Abstract
To better understand the effects of transient thermal stress in an aquatic insect, we first identified static temperatures associated with fitness deficits, and then reared larvae from egg hatch to adulthood under diurnally variable regimens including daily forays into deleterious temperatures. We sampled mature larvae at the coolest and warmest portions of their respective regimens for RNA-seq analysis. Few transcripts (28) were differentially expressed when larvae oscillated between favorable temperatures, while 614 transcripts were differentially expressed when experiencing daily transient thermal stress. Transcripts associated with N-glycan processing were downregulated while those associated with lipid catabolism and chitin turnover were significantly upregulated in heat stressed larvae. An across-regimen comparison of differentially expressed transcripts among organisms sampled at comparable temperatures demonstrated that the effects of daily thermal stress persisted even when larvae were sampled at a more optimal temperature (806 differentially expressed transcripts). The chronically stressed population had reduced expression of transcripts related to ATP synthesis, mitochondrial electron chain functions, gluconeogenesis and glycolytic processes while transcripts associated with cell adhesion, synaptic vesicle transport, regulation of membrane potential and lipid biosynthesis increased. Comparisons of constant vs. variable temperatures revealed that the negative consequences of time spent at stressful temperatures were not offset by more time spent at optimal temperatures.
Subject terms: Genetics, Physiology, Ecology, Environmental sciences
Introduction
Temperature is among the most important factors that determine the distribution and performance of ectothermic species1–8. In most aquatic ecosystems, organisms experience thermal regimes that include distinct diel cycles that are imbedded within the more commonly studied seasonal cycle9–12. As the global climate changes and human activities alter the thermal regimes of freshwater ecosystems13–16, it is increasingly likely that organisms are subjected to stressful temperatures on different temporal scales17(e.g. hourly, daily, seasonally, annually) that differentially affect physiological processes, developmental trajectories, life history outcomes, and ultimately species distributions18.
Insects play critical ecological roles and are the most widely used faunal group to evaluate ecological conditions of freshwater environments19–21. While insect thermal biology has been broadly studied, less has focused on aquatic insects. At the seasonal/annual scale, it appears that aquatic insects typically adhere to the temperature size rule (TSR)3,5,22,23, though some exceptions have been noted22. For a given species, relatively warm temperature accelerates growth and developmental rates, with development time decreasing at a disproportionately faster rate than biomass accumulation. As a result, warmer conditions produce smaller, less fecund individuals, relative to cooler temperatures, which produce larger, more fecund individuals. In life cycle rearing studies at constant temperatures with the mayfly Cloeon dipterum, Sweeney et al.8 defined a thermal “acclimation zone” where development and growth rates changed consistently with increasing temperature while degree-day requirements to complete metamorphosis were constant. Rearing at temperatures warmer than the thermal acclimation zone significantly reduced survival and fitness.
As thermal regimes continue to change, it is increasingly likely that some species will spend greater portions of their life cycle at daily high temperatures that are outside of the thermal acclimation zone. While some research in the thermal biology field has begun to incorporate transient thermal stress in recent years24, the fitness consequences of exposure to diel temperature fluctuations in insects still remains poorly understood. Understanding the physiological processes that occur under repeated, transient thermal stress are especially important because this situation is likely common as conditions warm.
Recent efforts have increased our knowledge of the thermal biology of mayflies and the development of a lab-reared mayfly model (Neocloeon triangulifer (Ephemeroptera: Baetidae) for physiological and ecological studies25–35. We have developed a better understanding of both short-term36,37 and long-term38 thermal challenge in N. triangulifer, but the more ecologically relevant situation of long-term development in a diel thermal regime with daily excursions out of the acclimation zone and into stressful temperatures has remained unstudied.
In this paper, we examine the consequences of daily, transient exposures to thermally challenging temperatures. We first used constant temperature rearing experiments (from egg hatch to adulthood) to identify a physiologically stressful and fitness-reducing temperature – in this case 28 °C which clearly fell outside the thermal acclimation zone for N. triangulifer. We then established variable temperature regimens that included a brief (hours), daily exposures to 28.5 °C. We used an RNA-seq approach to compare gene expression profiles of mature larvae sampled at the low and warm temperatures of their respective daily thermal regimes to better understand how short-term forays into sub-optimal (warm) conditions affected gene expression. We also made comparisons of gene expression profiles across thermal regimens to better understand how signatures of transient but chronic thermal challenge are retained at the transcriptomic level, even at “recovery” temperatures. We link these results to life history outcomes and highlight the important role of molting in mediating the thermal performance.
Materials and methods
Mayfly rearing
The parthenogenetic mayfly N. triangulifer (WCC-2 clone isolated from White Clay Creek, (Patent US5665555)) were reared at the Stroud Water Research Center (SWRC; Avondale, PA). Newly hatched eggs of N. triangulifer were partitioned into rearing jars using natural stream water from White Clay Creek (WCC) and natural WCC periphyton as a food source as described elsewhere39. Mayflies were reared at four constant temperature treatments (22°, 26°, 28°, and 30 °C ± 0.1 °C) and three variable temperature treatments with a 5 °C daily oscillation (0.5 °C/h) from 19.5 °C to 24.5 °C, mean = 22 °C (Regimen 1- diel fluctuation within the thermal acclimation zone) 23.5 °C to 28.5 °C, mean = 26 °C (Regimen2-diel fluctuation outside the thermal acclimation zone), and 25.5–30.5 °C, mean = 28 °C (Regimen 3 -diel fluctuation further outside the thermal acclimation zone). In each rearing condition, 5–11 replicate jars were used, and each jar contained 50 larvae. Temperature starts to rise at 6:30 am and reaches high soak at 4:30 pm for 2 h. Temperature starts to fall at 6:30 pm and reaches low soak at 4:30 am for 2 h. All temperature treatments were exposed 15:9 (L:D) photoperiod that began at 5:00 am and ended at 8:00 pm, simulating a light regime near the summer solstice at ~ 40°N). Survivorship, development time, and adult body size (fitness) were recorded for each replicate jar. Survivorship was the percentage of 1st-instar larvae surviving to the adult stage. These data were arcsine transformed for statistical analysis. Development time was the number of days from the start of the experiment (egg hatch) to the median day of adult emergence from a given replicate jar. Adult body size was mean individual biomass of emergent adults from a given replicate jar, dried at 60 °C for 48 h.
Larval rearing for molt counting
Hatchlings (< 12 h old) of N. triangulifer (WCC-2 clone) were reared from first instar larvae to the adult (subimago) stage in incubators (Thermo Scientific, MA) held at 18 °C and 26 °C ± 0.05 °C (trial 1), 22 °C and 26 °C ± 0.05 °C (trial 2) constant temperatures at NCSU. To conveniently monitor and collect mayfly exuviae, a single larva was put in a well containing artificial soft water (ASW) in a 12 well plate (Genesee Scientific, CA). Each temperature treatment contained three replicates (12-well plates). Larvae were transferred into larger 6-well plates as they developed. Simulated daylight was provided by fluorescent “grow lights” and all experiments involved a 15:9 h light:dark cycle. Food was provided every other day using periphyton shipped from SWRC. Water was filled and kept at the surface of each well to ensure larvae were oxygenated. Larvae molt count was conducted every day and exuviae were removed from wells.
Mayfly larval sampling for RNA-seq
Because temperature strongly influenced development time, it was essential for larvae in the different thermal treatments to be sampled at comparable developmental stages. While there are no perfect anatomical features that would allow for this developmental synchronization to be precise, the presence of developed but not darkened wing pads was used as a visual guide to sampling at the low soak and high soak temperatures of the respective thermal regimes. For the Regimen 1 group larvae were sampled on day 21.7 and 22.2 of larval development. The median larval emergence time for this thermal treatment was 23.7 days. Thus, we estimate that larvae from the 22 °C group were sampled at 92–94% completion of larval development. For the Regimen 2 group, larvae were sampled on day 18.2 and 18.7 of larval development. The median emergence time for this thermal treatment was 20.1 days. Thus, we estimate that larvae from the Regimen 2 group were sampled at 91–93% completion of larval development. Upon collection, all samples were flash frozen in liquid nitrogen and stored at − 80 °C freezer. All samples were packaged with dry ice and sent with overnight shipping to North Carolina State University (NCSU; Raleigh, NC) for RNA-seq at the NCSU Genomic Sciences Laboratory (GSL). Biological replicates were n = 4 for RNA-seq and n = 6 qPCR analysis.
RNA-seq and data analysis
To prepare samples for RNA-seq studies, total RNA was isolated from N. triangulifer following the SV Total RNA Isolation System protocol (Promega, WI). RNA quality was assured with Bioanalyzer RNA nano chips (Agilent, CA); Truseq libraries (Illumina, CA) were prepared; and 16 libraries were sequenced with paired-end reads using the Illumina NextSeq 500 at the NCSU GSL.
Transcriptome assembly
Transcriptome assembly and differential expression were performed in consultation with Bioinformatics Core at NCSU Center for Human Health and the Environment (CHHE). The quality of sequenced data was assessed using FastQC application, and the adapter sequence and quality trimming was preformed using Trimmomatic version 0.3640 with the following parameters (HEADCROP:12, LEADING:20, TRAILING:20, SLIDINGWINDOW:30:30, MINLEN:40). Transcriptome de novo assembly were conducted using trinity41. The transcriptome sequences were annotated using BLAST + command line utility (blastx and blastp; E-value cutoff 1e-5 and max target sequence -1)42 and the Trinotate pipeline (https://trinotate.github.io/). Since there was no N. triangulifer reference genome and mapping reads to another mayfly, E. danica resulted with less than 1% unique mapping, the raw reads were mapped back to the de novo assembled transcriptome. The count matrix was generated using align and estimate abundance Perl script in the Trinity software package using RSEM abundance estimation method and bowtie2 aligner.
Pairwise analysis
Differentially expressed genes were determined using the R package DESeq243. The Count data were normalized for sequencing depth and RNA composition, specifically the counts divided by sample-specific size factors determined by median ratio of gene counts relative to geometric mean per gene.
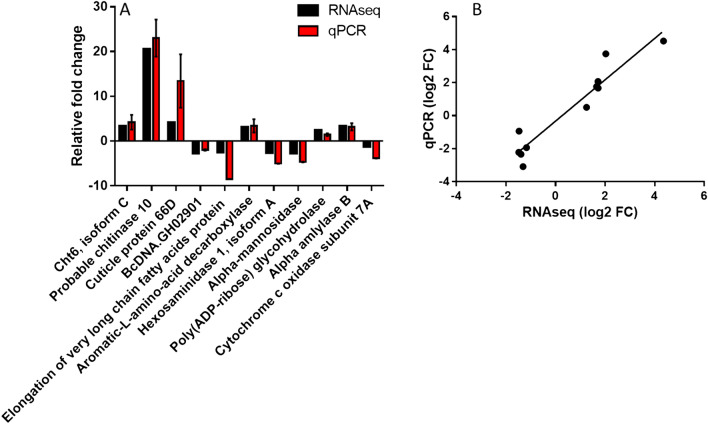
We fitted a linear model using the treatment levels, and differential expressed genes were identified after applying multiple testing correction using Benjamini–Hochberg procedure44 , padj < 0.05. Significant differentially-expressed genes (UniprotKB IDs) were assigned for Gene Ontologty (GO) analysis through comparison with annotated protein sequences from Drosophila melanogaster with online web tool PANTHER (https://geneontology.org/). Expression results were validated by conducting qPCR on a subset of genes (Fig. 4). Pathway analyses were conducted with two online database: reactome pathway analysis via PANTHER and KEGG pathway analysis via DAVID (https://david.ncifcrf.gov/summary.jsp). Both analyses showed similar result, and data presented in this study are shown as reactome pathways via PANTHER.
Figure 4.
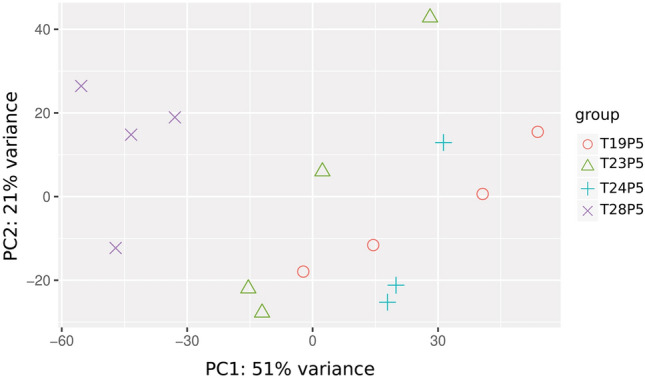
PCA plot showing similarity within Regimen 1 (19.5 and 24.5 °C) whereas the Regimen 2 shows strong effects of exposure to 28.5 °C. This transient 28.5 °C exposure appears to influence gene expression in the 23.5 °C group.
qPCR validation
To validate the expression results from RNA-seq analysis, we selected 11 genes for confirmation by conducting qPCR. We selected genes randomly from several functional categories of interest, including a mix of up- and down-regulated genes by our treatments. All primers were designed with IDT PrimerQuest Tool (https://www.idtdna.com/Primerquest/Home/Index) with the following parameters: length of 18–22 nt, melting temperature of 60 °C and product size of 150–220 bp. Primers were synthesized by Life technologies, USA and were tested using conventional PCR and gel electrophoresis for correct size product. cDNA for qPCR was generated from aliquots of the same RNA samples used for RNA-seq with 2 additional biological replicates for each treatment. First strand cDNA was synthesized from the same amount of each total RNA by MultiScribe MuLV reverse transcriptase using random primers (Applied Biosystems, (ABI), CA) and all thermocycling was done using a Bio-Rad iCycler (Bio-Rad, CA). The resulting cDNA samples were diluted 4 × before analysis and stored at − 20 °C. Quantitative real-time PCR (qRT-PCR) was performed on an ABI Prism 7700 Sequence Detection System (Applied Biosystems (ABI), CA) using default parameters. Amplification mixtures consisted of 5 µL of SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, CA), 10 µM primers, 20 ng template cDNA and nuclease free water in a total volume of 10 µL. qRT- PCR conditions were 2 min at 94 °C, followed by 40 cycles at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. Relative expression of each amplicon was calculated by the corrected delta delta Ct method (Pfaffl 2001), with EF1α serving as a reference gene38. Relative levels of EF1α were confirmed to be approximately equal across all treatments.
Results
Life history responses in constant temperature regimes.
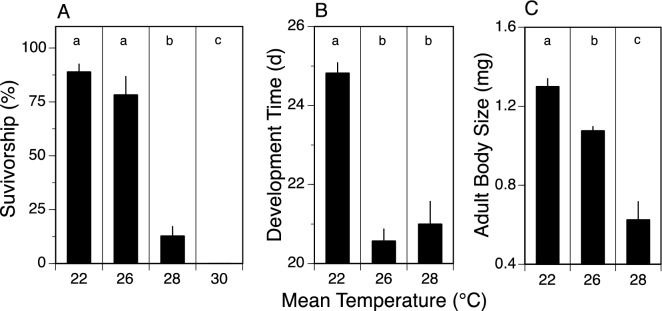
In constant temperature treatments, survivorship was 89% at 22° and 78% at 26 °C, but decreased to only 12.8% at 28 °C. No larvae survived to adulthood at 30 °C (Fig. 1A). Development time decreased from 24.8 d at 22 °C to 20.6 d at 26 °C, and then was unchanged (20.6 vs 21.0 d) between 26 and 28 °C (Fig. 1B). Adult body mass decreased gradually from 1.3 mg at 22 °C to 1.1 mg at 26 °C and 0.6 mg at 28 °C (Fig. 1C). Note that adult body size (as dry mass) is an excellent predictor of fecundity25; Funk, Jackson, Sweeney, unpublished data), and therefore individual fitness. When comparing 26° and 28 °C, the dramatic increase in mortality, absence of a decrease in development time, and decrease in adult body size establishes 28 °C as a clearly detrimental temperature based on these life history outcomes.
Figure 1.
Measures (mean ± 1SE) for survivorship (A), development time (B), and adult body size (dry weight) (C) across four constant temperature treatments (22, 26, 28, and 30 °C), with statistically significant differences (1-way ANOVA with Tukey’s multiple comparison test, p ≤ 0.05) between treatments indicated by different letters over bars (a,b,c). Survival, development time, and adult body size identify 28 °C as detrimental relative to 26 °C and/or 22 °C. Each mean represents the results for 7–9 replicate rearing jars, each containing 50 larvae.
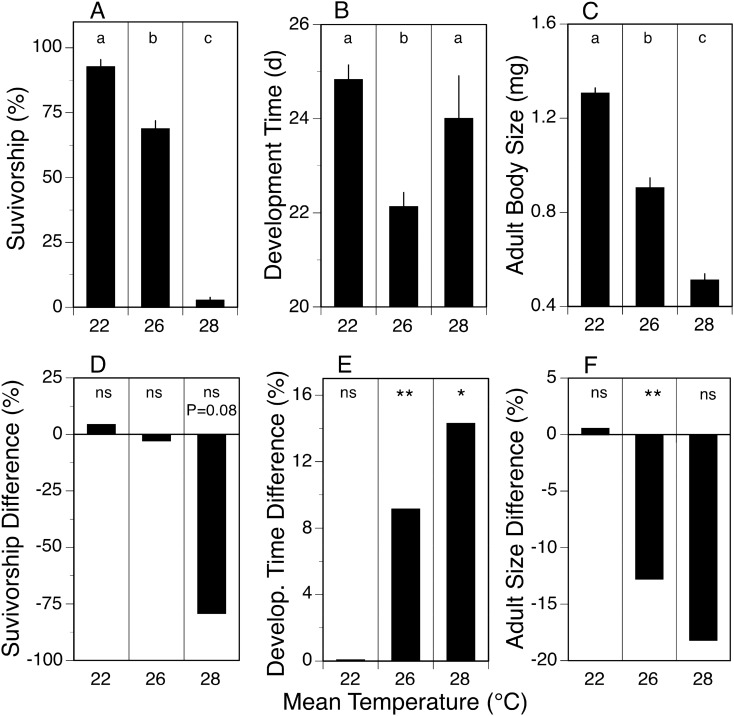
We used this information to rear N. triangulifer under three variable temperature regimens (Regimen 1: diel minimum of 19.5 °C and maximum of 24.5 °C, mean 22 °C; Regimen 2: 23.5–28.5 °C, mean 26 °C; Regimen 3: 25.5–30.5 °C, mean 28 °C) from egg hatch to adult. The responses for survivorship, development time, and body size in variable temperature treatments were similar to what was observed in constant temperature treatments – survivorship, development time, and body size decreased as mean temperature increased (Fig. 2A–C).
Figure 2.
Measures (mean ± 1SE) for survivorship (A), development time (B), and adult body size (dry weight) (C) across three variable temperature treatments (22, 26, and 28 °C). Each variable temperature treatment consisted of means from 4 replicate rearing jars, each containing 50 larvae. Statistically significant differences (1-way ANOVA with Tukey’s multiple comparison test, p ≤ 0.05) between variable treatments indicated by different letters over bars (a,b,c). Difference between constant and variable temperature treatments expressed as % difference (A–C relative to Fig. 1A–C) for survivorship (D), development time (E), and adult body size (F). Statistical significance (Student’s t-test) indicated by ns (p > 0.05), * (p ≤ 0.05) or ** (p ≤ 0.01).
Molting experiment
We reared mayflies in well plates so that we could monitor individual larvae on a daily basis and quantify the total number and frequency of molts. In two separate trials (trial 1 comparing 18 °C and 26 °C, and trial 2 comparing 22 °C and 26 °C), we found that there was no statistical difference in the total number of molts required to complete larval development within each trial. In trial 1, larvae averaged 13.4 ± 1.9 and 14.3 ± 1.3 molts at 18 °C and 26 °C, respectively. In trial 2, larvae averaged 13.5 ± 1.4 and 11.8 ± 1.6 molts respectively. Thus, because warmer temperature reduces development time and body size, there is less time between molts (e.g. 13 molts in 30 d vs 21 d), and molts later in development occur at a smaller size.
Life history outcomes in variable vs constant temperatures
The response to being exposed to a variable temperature regime of ± 2.5 °C versus a constant temperature regime depended on the mean temperature examined, and the life history parameter being measured. The variable temperature regime had no significant effect on survivorship, development time, and adult body size when mean temperature was 22 °C (Fig. 2D,E,F). In contrast when mean temperature was 26 °C, the variable temperature regime had no significant effect on survivorship (Fig. 2D), but development time was 9.1% longer (Fig. 2E) while adult body size was 12.8% less (Fig. 2F) than in the constant temperature treatment. Finally, survivorship in the variable temperature treatment at 28 °C was 79.2% lower (nearly significant at p = 0.08), development time was 14.3% greater, and adult body size was 18.2% smaller relative to the constant temperature treatment at 28 °C. The differences between the constant and variable temperature treatments at 28 °C was not always significant due to low survivorship in both treatments (i.e., few individuals survived). These results show that daily exposure of larvae to brief periods of 24.5 °C had no impact on the life history traits measured, but brief periods of 28.5 °C in the variable 26 °C treatment had a negative impact on development time and adult body size, and brief periods of 30.5 °C had a negative impact on survivorship, development time, and adult body size. Thus, larvae in warmer, variable temperature treatments responded negatively (e.g., reduced survivorship and adult size, increased development time) relative to constant temperature treatments, and the negative response intensified as temperature increased. The differences between variable and constant temperature treatments at 26 °C highlight that time spent at 23.5 °C does not compensate for the negative impact of a brief exposure to 28.5 °C.
Differential gene expression in variable thermal regimes
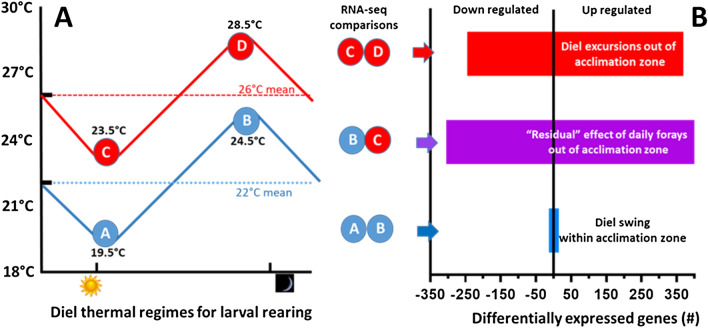
To better understand the influence of variable (diel) thermal regimes on global RNA expression patterns (Fig. 3), we first compared RNA-seq data in larvae that were reared entirely within the thermal acclimation zone (Regimen 1: diel cycles between 19.5 °C and 24.5 °C, daily mean 22 °C) (See Table S1). Remarkably few transcripts (28) were differentially expressed in larvae sampled at 19.5 °C vs. 24.5 °C that met our criteria of a false discovery rate (FDR) < 0.05. Of the 28 differentially expressed transcripts, 7 were upregulated and 19 were downregulated, but only 17 were named or have known or inferred function (A vs B in Fig. 3B). These few genes with known function (Table S1) are largely associated with functions related to circadian clock and/or visual functions such as rhodopsin-specific isozyme, peptidyl-prolyl cis–trans isomerase (protein folding, possible roles in co-chaperone activities and steroid hormone receptor trafficking), However, no thermal stress related responses were noted in this comparison.
Figure 3.
(A) Thermal regimens for rearing and sampling strategy for RNA-seq analyses. Circled letters represent the sampling temperatures for mature larvae reared for their entire larval development period. The 22 °C mean temperature regime (regimen 1) oscillated daily between 19.5 and 24.5 °C. The 26 °C mean temperature regime (regimen 2) oscillated daily between 23.5 and 28.5 °C. (B) The numbers of differentially expressed genes associated with each pairwise comparison.
In contrast, when larvae were subjected to daily excursions out the thermal acclimation zone (Regimen 2: diel fluctuations between 23.5 and 28.5 °C, daily mean 26 °C), we identified 514 differentially expressed genes, with 369 (60%) upregulated and 245 (40%) downregulated genes (C vs D in Fig. 3B) (Table S2). A cross regimen (regimen 3) comparison was made between larvae sampled at 24.5 °C in regimen 1, and larvae sampled at 23.5 °C in regimen 2. Here we identified 806 differentially expressed genes, with 501 (62%) upregulated and 305 (38%) downregulated genes (B vs C in Fig. 3B) (Table S3). A principal components analysis of the gene expression data (Fig. 4) shows similarities within regimen 1. However, within regimen 2, responses to 28.5 °C are clearly separate from 23.5 °C. For completeness, the other pairwise comparisons are provide in Tables S4−S6.
Functional comparisons of diel fluctuations outside of the thermal acclimation zone
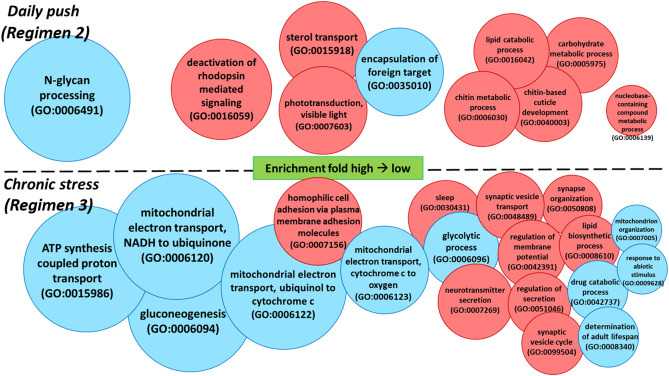
To further analyze and distinguish between functional groups of the differentially expressed genes that responded to transient daily thermal challenge (Fig. 3; Regimen 2), we separated the gene ontology (GO) enrichment analysis into lists of up- and down-regulated genes (Table S3). Comparing the daily high soak (28.5 °C) to low soak (23.5 °C) samples in regimen 2, GO enrichment analysis through web-based tool (PANTHER) identified up-regulated genes associated with GO enrichment analyses for biological processesof sterol transport, chitin metabolic process, lipid catabolic process, chitin-based cuticle development, carbohydrate metabolic process and nucleobase-containing compound metabolic process (Fig. 5 upper panel, red circles). Other up-regulated processes were associated with clock/time of day as described above and included deactivation of rhodopsin mediated signaling and phototransduction. The GO enrichment analysis identified down-regulated genes with major GO enrichment analyses for biological processesin N-glycan processing (packaging of monosaccharides) and encapsulation of foreign target (Fig. 5 upper panel, blue circles). Reactome pathway analysis combining both up- and down-regulated genes suggested that pathways involved in lipid and lipoprotein metabolism are significantly enriched during thermal challenge. Of the genes residing in the pathway, 63% were up-regulated while 37% were down regulated.
Figure 5.
Gene ontology (GO) enrichment analysis results showing GO enrichment analyses for biological processes affected in regimen 2 (top panel and C–D comparison from Fig. 3), and regimen 3 (bottom panel, B–C comparison from Fig. 3). Biological functions are represented as upregulated (red) and downregulated (blue) and are size scaled based on calculated fold enrichment of our differentially expressed genes dataset compared to drosophila database.
Functional comparisons of the residual effects of chronic but transient thermal stress
To assess the lingering or persistent effects of transient daily forays into challenging temperatures, we compared RNA-seq profiles of larvae sampled at 23.5 °C (the low soak (cool) portion of regimen 2) with those sampled at 24.5 °C (the high soak (hot) portion of regimen 1) (See B-C comparison from Fig. 3) (Table S4). GO enrichment analysis identified up-regulated genes associated with several biological process such as neurotransmitter secretion, synaptic vesicle transport/cycle/organization, and lipid biosynthetic process (Fig. 4, lower panel, red circles). Down-regulated genes were enriched in GO enrichment analyses for biological processes such as ATP synthesis coupled proton transport, mitochondrial electron transport gluconeogenesis, glycolytic process, drug catabolic process, and determination of adult lifespan (Fig. 4, lower panel, blue circles). Reactome pathway analysis combing both up and down-regulated genes suggested enrichment in pathways including acetylcholine neurotransmitter release cycle, formation of ATP by chemiosmotic coupling, complex l biogenesis, PLC beta mediated events and glycolysis. Of the pathways involved, 80% and 71% of the genes are up-regulated in the acetylcholine neurotransmitter release cycle and the PLC beta mediated events, respectively. Genes involved in metabolism pathways- formation of ATP by chemiosmotic coupling, complex l biogenesis and glycolysis, are all strongly down-regulated (92%, 84% and 85%, respectively).
qPCR confirmation
To validate the RNA sequencing results, we used separate aliquots of the biological samples for sequencing along with two additional samples, and randomly selected 11 genes from either regime 2 or regimen 3 pairwise-GO enrichment analysis groups for quantitative real-time PCR confirmation. The genes chosen were significantly differentially expressed from both groups. The gene set is comprised of different functional groups and included 5 down-regulated genes and 6 upregulated genes. For both RNAseq and qPCR shown in Fig. 6A, genes cht 6 isoform C, probable chitinase 10, cuticle protein 66D, aromatic-L-amino-acid decarboxylase and alpha amylase B expressions are pairwise comparison of daily high soak (28.5 °C) relative to low soak (23.5 °C) in regime 2; whereas genes bcDNA.GH02901, elongation of very long chain fatty acids protein, hexosaminidase 1 isoform A, poly (ADP-ribose) glycohydrolase and cytochrome c oxidase subunit 7A are pairwise comparison of regimen 3: 23.5 °C relative to 24.5 °C. EF1α was used as an internal control for qPCR to normalize expression before pairwise comparison. While Fig. 6A showed that our qPCR results had relatively stronger expression levels in some genes (we added two more biological replicates in addition to those sent for RNAseq for qPCR analysis), overall the RNA-seq and qPCR results were consistent. Figure 6B shows the relationship between RNA-seq and qPCR gene expression (y = 1.245x − 0.31, R2 = 0.90). Primer sequences used for this validation are provided in Table S5).
Figure 6.
qPCR analysis confirming transcriptomic results. (A) The 11 genes selected for qPCR analysis showed overall consistency with RNA-seq despite some of the gene expression levels appeared stronger. (B) Correlation between RNA-seq and qPCR gene expression. EF1a is used as a housekeeping gene to calculate relative fold change.
Discussion
The changing thermal regimes of freshwater ecosystems require that we better understand species responses to temperature at different time scales (e.g. hourly, daily, seasonally, annually). Recent efforts have made progress in our understanding of both short-term36,37 and long-term38 thermal challenge in N. triangulifer. Even so, the effect of ecologically relevant diel thermal variation with daily excursions into stressful temperatures on long-term survival and development remains poorly understood. It was critical for this study to establish an unambiguously stressful temperature in N. triangulifer (28 °C) such that we could explore the consequences of ecologically relevant transient exposures at both the levels of transcript expression and associated life history outcomes.
Previous studies in this species explored physiological processes associated with chronic thermal stress and indicated that lipid depletion, reduced trehalose synthase and increased histamine and heat shock protein (HSP) gene expression were associated with chronic thermal stress38. Here we opted for the RNA-seq approach so that we could obtain an unbiased view of transcript expression changes and assess even low-abundance transcripts45–49.
Within regimen comparisons
Few (28) transcripts were differentially expressed in Regimen 1, where rearing temperatures fluctuated well within the typical temperatures experienced in the source population of this lab-reared clone. In contrast, mayflies reared under Regimen 2—with daily forays outside the thermal acclimation zone (diel fluctuations between 23.5 and 28.5 °C, daily mean 26 °C) resulted in a total of 614 significantly differentially expressed transcripts, with more transcripts being upregulated at the warmer temperature than down-regulated. Upregulated processes included the deactivation of rhodopsin mediated signaling and phototransduction. Rhodopsin has long been known as a light-sensitive receptor protein involved in visual phototransduction50. Interestingly, research have also showed that the rhodopsin signaling pathway is involved in light-independent roles, such as thermosensory signaling51. Moreover, a daily temperature oscillation (< 5 °C) within a physiological range synchronizes circadian rhythms in D. melanogaster and can also be independent from light-entrainable oscillators52,53. However, we suspect that in our case the enriched biological function is independent from thermal effects. Our sampling times and thermal fluctuation range (5 °C) were the same in pairwise comparison groups both within and outside the thermal acclimation zone. While transcript numbers were not sufficient enough for GO enrichment analyses for biological processes in the Regimen 1 comparison, some individual transcripts related to circadian clock and/or visual functions were also differentially expressed. This is not surprising because sampling time A is just after lights on (sunrise) and sampling time B is just before lights off (sunset), and are timed to important elements in a circadian rhythm or diel cycle. Therefore, this suggests that these GO enrichment analyses for biological processes observed for the variable 22 °C treatment are independent from thermal effects.
During the transient thermal stress in regimen 2, there was a reduction of transcripts related to N-glycan processing in the GO enrichment analyses for biological processes. N-glycan plays an extremely important role in proper protein folding, and the data suggests that this daily thermal push, while transient, is stressful enough to effect physiological processes at the molecular level in N. triangulifer. This is also consistent with our RNAseq data as well as previous findings, that chaperone protein HSP90 was upregulated under thermal stress to aid for proper protein folding36. Increased expression of transcripts associated with lipid catabolism and carbohydrate metabolism was observed during transient thermal stress. Reactome pathway analysis suggests that metabolism of lipids and lipoproteins is highly enriched, and that more than half of the transcripts involved in the pathway are enriched, supporting the GO results. These responses are consistent with previous metabolomic studies of chronic heat stress in N. triangulifer36 where depletion of certain lipids was observed. We did not find evidence of hypoxia signaling at chronic thermal limits (see54–56).
Transient thermal stress in regimen 2 also simultaneously stimulated chitin metabolic processes and chitin- based cuticle development. The exoskeleton of insects is an assembly of chitin and cuticle proteins57. Interestingly, nearly half of the top 20 most upregulated transcripts in this pairwise comparison group were related to chitin catabolism or cuticle development. It has been suggested that cuticle is sensitive to temperature changes58–61, and these changes in cuticular transcript expression are intriguing because our separate molting experiments suggest that the number of molts required to reach adulthood is “fixed”, with warmer temperatures simply accelerating the process. Under warmer conditions, larvae molt more frequently and at smaller body sizes than larvae reared under cooler conditions. Thus, molting is not determined by body size. It is interesting that even brief thermal ramping stimulates molting. Camp et al.62 observed that larvae were more likely to molt when put on the thermal ramp than when maintained at static temperatures.
Hormone signaling (i.e. juvenile hormone and ecdysteriods) and its involvement with cuticle proteins in regulating insect molting process has been well studied63–65. However, the linkage between thermal effects and molting frequency (perhaps through hormone signaling up-regulating cuticle protein genes or other unknown physiological mechanisms) has not been elucidated. The transcriptome analysis in our study may have revealed that thermal stress affects chitin catabolic process and molting cycle, still it remains unknown why mayflies tend to molt more frequently at warmer temperatures when the process of molting itself requires high energy cost. Regardless, the findings that total molts per life cycle is relatively fixed and time between molts decreases as temperature increases may help explain why mayflies result in smaller body size under warm temperatures (Fig. 1).
Across regimen comparisons
A second goal of our study was to understand lingering effects of chronic but transient thermal stress. To achieve this goal, we compared transcript expression profiles between the warmer temperature of Regimen 1 (24.5 °C) and the cooler recovery temperature of Regimen 2 (23.5 °C) (regimen 3, e.g. the BC comparison in Fig. 3). We were surprised by how many genes were differentially expressed between these groups.
We found that the chronically stressed population had significant reductions in the expression of transcripts associated with ATP synthesis and the mitochondrial electron transport chain relative to the unstressed population. Both gluconeogenesis and glycolytic processes were simultaneously lower in the chronically stressed population. However, lipid biosynthesis was actually upregulated in the chronically stressed population, which is interesting because lipid catabolism was experienced in this population during the thermal challenge at 28.5 °C. It may be that the increased activity of lipid biosynthesis at the recovery temperature reflects a compensatory response to the heat stress. The finding of downregulation in energy metabolism was consistent with our previous study where we analyzed targeted metabolite end products in N. triangulifer exposed to chronic thermal stress statically38.
The other major difference between the two treatments was in the increased activity of neurotransmitter secretion, synaptic vesicle cycle and transport, and regulation of membrane potential. Many studies have provided evidence of hormonal and neurotransmitter change from the endocrinological aspect of insect stress response. Davenport and Evans66 linked the secretion of biogenic amines, which can function as neurohormones in response to stress. However, the stress response reaction depends on the speed of carbohydrate and lipid metabolic responses. The data from our pairwise comparison group in regimen 2 may suggest that while temperature rises slowly to a stressful level, the increase of energy-related metabolic processes to cope with that increasing thermal challenge may increase slowly as well. This helps explain the absences of neurotransmission related GO enrichment analyses for biological processesin regimen 2 (Figs. 3, 5). Interestingly, in our previous study where mayflies were subjected to chronic thermal stress, we found an increase in histamine and dopamine, both biogenic amines play the role as neurotransmitter38. Consistent with our current findings in this study, these data suggest that the thermal stress-induced neurological activities are also affected under chronically stressful conditions.
Life history outcomes
We found that in regimen 1 (within the thermal acclimation zone), it did not matter if larvae experience constant vs. variable temperature. Survival, development time and adult sizes were not statistically different. However, when larvae are transiently but repeatedly pushed outside their thermal acclimation zone (regimen 2), both development time and fitness (inferred from adult body sizes) are negatively affected. A more detailed treatment life history outcomes across more thermal treatments is needed, however these results suggest that time spent at harmful temperatures is not offset by time spent at more ideal temperatures.
Together, our study shows that N. triangulifer larvae do not recover from daily forays into thermally challenging conditions. RNA-seq and GO enrichment analysis support our previous findings of energy source re-allocation under thermal stress. In addition, the study also emphasizes the role of molting in mediating thermal performance. Our study helps elucidate how a modest increase in daily thermal fluctuation affects transcript expression and its associated GO enrichment analyses for biological processes and ultimately life history outcomes in N. triangulifer and likely other aquatic insects.
Supplementary information
Acknowledgements
The authors acknowledge the support of the National Science Foundation (IOS-1456191 and IOS-1455906), the NIEHS funded NC State Center for Human Health and the Environment (P30ES025128), and the Stroud Endowment for Environmental Research. Sarah Orr and Gerald LeBlanc and anonymous reviewers provided valuable editorial comments.
Author contributions
D.B.B. and H.C. wrote the manuscript. D.H.F., J.K.J. and B.W.S. conducted the rearing experiments and associated data analysis. J.K.J. edited the manuscript. H.C. and D.D.J. analyzed the RNA-seq data. D.B.B. conceived of the research strategy.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-75064-y.
References
- 1.Angilletta MJ., Jr . Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford: Oxford University Press; 2009. [Google Scholar]
- 2.Atkinson D. Temperature and organism size: a biological law for ectortherms? Adv. Ecol. Res. 1994;25:1–58. doi: 10.1016/S0065-2504(08)60212-3. [DOI] [Google Scholar]
- 3.Atkinson D, Sibly RM. Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol. Evol. 1997;12:235–239. doi: 10.1016/S0169-5347(97)01058-6. [DOI] [PubMed] [Google Scholar]
- 4.Hynes HBN. The ecology of stream insects. Ann. Rev. Entomol. 1970;15:25–42. doi: 10.1146/annurev.en.15.010170.000325. [DOI] [Google Scholar]
- 5.Sweeney BW, Vannote RL. Size variation and the distribution of hemimetabolous aquatic insects: two thermal equilibrium hypotheses. Science. 1978;200:444–446. doi: 10.1126/science.200.4340.444. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney BW, Vannote RL. Ephemerella mayflies of Whie Clay Creek: bioenergetic and ecological relationships among six coexisting species. Ecology. 1981;62:1353–1369. doi: 10.2307/1937299. [DOI] [Google Scholar]
- 7.Sweeney BW, Vannote RL. Influence of food quality and temperature on life-history characteristics of the parthenogenetic mayfly Cloeon triangulifer. Freshw. Biol. 1984;14:621–630. doi: 10.1111/j.1365-2427.1984.tb00181.x. [DOI] [Google Scholar]
- 8.Sweeney BW, Funk DH, Jackson JK, Camp AA, Buchwalter D. Why a mayfly Cloeon dipterum (Ephemeroptera: Baetidae) gets smaller as temperatures warm. Freshw. Sci. 2018;37:64–81. doi: 10.1086/696611. [DOI] [Google Scholar]
- 9.Caissie D. The thermal regime of rivers: a review. Freshw. Biol. 2006;51:1389–1406. doi: 10.1111/j.1365-2427.2006.01597.x. [DOI] [Google Scholar]
- 10.Haidekker A, Hering D. Relationship between benthic insects (Ephemeroptera, Plecoptera, Coleoptera, Trichoptera) and temperature in small and medium-sized streams in Germany: a multivariate study. Aquat. Ecol. 2008;42:463–481. doi: 10.1007/s10452-007-9097-z. [DOI] [Google Scholar]
- 11.Woodward G, Perkins DM, Brown LE. Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philos. Trans. R. Soc. B Biol. Sci. 2010;365:2093–2106. doi: 10.1098/rstb.2010.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodward G, et al. The effects of climatic fluctuations and extreme events on running water ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2016;371:20150274. doi: 10.1098/rstb.2015.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenter SR, Fisher SG, Grimm NB, Ktchell JF. Global change and freshwater ecosystems. Annu. Rev. Ecol. Syst. 1992;23:119–139. doi: 10.1146/annurev.es.23.110192.001003. [DOI] [Google Scholar]
- 14.El-Jabi N, Caissie D, Turkkan N. Water quality index assessment under climate change. J. Water Resour. Prot. 2014;06:533–542. doi: 10.4236/jwarp.2014.66052. [DOI] [Google Scholar]
- 15.Madden N, Lewis A, Davis M. Thermal effluent from the power sector: an analysis of once-through cooling system impacts on surface water temperature. Environ. Res. Lett. 2013;8:035006. doi: 10.1088/1748-9326/8/3/035006. [DOI] [Google Scholar]
- 16.Null SE, Ligare ST, Viers JH. A method to consider whether dams mitigate climate change effects on stream temperatures. JAWRA J. Am. Water Resour. Assoc. 2013;49:1456–1472. doi: 10.1111/jawr.12102. [DOI] [Google Scholar]
- 17.Schulte PM, Healy TM, Fangue NA. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 2011;51:691–702. doi: 10.1093/icb/icr097. [DOI] [PubMed] [Google Scholar]
- 18.Sweeney BW, Jackson JK, Newbold JD, Funk DH. Climate change and the life histories and biogeography of aquatic insects in Eastern North America. In: Firth P, Fisher S, editors. Global Cilmate Change and Freshwater Ecosystems. Berlin: Springer; 1990. pp. 143–176. [Google Scholar]
- 19.Hawkins CP, Norris RH, Hogue JN, Feminella JW. Development and evaluation of predictive models for measuring the biological integrity of streams. Ecol. Appl. 2000;10:1456–1477. doi: 10.1890/1051-0761(2000)010[1456:DAEOPM]2.0.CO;2. [DOI] [Google Scholar]
- 20.Hodkinson ID, Jackson JK. Terrestrial and aquatic invertebrates as bioindicators for environmental monitoring, with particular reference to mountain ecosystems. Environ. Manag. 2005;35:649–666. doi: 10.1007/s00267-004-0211-x. [DOI] [PubMed] [Google Scholar]
- 21.Bonada N, Prat N, Resh VH, Statzner B. Developments in aquatic insect biomonitoring: a comparative analysis of recent approaches. Annu. Rev. Entomol. 2006;51:495–523. doi: 10.1146/annurev.ento.51.110104.151124. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson D. Effects of temperature on the size of aquatic ectotherms: exceptions to the general rule. J. Therm. Biol. 1995;20:61–74. doi: 10.1016/0306-4565(94)00028-H. [DOI] [Google Scholar]
- 23.Kingsolver JG, Huey RB. Size, temperature, and fitness: three rules. Evol. Ecol. Res. 2008;10:251–268. [Google Scholar]
- 24.Colinet H, Sinclair BJ, Vernon P, Renault D. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 2015;60:123–140. doi: 10.1146/annurev-ento-010814-021017. [DOI] [PubMed] [Google Scholar]
- 25.Conley JM, Funk DH, Buchwalter DB. Selenium bioaccumulation and maternal transfer in the mayfly Centroptilum triangulifer in a life-cycle, periphyton-biofilm trophic assay. Environ. Sci. Technol. 2009;43:7952–7957. doi: 10.1021/es9016377. [DOI] [PubMed] [Google Scholar]
- 26.Conley JM, Funk DH, Cariello NJ, Buchwalter DB. Food rationing affects dietary selenium bioaccumulation and life cycle performance in the mayfly Centroptilum triangulifer. Ecotoxicology. 2011;20:1840–1851. doi: 10.1007/s10646-011-0722-1. [DOI] [PubMed] [Google Scholar]
- 27.Xie LT, et al. Cadmium biodynamics in the oligochaete Lumbriculus variegatus and its implications for trophic transfer. Aquat. Toxicol. 2008;86:265–271. doi: 10.1016/j.aquatox.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Xie L, Buchwalter DB. Cadmium exposure route affects antioxidant responses in the mayfly Centroptilum triangulifer. Aquat. Toxicol. 2011;105:199–205. doi: 10.1016/j.aquatox.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Kim KS, Funk DH, Buchwalter DB. Dietary (periphyton) and aqueous Zn bioaccumulation dynamics in the mayfly Centroptilum triangulifer. Ecotoxicology. 2012;21:2288–2296. doi: 10.1007/s10646-012-0985-1. [DOI] [PubMed] [Google Scholar]
- 30.Wesner JS, Kraus JM, Schmidt TS, Walters DM, Clements WH. Metamorphosis enhances the effects of metal exposure on the mayfly Centroptilum triangulifer. Environ. Sci. Technol. 2014;48:10415–10422. doi: 10.1021/es501914y. [DOI] [PubMed] [Google Scholar]
- 31.Soucek DJ, Dickinson A. Full-life chronic toxicity of sodium salts to the mayfly Neocloeon triangulifer in tests with laboratory cultured food. Environ. Toxicol. Chem. 2015;34:2126–2137. doi: 10.1002/etc.3038. [DOI] [PubMed] [Google Scholar]
- 32.Kunz JL, et al. Use of reconstituted waters to evaluate effects of elevated major ions associated with mountaintop coal mining on freshwater invertebrates. Environ. Toxicol. Chem. 2013;32:2826–2835. doi: 10.1002/etc.2391. [DOI] [PubMed] [Google Scholar]
- 33.Orr SE, Buchwalter DB. It’s all about the fluxes: temperature influences ion transport and toxicity in aquatic insects. Aquat. Toxicol. 2020;221:105405. doi: 10.1016/j.aquatox.2020.105405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchwalter D, Scheibener S, Chou H, Soucek D, Elphick J. Are sulfate effects in the mayfly Neocloeon triangulifer driven by the cost of ion regulation? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;374:20180013. doi: 10.1098/rstb.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson John K, Funk David H. Temperature affects acute mayfly responses to elevated salinity: implications for toxicity of road de-icing salts. Philos. Trans. R. Soc. B Biol. Sci. 2019;374:20180081. doi: 10.1098/rstb.2018.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim KS, et al. Physiological responses to short-term thermal stress in mayfly (Neocloeon triangulifer) larvae in relation to upper thermal limits. J. Exp. Biol. 2017;220:2598–2605. doi: 10.1242/jeb.156919. [DOI] [PubMed] [Google Scholar]
- 37.Chou H, Pathmasiri W, Deese-Spruill J, Sumner S, Buchwalter DB. Metabolomics reveal physiological changes in mayfly larvae (Neocloeon triangulifer) at ecological upper thermal limits. J. Insect. Physiol. 2017 doi: 10.1016/j.jinsphys.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou H, et al. The good, the bad, and the lethal: gene expression and metabolomics reveal physiological mechanisms underlying chronic thermal effects in mayfly larvae (Neocloeon triangulifer) Front. Ecol. Evol. 2018;101:107–112. [Google Scholar]
- 39.Funk DH, Jackson JK, Sweeney BW. Taxonomy and genetics of the parthenogenetic mayfly Centroptilum triangulifer and its sexual sister Centroptilum alamance (Ephemeroptera: Baetidae) J. North Am. Benthol. Soc. 2006;25:417–429. doi: 10.1899/0887-3593(2006)25[417:TAGOTP]2.0.CO;2. [DOI] [Google Scholar]
- 40.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haas BJ, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013;8:1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camacho C, et al. BLAST+: architecture and applications. BMC Bioinform. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- 45.Marioni JC, Mason CE, Mane SM, Stephens M, Gilad Y. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008;18:1509–1517. doi: 10.1101/gr.079558.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagalakshmi U, et al. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science. 2008;320:1344–1349. doi: 10.1126/science.1158441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramani AK, et al. High resolution transcriptome maps for wild-type and nonsense-mediated decay-defective Caenorhabditis elegans. Genome Biol. 2009;10:R101. doi: 10.1186/gb-2009-10-9-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yassour M, et al. Ab initio construction of a eukaryotic transcriptome by massively parallel mRNA sequencing. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3264–3269. doi: 10.1073/pnas.0812841106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katz B, Minke B. Drosophila photoreceptors and signaling mechanisms. Front. Cell. Neurosci. 2009;3:2. doi: 10.3389/neuro.03.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen WL, et al. Function of rhodopsin in temperature discrimination in drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- 52.Wheeler DA, Hamblen-Coyle MJ, Dushay MS, Hall JC. Behavior in light–dark cycles of drosophila mutants that are arrhythmic, blind, or both. J. Biol. Rhythms. 2016 doi: 10.1177/074873049300800106. [DOI] [PubMed] [Google Scholar]
- 53.Miyasako Y, Umezaki Y, Tomioka K. Separate sets of cerebral clock neurons are responsible for light and temperature entrainment of drosophila circadian locomotor rhythms. J. Biol. Rhythms. 2007;22:115–126. doi: 10.1177/0748730407299344. [DOI] [PubMed] [Google Scholar]
- 54.Verberk WC, Bilton DT. Oxygen-limited thermal tolerance is seen in a plastron-breathing insect and can be induced in a bimodal gas exchanger. J. Exp. Biol. 2015;218:2083–2088. doi: 10.1242/jeb.119560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verberk WC, Bilton DT, Calosi P, Spicer JI. Oxygen supply in aquatic ectotherms: partial pressure and solubility together explain biodiversity and size patterns. Ecology. 2011;92:1565–1572. doi: 10.1890/10-2369.1. [DOI] [PubMed] [Google Scholar]
- 56.Verberk WCEP, Sommer U, Davidson RL, Viant MR. Anaerobic metabolism at thermal extremes: a metabolomic test of the oxygen limitation hypothesis in an aquatic insect. Integr. Comp. Biol. 2013;53:609–619. doi: 10.1093/icb/ict015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merzendorfer H, Zimoch L. Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003;206:4393–4412. doi: 10.1242/jeb.00709. [DOI] [PubMed] [Google Scholar]
- 58.White, B. J. et al. Localization of candidate regions maintaining a common polymorphic inversion (2La) in Anopheles gambiae. PLoS Genet.preprint, e217 (2005). [DOI] [PMC free article] [PubMed]
- 59.Zhao L, Wit J, Svetec N, Begun DJ. Parallel gene expression differences between low and high latitude populations of drosophila melanogaster and D. simulans. PLOS Genet. 2015;11:e1005184. doi: 10.1371/journal.pgen.1005184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beament JWL. The waterproofing mechanism of arthropods: I. The effect of temperature on cuticle permeability in terrestrial insects and ticks. J. Exp. Biol. 1959;36:391–422. [Google Scholar]
- 61.Dennis AB, Dunning LT, Sinclair BJ, Buckley TR. Parallel molecular routes to cold adaptation in eight genera of New Zealand stick insects. Sci. Rep. 2015;5:13965. doi: 10.1038/srep13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Camp AA, Funk DH, Buchwalter DB. A sressful shortness of breath: molting disrupts breathing in the mayfly Cloeon dipterum. Freshw. Sci. 2014;33:695–699. doi: 10.1086/677899. [DOI] [Google Scholar]
- 63.Butenandt A, Karlson P. Über die Isolierung eines Metamorphose-Hormons der Insekten in kristallisierter Form. Z. Für Naturforschung B. 1954;9:389–391. doi: 10.1515/znb-1954-0601. [DOI] [Google Scholar]
- 64.Charles JP. The regulation of expression of insect cuticle protein genes. Insect. Biochem. Mol. Biol. 2010;40:205–213. doi: 10.1016/j.ibmb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Rees HH. Zooecdysteroids: structure and occurrence. In: Koolman J, editor. Ecdysone: From Chemistry to Mode of Action. Berlin: Thieme; 1989. [Google Scholar]
- 66.Davenport AP, Evans PD. Stress-induced changes in the octopamine levels of insect haemolymph. Insect. Biochem. 1984;14:135–143. doi: 10.1016/0020-1790(84)90021-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.