosterone had slightly more pain reduction at 1 month, but there were no significant differences at any other time.
Figure 2.
Mean percent change from baseline in BPI average pain scores over time.
Using repeated measures models, the BPI-AIA average pain score was significantly lower each month, from baseline (p < 0.01), independent of treatment arm, age, race or AI duration. There were no significant differences in the changes from baseline in the scores of any other BPI-AIA questions.
Hot flashes and quality of life
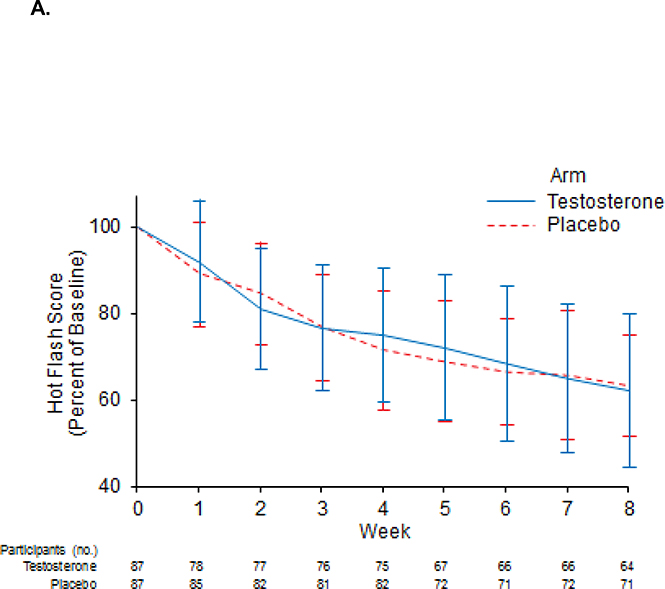
Hot flash scores were not significantly different between arms (Figure 3A), nor were there significant differences between hot flash frequencies. Participants who received testosterone reported more favorable relations with others compared to placebo with a mean (CI) change from baseline to month 3 of −1.3 (−1.8,−0.8) for patients on testosterone compared to −0.3 (0.9,0.3) for patients on placebo, p=0.05. Otherwise, there were no significant differences in the mean HFRDIS score at 3 months, or in any of its items evaluating the impact of hot flashes in quality of life.
Figure 3.
Mean percent of baseline hot flash score for the entire treatment group (A) and the subset of patients who received the subcutaneous testosterone preparation (B).
Regarding MENQOL data at 3 months (Supplemental Table S3), testosterone patients had significantly more improvement in ‘Decrease in Physical Strength’ and ‘Lack of Energy’. There were no significant differences on any of the items evaluated in the POMS instrument.
Testosterone toxicity evaluation
There were no significant differences between treatment arms in any of the following symptoms at 3 or 6 months, on the Symptom Experience Diary: stomach pain or cramps, nausea, diarrhea, dizziness, decrease in appetite, abnormal sweating, trouble sleeping, mood swings, trouble concentrating, hot flashes, deepening of voice, unwanted weight gain, acne, hand or feet swelling, or undesirable hair growth. Patients on testosterone did report less fatigue at 6 months, with a mean (±SD) fatigue score of 5.4±2.6 in the testosterone arm, as compared with a mean fatigue score of 6.1±2.7 with placebo (lower scores representing less fatigue; p = 0.04).
Genetic polymorphisms, distribution of variants and genetic associations
A total of 191 out of 207 patients on study had DNA samples genotyped. The 191 DNA samples genotyped comprised 178 Caucasians, 6 African-Americans, 4 Asians and 3 with unknown race. The distribution of genotypes, allele frequencies, and frequency comparisons for the Caucasian patients on the study is shown in Supplementary Table S1–A. Except for 5 SNPs, all other SNPs genotyped were in Hardy Weinberg Equilibrium (HWE) [Table S1–A].
When SNP frequencies in this study were compared to that for Caucasian control samples from the MA.27 AIMSS study, 4 SNPs [namely rs11632698 and rs900798 (CYP11A1), rs2073618 (TNFRSF11B) and rs7984870 (TNFSF11)] showed significant association with AIMSS (unadjusted P <0.05; Supplementary Table S1–B). The two CYP11A1 SNPs did not show any association after adjusting for multiple comparisons. The GG genotypes of TNFRSF11B rs2073618 and TNFSF11 rs7984870 remained significantly associated with AIMSS after adjusting for multiple comparisons (P<0.01 for both). The allele frequency for TCL1A rs11849538 SNP was increased compared to the MA.27 control but its association with AIMSS was marginal (unadjusted P value for GG genotype =0.08, Table S1–B).
In testosterone-treated patients on study (Table 2), the TNFSF11 rs7984870 CC genotype was associated with greater reduction in hot flash frequency than the CG+GG genotype (p=0.04).
Table 2:
TNFSF11 (RANKL)1 rs7984870 SNP: Association with Improvement in Study Endpoints for Patients on Testosterone.
| Event | Genotype (N) | N Events (% Between Allele Groups) | Odds Ratio (95% CI) | Fisher’s Exact Pvalue |
|---|---|---|---|---|
| Any Hot Flash Frequency Reduction From Baseline to Week 8 | CC (14) GC+GG (78) |
4 (9.1) 40 (90.9) |
3.53 (0.88–14.12) Reference2 |
0.08 |
| Any Hot Flash Frequency Reduction From Baseline to Week 8 | CC (16) GC+GG (80) |
4 (8.7) 42 (91.3) |
4.32 (1.12–16.70) Reference |
0.044
|
| Any Hot Flash Score Reduction From Baseline to Week 8 | CC (16) GC+GG (80) |
5 (10.2) 44 (89.8) |
3.52 (0.94–13.22) Reference3 |
0.07 |
| Any Joint Pain Reduction From Baseline to Month 3 | CC (16) GC+GG (80) |
7 (12.3) 50 (87.7) |
2.86 (0.83–9.81) Reference3 |
0.10 |
A total of 104 patients were treated with testosterone and 8 of them did not have any genetic information.
TNFSF11( RANKL): oesteoprotegerin ligand and a member of the tumor necrosis factor (TNF) superfamily 11.
Caucasian samples alone
All patients on testosterone
association reaching statistical significance, p<,0.05
Subset analysis of patients who received subcutaneous treatment
A subset analysis was performed, evaluating the 55 patients enrolled on the study prior to the January 15, 2016, amendment, who were randomized to receive surgically implanted pellets containing either testosterone/anastrozole or placebo. Of these, 4 patients cancelled (2 on testosterone and 2 on placebo), leaving 51 patients who started subcutaneous treatment (25 on testosterone and 26 on placebo).
The median age of these patients was 59±7.1 years. Baseline patient characteristics were well balanced between the two arms, with the exception that patients assigned to subcutaneous placebo reported more backache (p=0.05).
While patients on subcutaneous testosterone/anastrozole had more reduction in the BPI-AIA average pain scores during the first month (p=0.04), there were no significant differences in the subsequent months (Supplementary Figure S2). Patients on subcutaneous testosterone had significantly more reduction in the percent of baseline hot flash frequency and score after 8 weeks (Figure 3B). Patients on subcutaneous testosterone/anastrozole also reported significantly less nausea (p=0.02), fatigue (p=0.04), mood swings (p=0.03), hand/feet swelling (p=0.01), stress urinary incontinence (p=0.04) and changes in appearance, texture or tone of their skin (p=0.01), than did patients on subcutaneous placebo (Table 3).
Table 3.
Endpoint Comparisons for Subcutaneous Placebo vs Subcutaneous Testosterone (Means and 95% Confidence Intervals)
| Endpoint | Placebo (N=26) | Testosterone (N=25) | p-value |
|---|---|---|---|
| Change in BPI-AIA Average Pain Score from Baseline to Month 1 | −0.8 (−1.6,−0.1) | −2.0 (−2.8,−1.2) | 0.04 |
| Percent of Baseline Hot Flash Frequency at Week 8 | 59.9% (44%,76%) | 35.8% (20%,52%) | 0.03 |
| Percent of Baseline Hot Flash Scores at Week 8 | 48.1% (35%,61%) | 28.1% (15%,41%) | 0.03 |
| Maximum SED Nausea at Month 3 | 3.0 (1.8,4.1) | 1.1 (0.5,1.7) | 0.02 |
| Maximum SED Fatigue at Month 3 | 7.0 (5.9,8.0) | 5.3 (4.2,6.5) | 0.04 |
| Maximum SED Mood Swings at Month 3 | 5.4 (4.2,6.7) | 3.5 (2.3,4.7) | 0.03 |
| Maximum SED Hand or Feet Swelling at Month 3 | 4.2 (2.9,5.5) | 1.7 (0.8,2.6) | 0.01 |
| MENQOL Incidence of Stress Urinary Incontinence at Month 3 | 42% (22%,63%) | 10% (1%,32%) | 0.04 |
| MENQOL Incidence of Changes in Appearance, Texture or Tone of Skin at Month 3 |
46% (26%,67%) | 10% (1%,29%) | 0.01 |
BPI-AIA = Modified Brief Pain Inventory for Aromatase Inhibitor Arthralgia
SED = Symptom Experience Diary
MENQOL = Menopause Specific Quality of Life Questionnaire
DISCUSSION
In contrast to the prior double blind, phase II clinical trial, which suggested testosterone undecanoate may improve AIMSS, in the current study testosterone supplementation did not improve the average pain or joint stiffness, compared to placebo [28]. The discrepancy between the prior phase II and the current trial may be partly explained by the differences in the testosterone preparations between the two studies. The current trial did not evaluate systemic testosterone or DHEAS concentrations of participants (for instance, in patients who received the subcutaneous versus topical testosterone preparations, or versus the testosterone administered in the previous phase II trial), which may explain some of the differences observed. The topical preparation may have led to lower systemic androgen exposure and decreased anti-arthralgia efficacy. The potential need for higher systemic doses is supported by the fact that the 80-mg daily dose in the prior phase II study appeared to be superior to the placebo, but the 40-mg daily dose only achieved borderline statistical significance. Also, the improvement in the mean BPI pain score at month one in the subcutaneous implant group may be explained by the higher levels of testosterone released earlier in the implant cycle.
Data from the current study, which demonstrated that protocol patients experienced a consistent improvement in pain every month independently of treatment assignment, confirms a phenomenon observed in recently reported studies, in which patients in both placebo and intervention arms reported AIMSS improvement over time.[35–37]
Interestingly, the subset analysis of patients receiving subcutaneous testosterone/anastrozole in the current trial reported improvements in hot flashes, as well as several other menopausal symptoms including fatigue, mood swings, urinary incontinence, and skin appearance, tone, and texture. These results suggest that subcutaneous testosterone may have a potential benefit in the relief of hormone deficiency symptoms. Testosterone’s therapeutic effect is dose dependent [38, 39], and an observational study using higher doses of subcutaneous testosterone (169 mg + 32 mg) in breast cancer survivors reported significant improvements in psychological, somatic, and urogenital symptoms [40].
The improvements in untoward urogenital symptoms (polyuria, stress incontinence and vaginal dryness) in the patients who received testosterone are consistent with data from a small randomized trial of vaginal testosterone to treat vaginal dryness [41], and data from studies on vaginal DHEA, a testosterone precursor, which was also shown to help vaginal symptoms [42, 43].
The previously described association between TCL1A rs11849538 SNP and AIMSS [10] was not replicated in the present study; however, a marginal trend for an association with AIMSS was observed in Caucasian patients, which disappeared after adjustment for multiple comparisons. Our observation, that the GG genotypes for both TNFSF11 rs7984870 and TNFRSF11B rs2073618 are significantly associated with increase in AIMSS in our study of Caucasian patients conflicts with an Asian cohort study [11] that reported that the GG genotypes of rs7984870 and rs2073618 are protective for AIMSS, thus making the CC genotype the at risk genotype for AIMSS in the Asian study. In another study with a mostly Caucasian AI-treated patient cohort [44] a nominal protective association was observed for the TNFSF11 rs7984870 GG genotype with AIMSS but no association was seen for the TNFRSF11B rs2073618 SNP. However, our observation for TNFRSF11B rs2073618 GG genotype is in agreement with an European study [45] where carriers of the CG and GG genotypes of TNFRSF11B rs2073618 exhibited increased risk of AIMSS. Finally, after testosterone supplementation for AIMSS patients, those carrying the TNFSF11 rs7984870 CC genotype showed improvement in hot flashes reduction. These results warrant further studies with a larger set of patients to validate the subset of patients who might benefit from testosterone supplementation for hot flashes.
Since the time that the current trial was developed, other therapies have shown promise in alleviating the prominent clinical problem of AIMSS. Exercise was shown to decrease AIMSS pain scores when compared with usual care in a randomized controlled trial [46]. Zoledronic acid appeared to improve AIMSS in a nonrandomized pilot study, but no subsequent trial has been done to confirm this finding [47]. A third trial, evaluating omega-3 fatty acids versus placebo, did not find benefit in all protocol patients, but suggested benefit over placebo in the subset of women with a body mass index over 30 [36, 48]. Even more encouragingly, the favorable results of pilot studies evaluating acupuncture and duloxetine led to definitive placebo-controlled clinical trials, which have established these two strategies as efficacious treatments for AIMSS [37, 49].
Supplementary Material
Acknowledgments
Support: The research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), UG1CA232760, U24CA196171, UG1CA189858, UG1CA189997, U10CA180820 and UG1CA189861 (ECOG-ACRIN), U10CA180868 (NRG), and U24CA196171, as well as the Alliance biorepository resource.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The United States NCI provided funding for this trial.
The authors have no financial relationship with any private company regarding this research with the exception that Dr Birrell reports personal fees from Havah Therapeutics, that is developing androgen-based therapies for women, and has a patent AU2005905768A0; additionally, Dr Glaser has a patent (US 10,071,104 B2) related to this topic.
Charles Loprinzi, with Mayo statistical colleagues, has full control of all primary data and agrees to allow the journal to review the data, if requested.
Footnotes
Clinical Relevance: This study evaluates whether testosterone therapy improves the widespread clinical problem of aromatase inhibitor musculoskeletal symptoms.
ClinicalTrials.gov Identifier: NCT01573442
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Baum M, et al. , Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet, 2002. 359(9324): p. 2131–9. [DOI] [PubMed] [Google Scholar]
- 2.Howell A, et al. , Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet, 2005. 365(9453): p. 60–2. [DOI] [PubMed] [Google Scholar]
- 3.Harris PF, et al. , Prevalence and treatment of menopausal symptoms among breast cancer survivors. J Pain Symptom Manage, 2002. 23(6): p. 501–9. [DOI] [PubMed] [Google Scholar]
- 4.Kligman L and Younus J, Management of hot flashes in women with breast cancer. Curr Oncol, 2010. 17(1): p. 81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crew KD, et al. , Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. Journal of Clinical Oncology, 2007. 25(25): p. 3877–3883. [DOI] [PubMed] [Google Scholar]
- 6.Henry NL, et al. , Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast cancer research and treatment, 2008. 111(2): p. 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry NL, et al. , Genetic associations with toxicity-related discontinuation of aromatase inhibitor therapy for breast cancer. Breast Cancer Res Treat, 2013. 138(3): p. 807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gervasini G, et al. , Polymorphisms in ABCB1 and CYP19A1 genes affect anastrozole plasma concentrations and clinical outcomes in postmenopausal breast cancer patients. Br J Clin Pharmacol, 2017. 83(3): p. 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leyland-Jones B, et al. , ESR1 and ESR2 polymorphisms in the BIG 1–98 trial comparing adjuvant letrozole versus tamoxifen or their sequence for early breast cancer. Breast Cancer Res Treat, 2015. 154(3): p. 543–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingle JN, et al. , Genome-wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitors. J Clin Oncol, 2010. 28(31): p. 4674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, et al. , RANKL and OPG Polymorphisms Are Associated with Aromatase Inhibitor-Related Musculoskeletal Adverse Events in Chinese Han Breast Cancer Patients. PLoS One, 2015. 10(7): p. e0133964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crandall CJ, Crawford SL, and Gold EB, Vasomotor symptom prevalence is associated with polymorphisms in sex steroid-metabolizing enzymes and receptors. Am J Med, 2006. 119(9 Suppl 1): p. S52–60. [DOI] [PubMed] [Google Scholar]
- 13.Fontein DYB, Houtsma D, and Nortier JW, Germline variants in the CYP19A1 gene are related to specific adverse events in aromatase inhibitor users: a substudy of Dutch patients in the TEAM trial. Breast Cancer Res Treat, 2014. 144 (3): p. 599–606. [DOI] [PubMed] [Google Scholar]
- 14.H., J., Gray KP, and Pagani O, Impact of CYP19A1 and ESR1 variants on early-onset side effects during combined endocrine therapy in the TEXT trial.. Breast Cancer Res, 2016. 18(1): p. 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crandall C, et al. , Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the women’s health initiative study. Menopause, 2017. 24: p. 252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bordeleau L, et al. , Multicenter, randomized, cross-over clinical trial of venlafaxine versus gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol, 2010. 28(35): p. 5147–52. [DOI] [PubMed] [Google Scholar]
- 17.Cirillo DJ, et al. , Effect of hormone therapy on risk of hip and knee joint replacement in the Women’s Health Initiative. Arthritis & Rheumatology, 2006. 54(10): p. 3194–3204. [DOI] [PubMed] [Google Scholar]
- 18.Neugarten BL and Kraines RJ, “ Menopausal Symptoms” in Women of Various Ages. Psychosomatic Medicine, 1965. 27(3): p. 266–273. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich W, et al. , Estrogen receptor-β is the predominant estrogen receptor subtype in normal human synovia. Journal of the Society for Gynecologic Investigation, 2006. 13(7): p. 512–517. [DOI] [PubMed] [Google Scholar]
- 20.Dawson-Basoa M and Gintzler AR, Involvement of spinal cord delta opiate receptors in the antinociception of gestation and its hormonal simulation. Brain Res, 1997. 757(1): p. 37–42. [DOI] [PubMed] [Google Scholar]
- 21.Sniekers Y, et al. , Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment–a systematic approach. Osteoarthritis and cartilage, 2008. 16(5): p. 533–541. [DOI] [PubMed] [Google Scholar]
- 22.Oestergaard S, et al. , Effects of ovariectomy and estrogen therapy on type II collagen degradation and structural integrity of articular cartilage in rats: implications of the time of initiation. Arthritis & Rheumatology, 2006. 54(8): p. 2441–2451. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt M, et al. , Inflammation and sex hormone metabolism. Ann N Y Acad Sci, 2006. 1069: p. 236–46. [DOI] [PubMed] [Google Scholar]
- 24.Islander U, et al. , Estrogens in rheumatoid arthritis; the immune system and bone. Molecular and cellular endocrinology, 2011. 335(1): p. 14–29. [DOI] [PubMed] [Google Scholar]
- 25.Cutolo M, et al. , Synovial fluid estrogens in rheumatoid arthritis. Autoimmunity reviews, 2004. 3(3): p. 193–198. [DOI] [PubMed] [Google Scholar]
- 26.Cutolo M, et al. , Anti-TNF and Sex Hormones. Annals of the New York Academy of Sciences, 2006. 1069(1): p. 391–400. [DOI] [PubMed] [Google Scholar]
- 27.Gallicchio L, et al. , Androgens and musculoskeletal symptoms among breast cancer patients on aromatase inhibitor therapy. Breast Cancer Res Treat, 2011. 130(2): p. 56977. [DOI] [PubMed] [Google Scholar]
- 28.Birrell S and Tilley W, Testosterone Undecanoate Treatment Reduces Joint Morbidities Induced by Anastrozole Therapy in Postmenopausal Women with Breast Cancer: Results of a Double-Blind, Randomized Phase II Trial. 2009, AACR. [Google Scholar]
- 29.Glaser RL, Subcutaneous testosterone-anastrozole implant therapy in breast cancer survivors. ASCO Breast Cancer Conference September 2010: p. Abstract # 221. [Google Scholar]
- 30.Carpenter JS, The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. Journal of pain and symptom management, 2001. 22(6): p. 979–989. [DOI] [PubMed] [Google Scholar]
- 31.Bauml J, et al. , Arthralgia among women taking aromatase inhibitors: is there a shared inflammatory mechanism with co-morbid fatigue and insomnia? Breast Cancer Research, 2015. 17(1): p. 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biehl B and Landauer A, Das Profile of Mood States (POMS). Mannheim (Unveröffentlichtes Manuskript), 1975. [Google Scholar]
- 33.Hilditch JR, et al. , A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas, 1996. 24(6): p. 161–175. [DOI] [PubMed] [Google Scholar]
- 34.Benjamini Y and Hochberg Y, Controlling the false discovery rate: a practical and powerful approach to multiple hypothesis testing. J R Stat SocB, 1995. 57: p. 289–300. [Google Scholar]
- 35.Henry NL, et al. , Randomized, Multicenter, Placebo-Controlled Clinical Trial of Duloxetine Versus Placebo for Aromatase Inhibitor-Associated Arthralgias in Early-Stage Breast Cancer: SWOG S1202. J Clin Oncol, 2018. 36(4): p. 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hershman DL, et al. , Randomized Multicenter Placebo-Controlled Trial of Omega-3 Fatty Acids for the Control of Aromatase Inhibitor-Induced Musculoskeletal Pain: SWOG S0927. J Clin Oncol, 2015. 33(17): p. 1910–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hershman DL, et al. , Randomized Blinded Sham- and Waitlist-Controlled Trial of Acupuncture for Joint Symptoms Related to Aromatase Inhibitors in Women with Early Stage Breast Cancer (SWOG 1200). Oral Presentation at: San Antonio Breast Cancer Symposium December 2017. [Google Scholar]
- 38.Huang G, et al. , Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause, 2014. 21(6): p. 612–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glaser R, York AE, and Dimitrakakis C, Beneficial effects of testosterone therapy in women measured by the validated Menopause Rating Scale (MRS). Maturitas, 2011. 68(4): p. 355–61. [DOI] [PubMed] [Google Scholar]
- 40.Glaser RL, York AE, and Dimitrakakis C, Efficacy of subcutaneous testosterone on menopausal symptoms in breast cancer survivors. J Clin Oncol, 2014(32(Suppl 2)): p. 109. [Google Scholar]
- 41.Melisko ME, et al. , Vaginal Testosterone Cream vs Estradiol Vaginal Ring for Vaginal Dryness or Decreased Libido in Women Receiving Aromatase Inhibitors for Early-Stage Breast Cancer: A Randomized Clinical Trial. JAMA Oncol, 2017. 3(3): p. 313–319. [DOI] [PubMed] [Google Scholar]
- 42.Labrie F, et al. , Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause, 2018. 25(11): p. 1339–1353. [DOI] [PubMed] [Google Scholar]
- 43.Barton DL, et al. , Systemic and local effects of vaginal dehydroepiandrosterone (DHEA): NCCTG N10C1 (Alliance). Support Care Cancer, 2018. 26(4): p. 1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dempsey JM, et al. , Attempted replication of SNPs in RANKL and OPG with musculoskeletal adverse events during aromatase inhibitor treatment for breast cancer. Physiol Genomics, 2018. 50(2): p. 98–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lintermans A and Neven P, Safety of aromatase inhibitor therapy in breast cancer. Expert Opin Drug Saf, 2015. 14(8): p. 1201–11. [DOI] [PubMed] [Google Scholar]
- 46.Irwin ML, et al. , Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol, 2015. 33(10): p. 1104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santa-Maria CA, et al. , A phase II study evaluating the efficacy of zoledronic acid in prevention of aromatase inhibitor-associated musculoskeletal symptoms: the ZAP trial. Breast Cancer Res Treat, 2018. 171(1): p. 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen S, et al. , Omega-3 fatty acid use for obese breast cancer patients with aromatase inhibitor-related arthralgia (SWOG S0927). Breast Cancer Res Treat, 2018. 172(3): p. 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith EM, et al. , Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA, 2013. 309(13): p. 1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.