Abstract
Objective
To examine the association between weight change from young adulthood to midlife and risk of incident arthritis.
Methods
Using data from the National Health and Nutrition Examination Survey (NHANES), we categorized participants into weight change categories based on their recalled weight during young adulthood and midlife. We estimated the association of weight change and developing an arthritis condition over 10 years using adjusted Cox models. Findings were extrapolated to the US population to determine the proportion of incident arthritis cases that could be averted if the entire population maintained a normal BMI in young adulthood and midlife.
Results
Among our sample of adults who were 40–69 years old at their midlife weight measure (n=13,669), 3,603 developed an arthritis condition. Compared with adults who maintained a normal-normal BMI, the normal-overweight, normal-obese, overweight-obese, and obese-obese groups had significantly elevated risk of incident arthritis conditions. The obese-overweight group had lower risk of incident arthritis conditions compared with the obese-obese group and comparable risk to the overweight-overweight group. Nearly one quarter of incident arthritis cases, corresponding to 2.7 million individuals, would have been averted under the hypothetical scenario where all individuals maintained normal weight from young adulthood to midlife.
Conclusion
Weight loss from young adulthood to midlife was associated with substantially reduced risk of developing an arthritis condition. We found no evidence of residual risk from having been heavier earlier in life. Our findings highlight the critical need to expand obesity treatment and prevention to achieve meaningful reductions in the burden of arthritis.
Collectively, rheumatic and musculoskeletal diseases (RMDs) result in substantial disability and diminished quality of life. Their prevalence has also been increasing, particularly for osteoarthritis,1 the most common form of arthritis.2 As of 2015, joint pain and arthropathies were the most common diagnosis for ambulatory care visits,3 and osteoarthritis was the second most common reason for non-pregnancy/non-neonatal hospitalization in the United States (US).4
The prevalence of obesity has also increased dramatically from approximately 15% of US adults in 1980 to 40% in 2016.5,6 Obesity is associated with an increased risk of developing RMDs, including osteoarthritis,7,8 rheumatoid arthritis,9,10 gout,11,12 and psoriatic arthritis.13,14 For inflammatory arthritis diseases, this relationship is likely related to adipose-derived inflammation.14,15 The obesity-osteoarthritis association may result from both increased joint loading and low-grade inflammation.14–18 Weight gain increases the risk of arthritis broadly,19 including osteoarthritis-related hip and knee replacement20,21 and gout.12 Additionally, prior studies have shown weight loss is associated with reduced risk of osteoarthritis22,23 and gout.12
In addition to increases in the overall prevalence of obesity, recent US birth cohorts are becoming obese earlier in life and, thus, spending greater portions of their lives with excess weight. The effects of these weight shifts on the risk of arthritis are largely unknown. Using data from the Nurses’ Health Study, one recent study estimated that weight gain from early to mid-adulthood of 2.5–10 kg, 10–20 kg, or more than 20 kg was associated with a 20%, 31%, and 40% increase in the likelihood of osteoarthritis-related total hip replacement.20 However, the study did not investigate the risk of other types of arthritis or the effects of weight loss. If the effects of obesity on arthritis conditions are cumulative, those who lose weight may experience residual risk due to irreversible pathologic processes from carrying excess weight earlier in life.
Additionally, although some studies have demonstrated how weight change modifies risk of arthritis conditions at the individual-level,12,19–21,23,24 the aggregate effect of population-level weight loss or obesity prevention remains uncertain. Hence, additional empirical estimates derived from nationally representative data sources are needed to assess the population-level effect of weight change across the life-course on arthritis risk.
Our study uses a novel application of the National Health and Nutrition Examination Survey (NHANES) data to test two hypotheses about the association between weight change from young adulthood to midlife and risk of incident arthritis. First, we hypothesize that individuals who lose weight are at a reduced risk of developing arthritis conditions relative to individuals who maintain a stable overweight or obese body mass index (BMI) (“risk reduction” hypothesis). Second, we hypothesize that individuals who are overweight or obese in young adulthood and lose weight are at a greater risk of arthritis conditions relative to individuals who started at a lower weight and maintained that weight (“residual risk” hypothesis). After testing these hypotheses, we extrapolate our findings to the population-level, estimating the percentage of incident arthritis cases that could be averted under hypothetical scenarios related to weight loss and comprehensive prevention of overweight/obesity across the life-course.
Materials and Methods
Design
The NHANES is a nationally representative survey of US adults containing information on demographic characteristics, weight history, and health behaviors/conditions.25 We combined cross-sectional data from NHANES III (1988–1994) with repeat cross-sectional data from the NHANES continuous waves collected in two-year cycles between 1999 and 2016, creating a sample that is representative of the US population during an average year of the combined survey period.
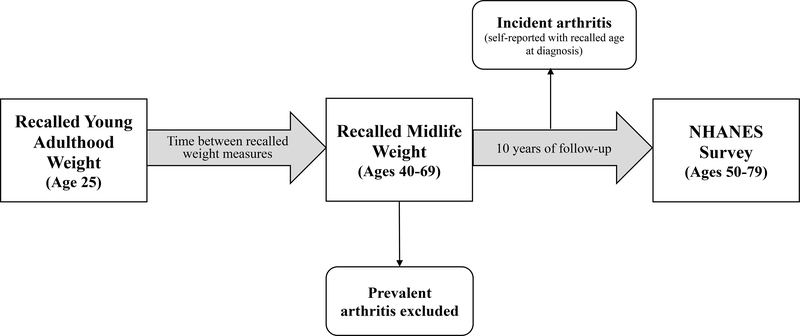
We used recall questions on weight history and age at arthritis diagnosis to create a retrospective cohort from the cross-sectional data. Specifically, we looked at recalled weight at age 25 and 10 years prior to the survey to measure weight change between young adulthood and midlife. We investigated the association between weight change and risk of incident arthritis over the 10-year period from the midlife measure to the time of survey. Age at midlife and self-reported age at arthritis diagnosis were used to determine the timing of incident events. This study design, depicted in Figure 1, was modified from a similar analysis of incident diabetes.26
Figure 1.
Study design for analysis of incident arthritis conditions (n= 13,669)
Figure 1 shows how cross-sectional, recall questions on weight history and age at arthritis diagnosis were leveraged to create a retrospective cohort of US adults. We studied individuals who participated in the NHANES III (1988–1994) or NHANES continuous (1999–2016) cross-sectional survey at ages 50–79 years. As part of the survey, individuals reported their recalled weight at age 25 (young adulthood) and at 10 year prior to survey (age 40–69 years, midlife), which were used to create a measure of weight change between young adulthood and midlife. We then investigated the association between this weight change and subsequent risk of developing an arthritis condition. ‘Follow-up’ for incident arthritis began at the midlife weight measure, which was 10 years prior to survey. Individuals who reported receiving a first diagnosis of arthritis more than 10 years prior to survey were considered prevalent cases and, thus, were excluded from the analysis of incident arthritis. Individuals who reported receiving a first diagnosis of arthritis during the follow-up period between midlife and time of survey were considered to have experienced incident arthritis over follow-up.
Sample
We included participants who were aged 50–79 years at the time of the NHANES survey so that their second recalled weight measure would correspond to midlife (aged 40–69 years). The sample was further restricted to participants with a BMI between 20 and 75 kg/m2 at both time points. A lower bound of 20 kg/m2 was chosen because people with lower BMIs may be experiencing weight loss related to illnesses such as chronic obstructive pulmonary diseases, heart failure, and cancer.27 Additionally, prior evidence suggests a j-shaped association between BMI and mortality with a BMI of approximately 20 kg/m2 having the lowest risk.28 Participants with missing information on weight, education level, or smoking status were excluded, as were those with unreliable BMI self-reports, defined as more than a 20% difference between BMI estimates calculated from self-reported weight and height and measured weight and height at the time of the survey. Adults who reported a diagnosis of arthritis more than 10 years prior to survey were considered prevalent cases and, thus, were excluded from the analysis of incident arthritis (Figure S1).
Weight Change Measures
We used recalled weights, which are strongly correlated with historically measured weight,29,30 to assess weight change between young adulthood and midlife.
Respondents were asked to recall their weight at age 25, which we considered young adulthood. For respondents in the NHANES continuous waves, we used recalled height at age 25 and recalled weight at age 25 to calculate BMI to account for the possibility of height decline with age. Because height at age 25 was not recorded during NHANES III, we used measured height at survey for these respondents.
Respondents were also asked to recall their weight from 10 years prior to the survey. Since participants’ age 10 years prior to survey ranged from 40–69 years, we considered this second time point to be a measure of midlife weight. We used measured height at survey to calculate BMI at midlife. BMI values at both young adulthood and midlife were categorized into normal weight (20.0–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (30.0–74.9 kg/m2).
We developed nine weight change categories: normal-normal, normal-overweight, normal-obese, overweight-normal, overweight-overweight, overweight-obese, obese-normal, obese-overweight, obese-obese. For example, someone classified as “normal-overweight” had a normal BMI in young adulthood but was overweight BMI in midlife. We grouped the categories into weight loss, weight maintenance, and weight gain (Table S1). For sensitivity analyses, we defined an alternative set of weight change categories based on percent weight change: > 10% weight loss, weight maintenance, and > 10% weight gain.
Assessment of Incident Arthritis Conditions
Arthritis conditions were defined based on the survey question, “has a doctor or other health professional ever told you that you had arthritis?” Reported age at diagnosis was used to determine arthritis onset.
Statistical Analysis
A Cox proportional hazard model was used to model incident arthritis conditions across the weight change categories over 10 years of follow-up between midlife weight and the time of survey, specifying the normal-normal category as the reference group. We adjusted for age at the midlife measure (40–44, 45–49, 50–54, 55–59, 60–64, 65–69 years), gender (male, female), race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Hispanic, Non-Hispanic Other), education at survey (less than high school, high school or equivalent, some college, college or higher), smoking status at the midlife measure (never, former, current), and categorical survey year. In addition to its importance as a confounder, adjusting for age allowed us to account for the difference in length of time between young adulthood and midlife weight measures which ranged from 15 to 44 years.
To better understand the differential risk between the weight change categories, we tested two hypotheses. First, we investigated whether individuals who lost weight between young adulthood and midlife were at a reduced risk of developing an arthritis condition relative to those who remained overweight or obese. To test this “risk reduction” hypothesis, we estimated the hazards of arthritis conditions for the overweight-normal group compared with the overweight-overweight group and the obese-overweight group relative to the obese-obese group.
Second, we investigated whether individuals with weight loss were at an increased risk of developing an arthritis condition relative to those who maintained a lower weight to determine if there is any residual risk associated with having previously been heavier. To test this “residual risk” hypothesis, we compared the hazards of arthritis conditions for the obese-overweight group to the overweight-overweight group. We also estimated the hazards for the overweight-normal group relative to the normal-normal group.
Hypothetical Scenarios
Using the formula for the population attributable fraction (PAF):
where pdi is proportion of total incident cases observed in the ith weight change category and HRi is the hazard ratio associated with that category, we estimated fraction of cases that would be eliminated if a weight change category were redistributed to another category. PAFs were then multiplied by the number of incident arthritis conditions in the overall population to determine the average number of cases that could be averted annually under two different hypothetical scenarios.
Under the obesity weight loss scenario, we estimated what would have happened if those who were obese at age 25 and during midlife instead lost down to an overweight BMI during midlife. This PAF calculation uses estimates from the primary Cox proportional hazard model, setting the obese-overweight group as the reference. Then, under the comprehensive prevention of overweight/obesity scenario, we examine the entire population had a normal BMI at age 25 and during midlife. Estimates for this calculation use the normal-normal group as the reference.
Sensitivity Analysis
To test the impact of excluding participants with missing covariates, we used multiple imputation by chained equations (10 imputations) to account for missing data in education and smoking status and refit our primary regression analysis using this imputed sample.31
Due to the wide range of ages at the midlife weight measure, we stratified our main results by age, comparing those who were 40–49 years at the midlife measure to those who were 50–69 years. We chose 50 years as the lower bound of the older age range because prior work has suggested weight gain levels off around age 50.32 Additionally, we stratified by smoking status to assess the possibility of confounding by smoking that may affect the risk of arthritis.
We also tested the robustness of our risk reduction findings by modeling incident arthritis conditions as a function of percent weight change categories among those who were obese at young adulthood.
We tested the proportional hazards assumption for the Cox model using a time-varying coefficients model. The interaction term between years of follow-up and several weight change categories were significant in this model, indicating a violation of proportionality. Therefore, we re-estimated PAF values using time-specific hazards at yearly intervals. The time-weighted PAF values were identical to the estimates derived from the primary models, suggesting we could proceed with the primary Cox models.
As the analyses used publicly available, de-identified data, Institutional Review Board approval was not required. Stata 15 (StataCorp) was used for all analyses. Following NHANES analytic guidelines,33 all estimates were sample weighted using pooled NHANES examination sample weights to account for unequal probabilities of selection and non-response adjustments. As a result, estimates are representative of the U.S. civilian, noninstitutionalized population during an average year of the combined survey period.
Results
Our sample included 13,669 US adults aged 50–79 at survey; 50.3% of the sample were under age 60, 56.7% were male, 78.2% were non-Hispanic white (Table 1). Additionally, 26.5% were former smokers and 26.6% were current smokers at midlife.
Table 1.
Sample characteristics, NHANES 1988–1994 & 1999–2016 (n=13,669)
| n | %a | |
|---|---|---|
| Age at survey | ||
| 50–54 years | 3012 | 28.4 |
| 55–59 years | 2330 | 21.9 |
| 60–64 years | 2938 | 17.9 |
| 65–69 years | 2196 | 14.0 |
| 70–74 years | 1948 | 11.0 |
| 75–79 years | 1245 | 6.9 |
| Gender | ||
| Female | 5706 | 43.3 |
| Male | 7963 | 56.7 |
| Race/ethnicity | ||
| Non-Hispanic White | 6549 | 78.2 |
| Non-Hispanic Black | 3054 | 9.5 |
| Hispanic | 3428 | 8.4 |
| Non-Hispanic Other | 638 | 3.8 |
| Educationb | ||
| Less than high school | 5444 | 26.1 |
| High school/equivalent | 2812 | 22.1 |
| Some college | 2858 | 24.8 |
| College or higher | 2555 | 27.0 |
| Smoking statusc | ||
| Never | 6344 | 46.8 |
| Former | 3537 | 26.5 |
| Current | 3788 | 26.6 |
| Weight change categoryd | ||
| Normal-normal | 3634 | 28.3 |
| Normal-overweight | 4191 | 30.0 |
| Normal-obese | 1575 | 10.6 |
| Overweight-normal | 241 | 1.6 |
| Overweight-overweight | 1587 | 12.0 |
| Overweight-obese | 1592 | 11.6 |
| Obese-normal | 30 | 0.2 |
| Obese-overweight | 126 | 0.8 |
| Obese-obese | 693 | 4.9 |
| Type of weight changee | ||
| Weight loss | 397 | 2.5 |
| Weight maintenance | 5914 | 45.3 |
| Weight gain | 7358 | 52.2 |
| Incident arthritis conditionsf | 3603 | 25.8 |
Percentages are sample weighted using NHANES examination weights. Counts are unweighted.
Education was reported at the time of the NHANES survey.
Smoking status was reported from the midlife weight measure (10 years prior to survey).
Weight was recorded at age 25 and 10 years prior to survey to determine weight change between young adulthood and midlife.
Weight loss includes overweight-normal, obese-normal, and obese-overweight. Weight maintenance includes normal-normal, overweight-overweight, and obese-obese. Weight gain includes normal-overweight, normal-obese, and overweight-obese.
Incident arthritis conditions reflects the number of new arthritis conditions that occurred over the 10-years of follow-up from the recalled midlife weight measure to the time of survey.
Less than half of the sample (45.3%) maintained their BMI category; 28.3% were normal-normal, 12.0% were overweight-overweight, and 4.9% were obese-obese. Weight gain was common (52.2%); 30.0% were normal-overweight, 10.6% were normal-obese, and 11.6% were overweight-obese. Weight loss, on the other hand, was rare (2.5%); 1.6% were overweight-normal, 0.2% were obese-normal, and 0.8% were obese-overweight. Table S2 shows the average BMI during young adulthood and midlife across weight change categories.
Weight Change and Incident Arthritis Conditions
A total of 3,603 cases of incident arthritis conditions (25.8%) were reported over 123,412 years of person-time (29.2 cases per 1,000 person-years). The unadjusted incidence of arthritis conditions overall was similar among adults who maintained weight (25.0 cases per 1,000 person-years) and adults who lost weight (23.0 cases per 1,000 person-years). The unadjusted incidence of arthritis was higher among adults who gained weight (33.0 cases per 1,000 person-years).
Table 2 shows the hazard ratios of developing an arthritis condition for each weight change category compared with adults who maintained a normal BMI. Results were suppressed for the obese-normal weight group as there were less than 10 incident arthritis cases in this group. The normal-overweight, normal-obese, overweight-obese, and obese-obese groups had significantly elevated risk of incident arthritis conditions. Figure S2 shows the cumulative hazard functions for each weight change group.
Table 2.
Weight change from young adulthood to midlife and risk of developing an arthritis conditions, NHANES 1988–1994 & 1999–2016 (n=13,669)
| Weight Changea | Number of Incident Arthritis Conditionsb | Incidence (95% CI)c | HR (95% CI)d | P value |
|---|---|---|---|---|
| Normal-normal | 783 | 23.3 (21.7–25.0) | Ref | - |
| Normal-overweight | 1071 | 28.2 (26.6–30.0) | 1.27 (1.10–1.46) | 0.001 |
| Normal-obese | 554 | 40.6 (37.3–44.1) | 1.73 (1.48–2.02) | <0.001 |
| Overweight-normal | 50 | 22.2 (16.8–29.3) | 1.06 (0.76–1.47) | 0.752 |
| Overweight-overweight | 333 | 22.8 (20.5–25.4) | 1.12 (0.93–1.35) | 0.229 |
| Overweight-obese | 539 | 38.8 (35.6–42.2) | 2.00 (1.71–2.34) | <0.001 |
| Obese-normal | - | - | - | - |
| Obese-overweight | 31 | 26.8 (18.9–38.2) | 1.13 (0.68–1.87) | 0.634 |
| Obese-obese | 238 | 40.0 (35.2–45.4) | 2.08 (1.73–2.51) | <0.001 |
Weight change categories based on BMI at age 25 (young adulthood) and BMI 10 years prior to the survey (midlife). Weight change categories are defined in Table S1.
Incident arthritis conditions reflects the number of new arthritis conditions that occurred over the 10-years of follow-up from the recalled midlife weight measure to the time of survey.
Arthritis conditions incidence rate per 1,000 person-years. Incidence rates are unadjusted.
Hazard of developing an arthritis condition, using normal-normal as the reference group. The Cox proportional hazard model was sample weighted and adjusted for categorical age at the midlife measure, gender, race/ethnicity, education level at survey, smoking status at the midlife measure, and survey year.
- indicates HR was suppressed because there were less than 10 incident cases in this group.
Risk Reduction Hypothesis
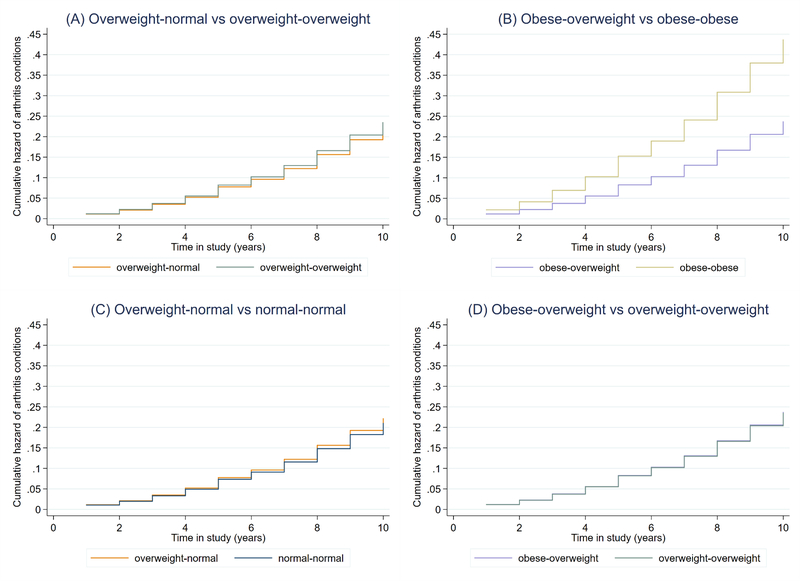
Compared with adults who remained obese, those who lost weight to an overweight BMI had lower risk of developing an arthritis condition (HR, 0.54; 95% CI, 0.32–0.92) (Table 3). However, adults who lost from overweight to normal did not have reduced risk of developing an arthritis condition (HR, 0.94; 95% CI, 0.67–1.34) compared with adults who were overweight at both time points. Figure 2a and 2b show the difference in cumulative hazard functions for the two risk reduction comparisons.
Table 3.
Risk reduction and residual risk: HRs for weight changea and incident arthritis conditionsb, NHANES 1988–1994 & 1999–2016 (n=13,669)
| Weight Change | HRc | 95% CI | P value |
|---|---|---|---|
| Risk Reduction Hypothesis (weight loss vs higher weight maintenance) | |||
| overweight-normal vs overweight-overweightd | 0.94 | 0.67–1.34 | 0.739 |
| obese-overweight vs obese-obese | 0.54 | 0.32–0.92 | 0.023 |
| Residual Risk Hypothesis (weight loss vs lower weight maintenance) | |||
| overweight-normal vs normal-normal | 1.06 | 0.76–1.47 | 0.752 |
| obese-overweight vs overweight-overweight | 1.01 | 0.61–1.68 | 0.972 |
Weight change categories based on BMI at age 25 (young adulthood) and BMI 10 years prior to the survey (midlife). Weight change categories are defined in Table S1.
Incident arthritis conditions reflects new arthritis conditions that occurred over the 10-years of follow-up from the recalled midlife weight measure to the time of survey.
Hazard ratios generated via post-estimation through the lincom command of the base model comparing all weight change categories to the normal-normal reference group. All estimates sample weighted and adjusted for categorical age at the midlife measure, gender, race/ethnicity, education level at survey, smoking status at the midlife measure, and survey year.
This model presents the hazards of developing an arthritis condition for individuals who were overweight in young adulthood and lost to normal weight at midlife compared with individuals who were overweight and remained overweight. The other comparisons were constructed in the same fashion.
Figure 2.
Cumulative hazards of arthritis conditions by weight change category: Testing (A-B) risk reduction hypothesis and (C-D) residual risk hypothesis, NHANES 1988–1994 & 1999–2016 (n=13,669)
Figure 2 shows the function for cumulative hazard of developing an arthritis condition by weight change category for the two comparisons testing the risk reduction hypothesis (A&B) and the two comparisons testing the residual risk hypothesis (C & D). Weight was recorded at age 25 and 10 years prior to survey to determine weight change between young adulthood and midlife. The x-axis corresponds to years between the midlife BMI measure, when participants’ ages were 40–69 years, and the time of survey, when participants’ ages were 50–79 years. Models were adjusted for age at the midlife measure, gender, race, education, smoking status at the midlife measure, and survey year.
Residual Risk Hypothesis
Compared with individuals who maintained a normal BMI, individuals who lost from overweight to normal (HR, 1.06; 95% CI, 0.76–1.47) had similar risk of developing an arthritis condition, suggesting there is little residual risk associated with having once had a higher BMI (Table 3). Adults who lost from obese to overweight also had comparable risk to adults who were overweight at both time points (HR, 1.01; 95% CI, 0.61–1.68). Figure 2c and 2d show the difference in cumulative hazard functions for the two residual risk comparisons.
Hypothetical Scenarios
If individuals who were obese in young adulthood and remained obese had instead lost weight and became overweight at midlife, 3.1% of incident arthritis conditions over the subsequent 10 years could have been averted (95% CI, 1.0% to 5.2%). Extrapolating these estimates to the population level, 335,276 cases (95% CI, 108,154 to 562,398) on average per year of the study period, out of a total of 10,815,354 incident cases, could have been averted under the hypothetical weight loss from obese scenario.
If the total population had a normal BMI at both young adulthood and midlife, 24.7% (95% CI, 17.6% to 31.2%) of incident arthritis conditions may be averted, corresponding to 2,671,392 cases on average per year at the population level (95% CI, 1,903,502 to 3,374,390).
Sensitivity Analyses
Our sensitivity analysis using imputed data for missing covariates (Table S3) was nearly identical to Table 2, suggesting that excluding participants without information on education or smoking status had no impact on our results.
Table S4 shows the associations between weight change category and arthritis conditions stratified by midlife age. The overall patterns are broadly similar, but the younger age group (40–49 years) has stronger risk in the weight gain categories. Additionally, being in the overweight-overweight group appears to increase risk for the younger age group but not for the older age group (50–69 years). Table S5 shows the associations stratifying by smoking status, which are broadly similar to the main results.
Using percent weight change to assess weight change, we found that obese adults who lost more than 10% had reduced risk compared with those who maintained their weight, though the association was not statistically significant, likely reflecting a lack of precision since few individuals achieved this degree of weight loss (Table S6).
Discussion
This nationally representative study of the relation of weight change to risk of incident arthritis had four principle findings. First, adults who lost weight from obese at age 25 to overweight at midlife had reduced risk of developing an arthritis condition compared with those who remained obese. The same protective effect was not observed for individuals who lost from overweight to normal, suggesting weight loss is more beneficial for individuals with higher BMI. Second, individuals who lost weight had a similar level of risk as those who maintained a lower weight, suggesting there may not be residual risk associated with having been at a higher weight previously. Together, these results suggest weight in midlife appears to be an important influencer of arthritis risk. Finally, we estimated that nearly one quarter of incident arthritis cases at the national level, corresponding to 2.7 million individuals, would have been averted under the hypothetical scenario where all individuals were normal weight in young adulthood and midlife.
As in previous studies,12,19,20 we demonstrated an increase in arthritis risk associated with weight gain. Additionally, the risk reduction we observed was consistent with prior studies on osteoarthritis and gout.12,22,23 Weight loss may reduce arthritis risk through reduced joint loading and inflammation. It is possible that the observed reduction in arthritis risk was because obese adults who lose to overweight started at lower weights than the obese adults who maintained their weight. However, Table S2 shows the obese-overweight and obese-obese categories had similar average BMIs at age 25. Additionally, our sensitivity analysis using 10% weight change suggest the findings are robust to different definitions of weight loss.
Prior evaluations of the residual risk hypothesis have been mixed. Analysis of an Australian cohort study concluded that childhood overweight measures were associated with adult knee pain independent of adult weight but only among men.34 Conversely, an analysis of the 1946 British birth cohort study concluded that there was no additional risk of knee osteoarthritis associated with adolescent BMI after accounting for adult BMI.35 Similarly, we found that those who lost weight had comparable risk of developing an arthritis condition to participants who maintained a lower weight.
We also estimated the potential health implications of several weight loss intervention and overweight/obesity prevention scenarios. We found that progressively more cases of incident arthritis conditions could be averted under more comprehensive intervention/prevention scenarios from 3.1% under the obesity weight loss scenario to 24.7% under a comprehensive overweight/obesity prevention scenario (i.e., maintaining normal weight).
These findings underscore the importance of primary and secondary prevention of overweight/obesity in reducing arthritis incidence and its attendant substantial morbidity. In addition to reducing arthritis risk, lifestyle modification strategies associated with weight loss have been established as effective methods of improving outcomes and reducing pain and disability at the individual level among those with RMDs. Increasing physical activity has been shown to reduce risk of disability in those with knee osteoarthritis.36 Exercise and diet interventions leading to weight loss have also been associated with reduction in knee pain.37,38 Bariatric surgery can reduce pain,39 improve joint function,40 increase overall functional health and wellbeing,39 and prevent gout and hyperuricaemia.41
Given the high cost of pharmacologic, including biologic, therapy for many RMDs and lack of effective therapies for osteoarthritis beyond guidelines recommending weight loss and physical activity, a concerted effort to achieve and maintain a healthy weight is necessary to cost-effectively manage arthritis conditions. However, we found that losing enough weight to drop BMI categories was rare (2.5%), potentially due to the set-point theory where the body calibrates to its metabolic activity to a given weight and resists weight loss.42 Thus, the efficacy of individual-level behavioral change strategies encouraging weight loss/maintenance remain low. Instead, priority should be placed on developing policy interventions that reverse the upstream systemic and environmental drivers of the obesogenic environment and, as a result, reduce obesity at the population-level.43 Policy approaches, including unhealthy food and beverage taxes, front-of-pack nutrition labeling, and reduction of junk food advertising to children, are generally more cost-effective than health promotion or clinical interventions that target patients who have already become overweight or obese.44
Our study had several notable strengths. First, we used a novel application of a large, nationally representative cross-sectional survey to create a retrospective cohort design. As a result, our estimates are broadly generalizable to the US population. Second, in addition to evaluating weight loss in relation to weight maintenance as most prior studies have done, we tested an additional hypothesis concerning the residual risk associated with having previously been at a higher weight. Third, since age-associated height loss can lead to spurious estimates of BMI,45 we used recalled height at age 25, which is strongly correlated with historically measured height,46,47 to calculate BMI at age 25.
Nonetheless, our study had several limitations. First, the definition of “arthritis” relied on self-reported doctor-diagnosed arthritis, which does not necessarily reflect the true incidence of arthritis conditions. Second, we investigated the association between weight change and risk of arthritis conditions broadly, obscuring differences in types of arthritis. However, since self-reported doctor diagnosis of specific forms of arthritis in the NHANES is not validated, this broader approach is prone to less misclassification. Nonetheless, we acknowledge that the majority of these arthritis conditions are likely to be osteoarthritis given its much higher prevalence (27 million in 2008 compared to 1.3 and 3 million cases of rheumatoid arthritis and gout).48,49 Third, reliance on self-reported historic weight measures may have introduced error into our weight change estimates. However, prior studies have shown that self-reports of both current weight50 and past body weights are strongly correlated with measured weight.29,30 Fourth, our midlife BMI measure corresponds to an age range of 40–69 years so our weight history period varies across individuals (between 15 and 44 years). However, analyses were adjusted for age at the midlife BMI measure to ensure that the associations of weight change with arthritis risk were made conditional on the length of the weight change period. Additionally, our age-stratified results were broadly similar. Fifth, exclusions of prevalent arthritis cases were differential by weight change category with the largest exclusions in the normal-obese, overweight-obese, and obese-obese groups. However, the higher prevalence among these groups is consistent with the increased incidence we see in the present analysis. Finally, weight loss was rare (2.5%) affecting the precision of our estimates, and, thus, limiting the ability to make inferences regarding the effects of weight loss on arthritis risk.
In conclusion, in this nationally representative sample of US adults, we found strong associations between weight change from young adulthood to midlife and the risk of developing an arthritis condition. Weight gain was associated with increased arthritis risk, whereas weight loss was associated with substantially reduced risk. Those who lost weight had a risk comparable to those who maintained a lower weight, suggesting there may not be residual risk associated with having previously been heavier. Extrapolating to the population-level, we estimate that a substantial portion of incident arthritis cases could be avoided through effective weight loss strategies for individuals and population-level policies that encourage primary prevention of overweight/obesity. Our findings highlight the critical need to expand obesity treatment and prevention activities in order to achieve meaningful reductions in the burden of arthritis in the US population.
Supplementary Material
Significance & Innovation.
We used a novel application of a large, nationally representative cross-sectional survey to create a retrospective cohort design where change in recalled weight from two earlier stages of life were used to study the incidence of arthritis conditions.
Weight loss from young adulthood to midlife was associated with substantially reduced risk of developing an arthritis condition, and we found no evidence of residual risk from having been heavier earlier in life.
Nearly one quarter of incident arthritis cases, corresponding to 2.7 million individuals at the national level, would have been averted under the hypothetical scenario where all individuals maintained normal weight from young adulthood to midlife.
Acknowledgments
Funding: This research was funded by Ethicon Endo-Surgery, Inc. Additionally, Ms. Berry was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) under Award Number 1T32HD095134–01A1 (Warren and Osypuk, PIs) and Award Number P2C HD041023. Dr. Neogi is supported by NIH AR070892. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Park J, Mendy A, Vieira ER. Various Types of Arthritis in the United States: Prevalence and Age-Related Trends From 1999 to 2014. Am J Public Health. 2018;108(2):256–258. doi: 10.2105/AJPH.2017.304179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Prim. 2016;2(1):16072. doi: 10.1038/nrdp.2016.72 [DOI] [PubMed] [Google Scholar]
- 3.Rui P, Okeyode T. National Ambulatory Medical Cary Survey: 2015 State and National Summary Tables. http://www.cdc.gov/nchs/ahcd/ahcd_products.htm.
- 4.McDermott K, Elixhauser A, Ruirui S. Trends in Hospital Inpatient Stays in the United States, 2005–2014. Vol 225. Rockville, MD; 2017. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb225-Inpatient-US-Stays-Trends.pdf. [Google Scholar]
- 5.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity and Trends in Body Mass Index Among US Children and Adolescents, 1999–2010. JAMA. 2012;307(5):483. doi: 10.1001/jama.2012.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. doi: 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveria SA, Felson DT, Cirillo PA, Reed JI, Walker AM. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology. 1999;10(2):161–166. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed4&NEWS=N&AN=1999073185. [PubMed] [Google Scholar]
- 8.Reyes C, Leyland KM, Peat G, Cooper C, Arden NK, Prieto-Alhambra D. Association Between Overweight and Obesity and Risk of Clinically Diagnosed Knee, Hip, and Hand Osteoarthritis: A Population-Based Cohort Study. Arthritis Rheumatol. 2016;68(8):1869–1875. doi: 10.1002/art.39707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowson CS, Matteson EL, Davis JM, Gabriel SE. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(1):71–77. doi: 10.1002/acr.21660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linauskas A, Overvad K, Symmons D, Johansen MB, Stengaard-Pedersen K, de Thurah A. Body fat percentage, waist circumference and obesity as risk factors for rheumatoid arthritis - A Danish cohort study. Arthritis Care Res (Hoboken). 2018;0(0):1–10. doi: 10.1002/acr.23694 [DOI] [PubMed] [Google Scholar]
- 11.Evans PL, Prior JA, Belcher J, Mallen CD, Hay CA, Roddy E. Obesity, hypertension and diuretic use as risk factors for incident gout: A systematic review and meta-analysis of cohort studies. Arthritis Res Ther. 2018;20(1):1–15. doi: 10.1186/s13075-018-1612-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, Weight Change, Hypertension, Diuretic Use, and Risk of Gout in Men. Arch Intern Med. 2005;165(7):742. doi: 10.1001/archinte.165.7.742 [DOI] [PubMed] [Google Scholar]
- 13.Jon Love T, Zhu Y, Zhang Y, et al. Obesity and the risk of psoriatic arthritis: a population-based study. Ann Rheum Dis. 2012;71(8):1273–1277. doi: 10.1136/annrheumdis-2012-201299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soltani-Arabshahi R, Wong B, Feng B-J, Goldgar DE, Duffin KC, Krueger GG. Obesity in Early Adulthood as a Risk Factor for Psoriatic Arthritis. Arch Dermatol. 2010;146(7):721–726. doi: 10.1001/archdermatol.2010.141 [DOI] [PubMed] [Google Scholar]
- 15.Gómez R, Conde J, Scotece M, Gómez-Reino JJ, Lago F, Gualillo O. What’s new in our understanding of the role of adipokines in rheumatic diseases? Nat Rev Rheumatol. 2011;7(9):528–536. doi: 10.1038/nrrheum.2011.107 [DOI] [PubMed] [Google Scholar]
- 16.Thijssen E, Van Caam A, Van Der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatol (United Kingdom). 2014;54(4):588–600. doi: 10.1093/rheumatology/keu464 [DOI] [PubMed] [Google Scholar]
- 17.Urban H, Little CB. The role of fat and inflammation in the pathogenesis and management of osteoarthritis. Rheumatol (United Kingdom). 2018;57(February):iv10–iv21. doi: 10.1093/rheumatology/kex399 [DOI] [PubMed] [Google Scholar]
- 18.Fowler-Brown A, Kim DH, Shi L, et al. The mediating effect of leptin on the relationship between body weight and knee osteoarthritis in older adults. Arthritis Rheumatol. 2015;67(1):169–175. doi: 10.1002/art.38913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mo F, Morrison H, Neutel IC. Population attributable risk from obesity to arthritis in the Canadian Population Health Longitudinal Survey 1994–2006. Int J Rheum Dis. 2014;17(6):628–634. doi: 10.1111/1756-185X.12372 [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Manson JE, Yuan C, et al. Associations ofweight gain from early to middle adulthood with major health outcomes later in life. JAMA - J Am Med Assoc. 2017;318(3):255–269. doi: 10.1001/jama.2017.7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apold H, Meyer HE, Nordsletten L, Furnes O, Baste V, Flugsrud GB. Weight gain and the risk of knee replacement due to primary osteoarthritis: A population based, prospective cohort study of 225,908 individuals. Osteoarthr Cartil. 2014;22(5):652–658. doi: 10.1016/j.joca.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 22.Felson D, Zhang Y, Anthony JM, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann Intern Med. 1992;116(7):535–539. [DOI] [PubMed] [Google Scholar]
- 23.Runhaar J, de Vos BC, van Middelkoop M, Vroegindeweij D, Oei EHG, Bierma-Zeinstra SMA. Prevention of Incident Knee Osteoarthritis by Moderate Weight Loss in Overweight and Obese Females. Arthritis Care Res. 2016;68(10):1428–1433. doi: 10.1002/acr.22854 [DOI] [PubMed] [Google Scholar]
- 24.Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framlingham study. Ann Intern Med. 1992;116:535–539. [DOI] [PubMed] [Google Scholar]
- 25.National Center for Health Statistics. National Health and Nutrition Examination Survey : Plan and Operations, 1999 – 2010. Vital Heal Stat. 2010;1(56):1999–2010. [PubMed] [Google Scholar]
- 26.Stokes A, Collins JM, Grant BF, et al. Obesity progression between young adulthood and midlife and incident diabetes: A retrospective cohort study of U.S. Adults. Diabetes Care. 2018;41(5):1025–1031. doi: 10.2337/dc17-2336/-/DC1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokes A, Preston SH. How Dangerous Is Obesity? Issues in Measurement and Interpretation. Popul Dev Rev. 2016;42(4):595–614. doi: 10.1111/padr.12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-Mass Index and Mortality among 1.46 Million White Adults. N Engl J Med. 2010;363(23):2211–2219. doi: 10.1056/NEJMoa1000367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casey VA, Dwyer JT, Berkey CS, Coleman KA, Gardner J, Valadian I. Long-term memory of body weight and past weight satisfaction: a longitudinal follow-up study. Am J Clin Nutr. 1991;53(6):1493–1498. doi: 10.1093/ajcn/53.6.1493 [DOI] [PubMed] [Google Scholar]
- 30.Perry GS, Byers TE, Mokdad AH, Serdula MK, Williamson DF. The validity of self-reports of past body weights by U.S. adults. Epidemiology. 1995;6(1):61–66. http://www.ncbi.nlm.nih.gov/pubmed/7888448. [DOI] [PubMed] [Google Scholar]
- 31.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. doi: 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dutton GR, Kim Y, Jacobs DR, et al. 25-year weight gain in a racially balanced sample of U.S. adults: The CARDIA study. Obesity. 2016;24(9):1962–1968. doi: 10.1002/oby.21573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson C, Paulose-Ram R, Ogden C. National Health and Nutrition Examination Survey: Analytic guidelines, 1999–2010. Natl Cent Heal Stat Vital Heal Stat. 2013;2(161). [PubMed] [Google Scholar]
- 34.Antony B, Jones G, Venn A, et al. Association between childhood overweight measures and adulthood knee pain, stiffness and dysfunction: A 25-year cohort study. Ann Rheum Dis. 2015;74(4):711–717. doi: 10.1136/annrheumdis-2013-204161 [DOI] [PubMed] [Google Scholar]
- 35.Wills AK, Black S, Cooper R, et al. Life course body mass index and risk of knee osteoarthritis at the age of 53 years: Evidence from the 1946 British birth cohort study. Ann Rheum Dis. 2012;71(5):655–660. doi: 10.1136/ard.2011.154021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunlop DD, Song J, Semanik PA, et al. Relation of physical activity time to incident disability in community dwelling adults with or at risk of knee arthritis: Prospective cohort study OPEN ACCESS. BMJ. 2014;348(April):1–11. doi: 10.1136/bmj.g2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. JAMA - J Am Med Assoc. 2013;310(12):1263–1273. doi: 10.1001/jama.2013.277669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White DK, Neogi T, Rejeski WJ, et al. Can an intensive diet and exercise program prevent knee pain among overweight adults at high risk? Arthritis Care Res. 2015;67(7):965–971. doi: 10.1002/acr.22544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King WC, Chen J-Y, Belle SH, et al. Change in Pain and Physical Function Following Bariatric Surgery for Severe Obesity. JAMA. 2016;315(13):1362. doi: 10.1001/jama.2016.3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Springer BD, Carter JT, McLawhorn AS, et al. Obesity and the role of bariatric surgery in the surgical management of osteoarthritis of the hip and knee: a review of the literature. Surg Obes Relat Dis. 2017;13(1):111–118. doi: 10.1016/j.soard.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 41.Maglio C, Peltonen M, Neovius M, et al. Effects of bariatric surgery on gout incidence in the Swedish Obese Subjects study: A non-randomised, prospective, controlled intervention trial. Ann Rheum Dis. 2017;76(4):688–693. doi: 10.1136/annrheumdis-2016-209958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity. 2016;24(8):1612–1619. doi: 10.1002/oby.21538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: Shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814. doi: 10.1016/S0140-6736(11)60813-1 [DOI] [PubMed] [Google Scholar]
- 44.Gortmaker SL, Swinburn BA, Levy D, et al. Changing the future of obesity: Science, policy, and action. Lancet. 2011;378(9793):838–847. doi: 10.1016/S0140-6736(11)60815-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorkin JD, Muller DC, Andres R. Longitudinal change in the heights of men and women: Consequential effects on body mass index. Epidemiol Rev. 1999;21(2):247–260. doi: 10.1093/oxfordjournals.epirev.a018000 [DOI] [PubMed] [Google Scholar]
- 46.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138(1):56–64. doi: 10.1093/oxfordjournals.aje.a116777 [DOI] [PubMed] [Google Scholar]
- 47.Heaney R, Ryan R. Relation between Measured and Recalled Body Height. N Engl J Med. 1988;319(12):795–796. doi: 10.1056/NEJM198809223191216 [DOI] [PubMed] [Google Scholar]
- 48.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177 [DOI] [PubMed] [Google Scholar]
- 49.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stokes A, Ni Y. Validating a summary measure of weight history for modeling the health consequences of obesity. Ann Epidemiol. 2016;26(12):821–826.e2. doi: 10.1016/j.annepidem.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.