Highlights
-
•
The serum lipid patterns of GCNEI differed from those of pure gastric adenocarcinoma significantly.
-
•
Serum lipid levels correlated to the progression of GCNEI.
-
•
Serum lipid levels impacted the risk of the occurrence of GCNEI.
Keywords: Stomach neoplasms, Adenocarcinoma, Neuroendocrine neoplasms, Serum lipids
Abstract
Purpose
Dyslipidemia was associated with gastric adenocarcinoma or neuroendocrine tumors, but its role in a more malignant entity, gastric cancer with neuroendocrine immunophenotypes (GCNEI), was unclarified. This study sought to explore the relationship between serum lipid levels and the biological behaviors of gastric cancer with neuroendocrine immunophenotypes (GCNEI).
Methods
Patients with neuroendocrine carcinoma (NEC), GC with NEC components (GC-NEC), or GC expressing NE marker(s) but no NE morphology (GC-NENM) were enrolled from three centers. Their preoperative serum lipid levels, demographic, and clinicopathological information were analyzed and compared with those of patients with pure adenocarcinoma (PAC) or a background population selected from 10,061 health-check people by propensity-score matching.
Results
A total of 342 GCNEI patients were enrolled. Compared with the background population, total cholesterol (TCHO) and high-density lipoprotein cholesterol (HDL-C) levels were lower in GCNEI. Compared with PAC, GC-NENM and GC-NEC showed lower triglyceride (TG) levels, while, carcinoma with NE morphology showed higher low-density lipoprotein cholesterol (LDL-C) levels. Among GCNEI subtypes, GC-NEC differed from the others by higher LDL-C and non-HDL-C levels. A higher LDL-C level and(or) lower TG, HDL-C levels correlated to higher stages or large tumor sizes in GC-NENM, and a lower HDL-C level correlated to large tumor sizes in GC-NEC. A higher LDL-C level, lower TG, HDL-C, and non-HDL levels increased the risk of GC-NEC, and lower TG, and HDL-C levels increased the risk of GC-NENM and NEC.
Conclusion
GCNEI had distinct and heterogeneous serum lipid patterns, which correlated to tumor development and progression.
Introduction
Gastric cancer with neuroendocrine immunophenotypes (GCNEI) is a distinct and heterogeneous cohort of gastric malignant tumors, characterized by the varying expression of the neuroendocrine-associated protein. According to the 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System, GCNEI includes neuroendocrine carcinoma (NEC) and mixed adeno-neuroendocrine carcinoma (MANEC), of which the entire or partial tumor showed NE morphology (NEM) in addition to NE immunophenotypes, and either component of the latter should exceed 30% [5]. In addition, GC with <30% NEC component and GC expressing NE marker(s) but no NEM (GC-NENM) also meet the criteria of GCNEI, although they have not been listed in WHO classification yet. Numerous previous studies and ours have revealed that, compared to pure adenocarcinoma (PAC), GCNEI showed more malignant clinicopathological features and worse prognosis [9,18,23]. However, the underlying mechanism remains elusive so far.
The significance of serum lipids, including triglyceride (TG), total cholesterol (TCHO), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and non-HDL-C, have been evaluated in various tumors, and several of them have been proven to be associated with the biological behaviors of PAC [13] and well-differentiated gastroenteropancreatic/rectal neuroendocrine tumor (NET) ([8], Santos, [20]), including tumorigenesis ([8,13], Santos, [20]), tumor size [22], histological differentiation [22], distal metastasis [6], neoadjuvant chemotherapy response [22] and prognosis [21]. Although, the association between serum lipids and stomach cancer or NET was identified, due to the relative lack of cases, rare studies have focused on the significance of serum lipids in GCNEI, leaving these patients’ lipid metabolic features unexplored.
In this study, we sought to investigate the relationship between serum lipids levels and the clinicopathological features or the occurrence of GCNEI. Samples of patients with NEC, GC with varying amounts of NEC components (GC-NEC), or GC-NENM were collected from three centers. The serum lipid levels were analyzed and compared with the matched PAC or a background population selected from 201 PAC and 10,061 health-check people by propensity-score matching (PSM). The risk factors of clinicopathological features and the occurrence of GCNEI were also explored.
Materials and methods
Patient and control case selection
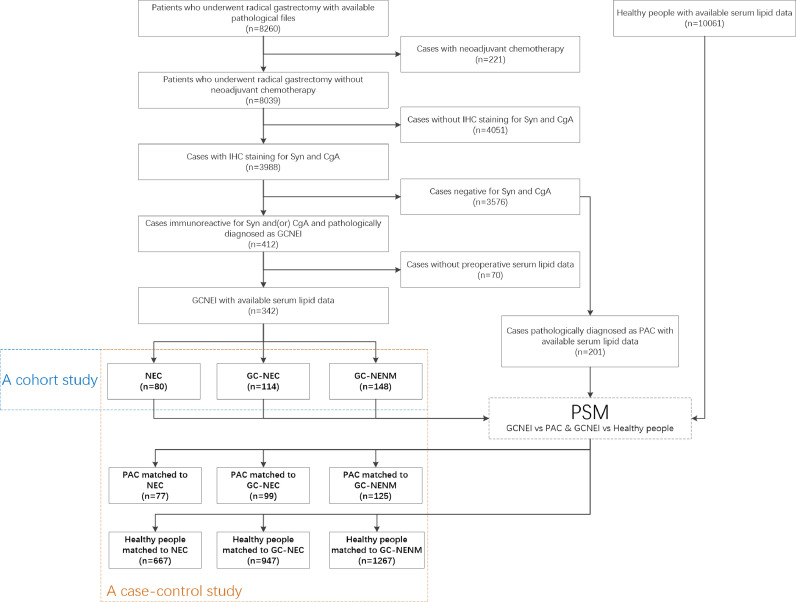
Pathological files of patients who underwent radical gastrectomy between 2010 and 2019 in Second Affiliated Hospital of Zhejiang University School of Medicine, Union Hospital of Fujian Medical University or Second Affiliated Hospital of Fujian Medical University were reviewed. Cases were selected according to the following criteria: i. neoadjuvant chemotherapy had not been applied; ii. pathological diagnoses were NEC, NEC with adenocarcinoma components, adenocarcinoma with NEC components, MANEC, or GC with NE differentiation, which were confirmed by immunohistochemical (IHC) staining for synaptophysin (Syn) and chromogranin A (CgA) [1]; iii. Preoperative lipid profile test was performed and the data were available (Fig. 1). The IHC staining was performed on Ventana BenchMark XT (Roche Diagnostics, USA), BOND-MAX (Leica Biosystems, USA), or Lab Vision Autostainer 720 (Thermo Scientific, USA) according to the corresponding protocols. The information on primary antibodies was listed in Table S1.
Fig. 1.
Flowchart of study object selection
GCNEI: gastric cancer with neuroendocrine immunophenotypes; GC-NENM: gastric adenocarcinoma expressing neuroendocrine markers but no neuroendocrine morphology; GC-NEC: mixed carcinoma with adenocarcinoma and neuroendocrine components; NEC: neuroendocrine carcinoma; PAC: pure adenocarcinoma; PSM: propensity-score matching.
A total of 201 patients who underwent radical gastrectomy and were pathologically diagnosed as PAC (negative for NE markers in IHC staining) between 2015 and 2019 in Second Affiliated Hospital of Zhejiang University School of Medicine were selected as the control groups of PAC. Another 10,061 people who underwent health check between 2015 and 2019 in the Center for Health Management, Second Affiliated Hospital of Fujian Medical University were selected as the control groups of background people (Fig. 1). Most of these people were employees of local government or companies, and the blood test was part of their annual health check program provided by employers.
This study was approved by the Institutional Review Board of Second Affiliated Hospital of Zhejiang University (2020-ERR-031), Union Hospital of Fujian Medical University (2020KY047), and Second Affiliated Hospital of Fujian Medical University (2020-SAHFMER-228). Patient consent was waived by the institutional review boards, as this study was retrospective and patients’ information was protected by a blind method.
Data collection and normalization
Information on age, sex, body mass index (BMI, weight(kg)/height(m)2) and preoperative distal metastasis was obtained from the Electronic Medical Record System of each center. Clinicopathological features, including tumor location (cardia and fundus, body and angle, or antrum were classified into the upper, middle or lower third stomach, respectively), tumor size, histological type, depth of invasion (T), lymphovascular invasion (LVI), node metastasis (LNM) and the results of IHC staining, were obtained from the Electronic Pathological Report System of each center.
TG, TCHO, LDL-C, and HDL-C levels were retrieved from the Lab Information System of each center directly. Non-HDL-C was calculated as total cholesterol minus HDL-C, and its thresholds were defined by adding 0.777 mmol/L to the LDL-C thresholds [4]. The raw data of serum lipid levels were normalized with min-max normalization using the following formula [7], and the reference ranges of serum lipids were shown in Table S2.
y: normalized data; x: raw data; RR: reference range
Propensity-score matching and statistical analysis
PMS was applied between each GCNEI subtype and PAC or the health-check people. For the control groups of PAC, the matching was according to age, sex, and pathological (pTNM) stage by the matching ratio of 1:1; for the control groups of background people, the matching was according to Age and Sex by the matching ratio of 1:10. The matching algorithm was Nearest Neighbors with calipers of width equal to 0.05 [17].
The distribution of demographic, BMI, clinicopathological features, and serum lipid levels were compared using the χ2 test or Fisher's exact test. One-way Analysis of Variance (ANOVA) was used in the analyses of data following a(n) (approximately) normal distribution, including age, tumor size, and the normalized level of TCHO, LDL-C, HDL-C and non-HDL-C of GCNEI patients. The non-parametric test was used in the analyses of data following an abnormal distribution, including TCHO, LDL-C, HDL-C, and non-HDL-C of the background population and all TG data. Risk analysis was performed with binary logistic regression. A P value less than 0.05 was considered statistically significant. Statistical analyses and PSM were performed with SPSS 26.0 (SPSS Inc., Chicago, IL, USA).
Results
The baseline characteristics of GCNEI patients
A total of 342 GCNEI patients, including 148 GC-NENM, 114 GC-NEC, and 80 NEC patients, were enrolled in this study, of which 126 were from Second Affiliated Hospital of Zhejiang University School of Medicine, 195 were from Union Hospital of Fujian Medical University, and 21 were from Second Affiliated Hospital of Fujian Medical University. The representative histological features of GCNEI were shown in Fig. 2.
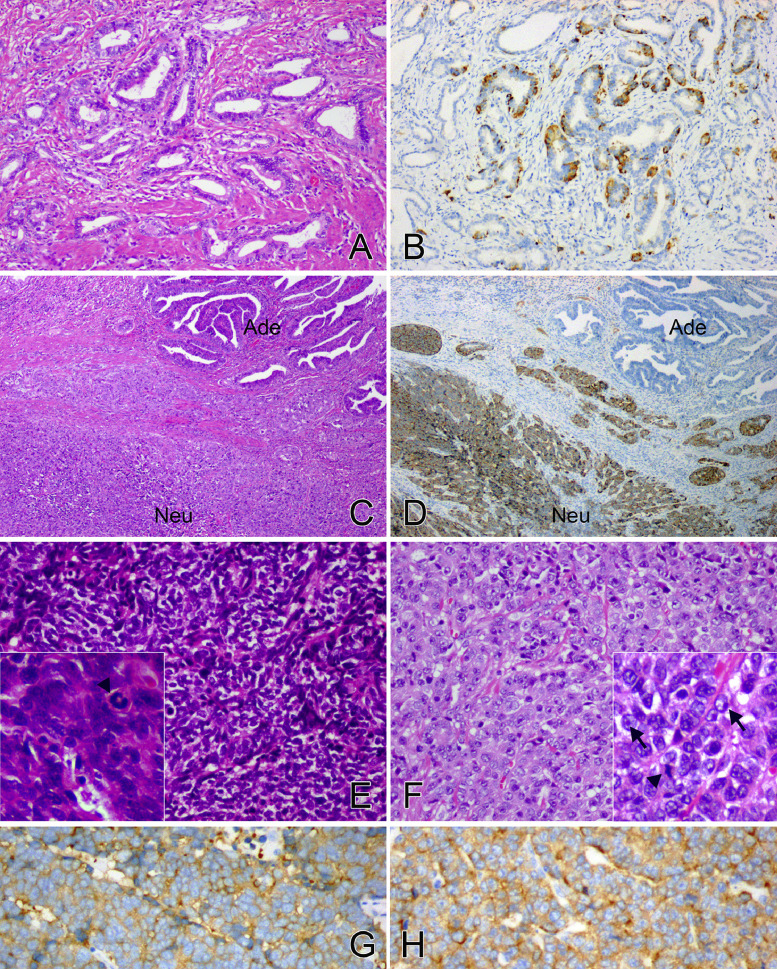
Fig. 2.
Histological features of GCNEI
GC-NENM showed the morphological manifestation of adenocarcinoma, such as glandular structures lined by columnar cells (A, H&E, × 200), and the NE cells were identified in IHC staining for Syn (B, × 200). GC-NEC comprised adenocarcinoma (Ade) and NEC (Neu) components simultaneously (C, H&E, × 40), but only the latter was immunoreactive for Syn (D, × 40). NEC was entirely composed of either small cells (E, H&E, × 200) or large cells (F, H&E, × 200). The former grew densely with dust-like chromatin (E, lower left inset H&E, × 400), while the latter arranged in a relatively loose pattern with abundant cytoplasm and prominent nucleoli (F, lower left inset H&E, × 400, arrow). Both the two types of NEC expressed Syn diffusely (G, H, Syn, × 200) with frequent mitoses (E, F, arrowhead).
The overall GCNEI patients aged 22–84 years with a mean of 59.98±10.41 years, and were composed of males predominantly. The BMI of GCNEI patients distributed between 16.04 kg/m2 and 38.05 kg/m2, of which 76.6% was in the normal range (18.5 kg/m2–25.0 kg/m2). The upper 1/3 of the stomach was favored by nearly half of the tumors, and the mean tumor size was 5.10±2.40 cm. Over 80% of the tumors invaded the muscularis propria and/or deeper layers. LVI and LNM were found in more than 50% and 75% of the cases, respectively, but distal metastasis only occurred in 4.1% of all GCNEI (Table 1).
Table 1.
Baseline characteristics of GCNEI patients.
| Characteristics | GC-NENM n = 148 | GC-NEC n = 114 | NEC n = 80 | P value |
|---|---|---|---|---|
| Age (yr, mean±SD) | 59.68±11.85 | 59.74±9.81 | 60.89±8.21 | 0.674 |
| Sex, n (%) | 0.291 | |||
| Male | 111 (75.0) | 93 (81.6) | 66 (82.5) | |
| Female | 37 (25.0) | 21 (18.4) | 14 (17.5) | |
| BMI (kg/m2) | ||||
| Low (<18.5) | 15 (10.1) | 6 (5.3) | 2 (2.5) | 0.235 |
| Normal (18.5–25.0) | 111 (75.0) | 88 (77.2) | 63 (78.8) | |
| High (>25.0) | 22 (14.9) | 20 (17.5) | 15 (18.8) | |
| Location, n (%) | 0.006 | |||
| Upper 1/3 | 52 (35.1) | 58 (50.9) | 44 (55.0) | |
| Middle 1/3 | 45 (30.4) | 24 (21.1) | 23 (28.7) | |
| Lower 1/3 | 51 (34.5) | 32 (28.1) | 13 (16.3) | |
| Tumor size (cm, mean±SD) | 5.19±2.59 | 4.99±2.40 | 5.08±2.02 | 0.801 |
| Depth of invasion, n (%) | 0.010 | |||
| T1 (LP, MM or SM) | 13 (8.8) | 7 (6.1) | 2 (2.5) | |
| T2 (MP) | 16 (10.8) | 17 (14.9) | 9 (11.3) | |
| T3 (SS) | 97 (65.5) | 60 (52.6) | 61 (76.3) | |
| T4 (perforates serosa) | 22 (14.9) | 30 (26.3) | 8 (10.0) | |
| LVI, n (%) | 0.287 | |||
| Negative | 73 (49.3) | 46 (40.4) | 33 (41.3) | |
| Positive | 75 (50.7) | 68 (59.6) | 47 (58.8) | |
| LNM, n (%) | 0.164 | |||
| Negative | 37 (25.0) | 22 (19.3) | 25 (31.3) | |
| Positive | 111 (75.0) | 92 (80.7) | 55 (68.8) | |
| Distal metastasis, n (%) | 0.414 | |||
| Negative | 143 (96.6) | 107 (93.9) | 78 (97.5) | |
| Positive | 5 (3.4) | 7 (6.1) | 2 (2.5) | |
| Stage, n (%) | 0.277 | |||
| I | 21 (14.2) | 13 (11.4) | 8 (10.0) | |
| II | 44 (29.7) | 23 (20.2) | 26 (32.5) | |
| III | 79 (53.4) | 71 (62.3) | 44 (55.0) | |
| IV | 4 (2.7) | 7 (6.1) | 2 (2.5) |
GCNEI: gastric cancer with neuroendocrine immunophenotypes; GC-NENM: gastric adenocarcinoma expressing neuroendocrine markers but no neuroendocrine morphology; GC-NEC: mixed carcinoma with adenocarcinoma and neuroendocrine components; NEC: neuroendocrine carcinoma; BMI: body mass index; LP: lamina propria; MM: muscularis mucosae; SM: submucosa; MP: muscularis propria; SS: subserosa; LVI: lymphovascular invasion; LNM: lymph mode metastasis.
The demographic and clinicopathological features were comparable among different GCNEI subtypes. Significant differences were only found in the terms of tumor location and depth of invasion: over half of GC-NEC and NEC were seen in the upper 1/3 of the stomach, while the locational distribution of GC-NENM was relatively even; the proportion of tumors invading serosa was significantly higher in GC-NEC than in other subtypes (Table 1).
Comparison of serum lipid levels between GCNEI patients and the background population
With PSM, the confounding factors of age and sex were adjusted, and there was no statistical difference of these factors between GCNEI subtypes and the background population after matching (Table S3).
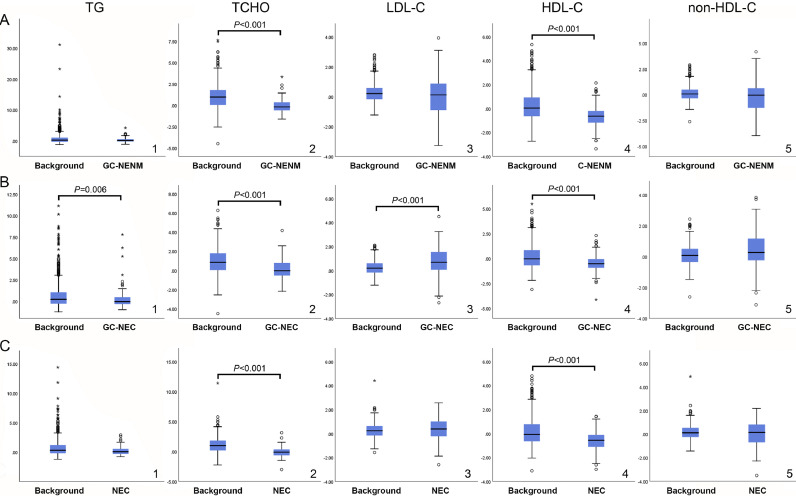
The serum lipid patterns of GCNEI patients were distinct. Compared with the matched background population, the TCHO and HDL-C levels were significantly lower in all GCNEI subtypes (Fig. 3. A2, A4, B2, B4, C2, and C4) with additional lower TG (Fig. 3. B1) and higher LDL-C levels (Fig. 3. B3) in GC-NEC. However, there was no statistically significant difference in terms of non-HDL-C between all GCNEI subtypes and the matched background population (Fig. 3. A5, B5, and C5).
Fig. 3.
Comparison of serum lipid levels between GCNEI patients and the background population
A. Comparison of the normalized serum lipid levels between GC-NENM and the background population; B. Comparison of the normalized serum lipid levels between GC-NEC and the background population; C: Comparison of the normalized serum lipid levels between NEC and the background population. *: values which are more than three box lengths from either end of the box receive; ○: values which are between one and a half and three box lengths from either end of the box.
Comparison of serum lipid levels between GCNEI and PAC
The confounding factors, including sex, age, and tumor stages, were minimized by PSM. No difference was found between each GCNEI subtype and the matched PAC (Table S4).
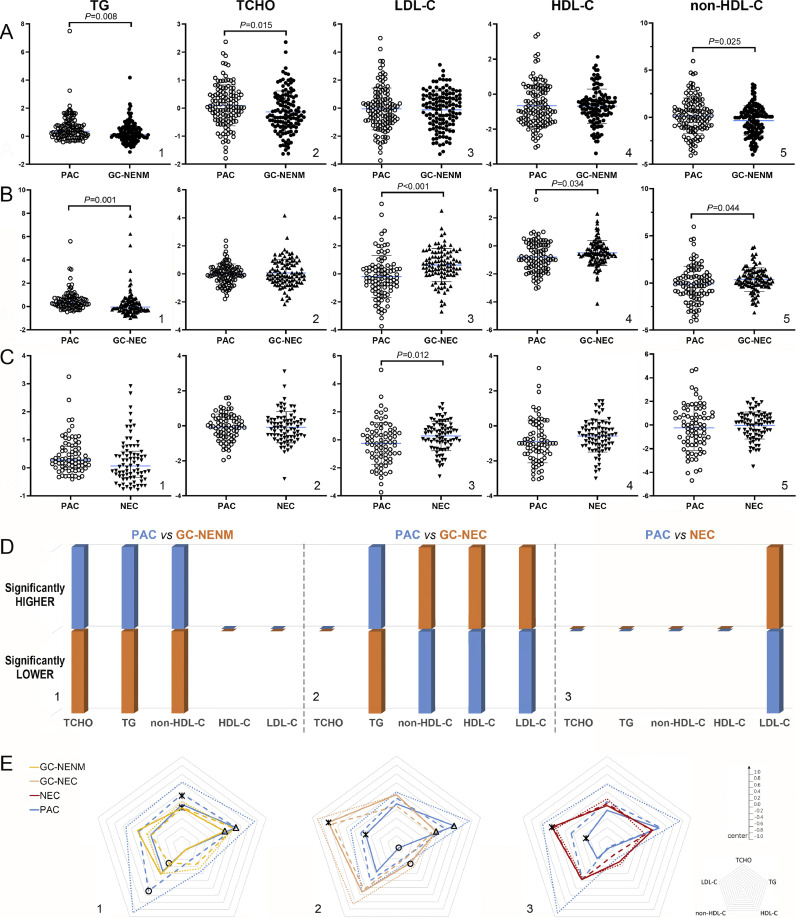
Compared with PAC, TG, TCHO, and non-HDL-C levels were significantly lower in GC-NENM (Fig. 4. A). GC-NEC possessed the most distinct serum lipid patterns, characterized by elevated LDL-C, HDL-C, and non-HDL-C levels, but a reduced TG level (Fig. 4. B). NEC was the subtype with a minimum difference from PAC, as only its LDL-C level was higher (Fig. 4. C). On the whole, all the differences above made a “colder” lipid patterns in GC-NENM patients (Fig. 4. D1), but much “hotter” counterparts in GC-NEC and NEC patients (Fig. 4. D2 and D3), which indicated that GC-NEC and NEC might be associated with a significantly different lipid microenvironment than that of GC-NENM, and that GC-NEC might have the closest relationship with serum lipids.
Fig. 4.
Comparison of serum lipid levels between GCNEI and PAC
A. Comparison of the normalized serum lipid levels between GC-NENM and PAC; B. Comparison of the normalized serum lipid levels between GC-NEC and PAC; C. Comparison of the normalized serum lipid levels between NEC and PAC; D. Summary of the significant differences between GCNEI subtypes and PAC; E. Comparison of the serum lipid patterns between GCNEI and PAC by pathological stages (dot line: stage I; dash line: stage II; solid line: stage III+IV). *, △, ○: P<0.05.
Between each GCNEI subtype and PAC stratified by stages, differences existed in all pairs at stage III+IV (Fig. 4. E1△; E2*, △, ○; and E3 *), besides, significantly lower non-HDL-C and TCHO levels were also seen in GC-NENM at stage I (Fig. 4. E1 *, ○). These differences were generally consistent with the tendency found between unstratified GCNEI subtypes and PAC (Fig. 4. D).
Comparison of serum lipid levels among GCNEI subtypes
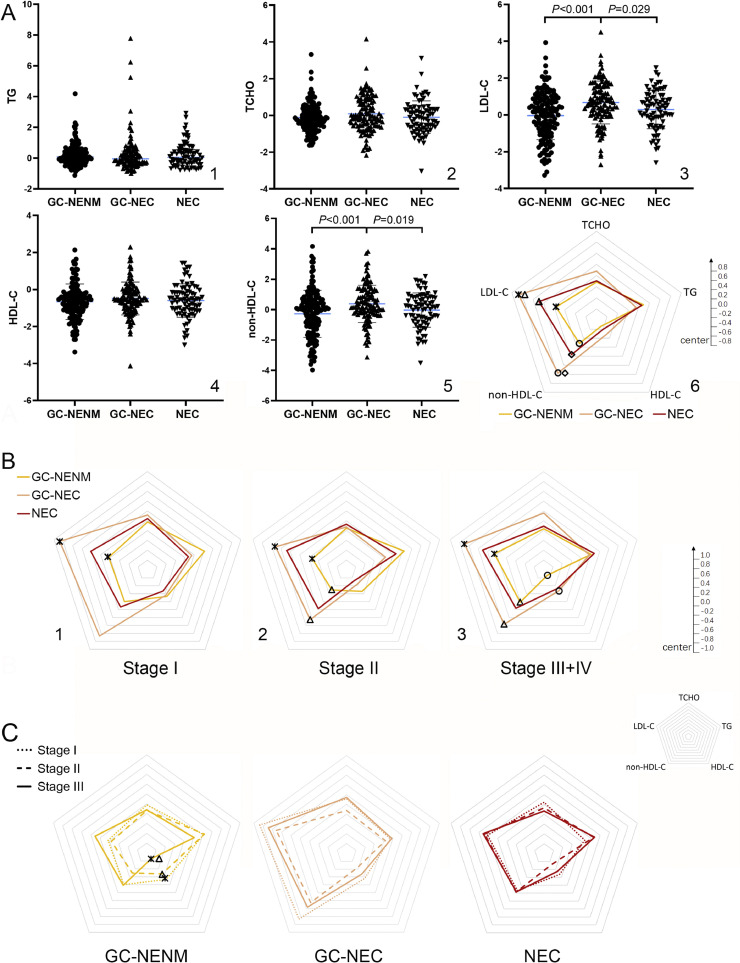
According to the reference ranges in the center samples originated, the distribution of TCHO, TG, and HDL-C levels were comparable among different GCNEI subtypes: the TCHO and TG levels of most GCNEI patients were normal, and a decreased HDL-C level was seen in 20%−35% of GCNEI patients. Significant differences were found in the distribution of LDL-C and non-HDL-C levels, whereas more GC-NENM patients had decreased levels than the other two subtypes (Table S5).
The TG, TCHO, and HDL-C levels were similar among GCNEI subtypes (Fig. 5, A1, A2, and A4), while, the LDL-C and non-HDL-C levels of GC-NEC were significantly higher than those of GC-NENM or NEC (Fig. 5, A3, and A5), which was reflected by the “lipid shapes” in the radar chart more prominently (Fig. 5, A6, LDL-C: *GC-NENM vs GC-NEC, △NEC vs GC-NEC; non-HDL-C: ○GC-NENM vs GC-NEC, ◇NEC vs GC-NEC). To evaluate if the differences of serum lipid levels changed among GCNEI subtypes with tumor progression, GCNEI were stratified by pathological stages (due to the small number of cases at stage IV, they are merged with those at stage III), and statistical differences between GC-NEC and GC-NENM increased with pathological stages. The LDL-C (*) level of GC-NEC was significantly higher through all stages (Fig. 5. B1, B2, and B3), and non-HDL-C (△) or HDL-C (○) showed significantly higher levels in GC-NEC from stage II (Fig. 5. B2 and B3) or at stage III+IV (Fig. 5. B3), respectively. Further, to evaluate the correlation between serum lipids and tumor progression of each GCNEI subtype, patients were stratified by pathological stages, and significant differences were only found in the HDL-C levels of GC-NENM between stage III+IV and stage I (Fig. 5. C1 *) or stage II (Fig. 5. C1 △).
Fig. 5.
Comparison of serum lipid levels and patterns among GCNEI subtypes
A. Comparison of the normalized serum lipid levels and patterns among GCNEI subtypes; B. Comparison of the normalized serum lipid patterns among GCNEI subtypes by pathological stages. C. Comparison of the normalized serum lipid patterns among pathological stages. *, △, ○, ◇: P<0.05.
The significance of serum lipid levels on the occurrence and progression of GCNEI
Logistic regression analysis based on GCNEI patients and the background population showed that serum lipids were associated with the occurrence of GCNEI independently (Table 2 and Table S6). The risk of all GCNEI subtypes was increased by a lower level of TG or HDL-C, and the risk of GC-NEC was also negatively associated with the non-HDL-C level, but positively with the LDL-C level. In addition, age showed a preventive role in the occurrence of all GCNEI subtypes but the hazard ratios were weak.
Table 2.
Independent risk factors of the occurrence of GCNEI.
| Subtype | Factor | P value | HR | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| GC-NENM | Age | <0.001 | 0.965 | 0.961 | 0.968 |
| TG | <0.001 | 0.459 | 0.368 | 0.572 | |
| HDL-C | <0.001 | 0.298 | 0.238 | 0.373 | |
| GC-NEC | Age | <0.001 | 0.956 | 0.951 | 0.962 |
| TG | <0.001 | 0.546 | 0.415 | 0.718 | |
| HDL-C | <0.001 | 0.369 | 0.282 | 0.483 | |
| LDL-C | <0.001 | 6.183 | 2.965 | 12.894 | |
| non-HDL-C | 0.012 | 0.407 | 0.202 | 0.818 | |
| NEC | Age | <0.001 | 0.967 | 0.962 | 0.971 |
| TG | <0.001 | 0.430 | 0.318 | 0.583 | |
| HDL-C | <0.001 | 0.332 | 0.242 | 0.454 | |
GC-NENM: gastric cancer expressing neuroendocrine markers but no neuroendocrine morphology; GC-NEC: mixed carcinoma with adenocarcinoma and neuroendocrine components; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; HR: hazard ratio; CI: confidential interval.
In the GCNEI cohort, the serum lipid levels showed independent significance on tumor size and tumor stages (Table 3 and Table S7-S9). A lower TG or HDL-C level increased the risk of large tumor size (>5 cm) in GC-NENM, but only the latter was significant in GC-NEC. A larger tumor size or lower TG level increased the risk of both advanced (pT>1) and late (III+IV) tumor stages of GC-NENM, but a lower HDL-C level or higher LDL-C level only increased the risk of late tumor stages. Moreover, younger age was also associated with late tumor stages independently. However, serum lipid levels were not associated with tumor size and tumor stages in NEC (Table S7-S9), as well as LNM (Table S10) and LVI (Table S11) in all GCNEI subtypes.
Table 3.
Independent risk factors of tumor size and tumor stages in multivariate analysis.
| Clinicopathological features | Subtype | Factor | P value | HR | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Tumor size >5cm | GC-NENM | TG | 0.014 | 0.499 | 0.287 | 0.867 |
| HDL-C | <0.001 | 0.493 | 0.348 | 0.699 | ||
| GC-NEC | HDL-C | 0.042 | 0.666 | 0.450 | 0.986 | |
| Advanced stages (pT>1) | GC-NENM | Size | <0.001 | 2.067 | 1.632 | 2.619 |
| TG | 0.023 | 0.399 | 0.181 | 0.879 | ||
| GC-NEC | Size | <0.001 | 2.189 | 1.660 | 2.886 | |
| Late stages (stage III+IV) | GC-NENM | Age | 0.001 | 0.969 | 0.951 | 0.987 |
| Size | <0.001 | 1.406 | 1.173 | 1.685 | ||
| TG | 0.002 | 0.279 | 0.124 | 0.628 | ||
| HDL-C | 0.001 | 0.363 | 0.203 | 0.648 | ||
| LDL-C | 0.002 | 1.899 | 1.274 | 2.830 | ||
| GC-NEC | BMI | 0.020 | 0.946 | 0.903 | 0.991 | |
| Size | <0.001 | 1.565 | 1.240 | 1.977 | ||
GC-NENM: gastric cancer expressing neuroendocrine markers but no neuroendocrine morphology; GC-NEC: mixed carcinoma with adenocarcinoma and neuroendocrine components; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; BMI: body mass index; HR: hazard ratio; CI: confidential interval.
Discussion
In the present study, the serum lipid levels of GCNEI patients were analyzed and compared with matched PAC or a background population selected by PSM. To the best of our knowledge, this is the first study to reveal the significance of serum lipid levels in the whole spectrum of GCNEI subtypes. Generally, the serum lipid patterns of GCNEI differed from those of PAC or the background population, and GC-NEC had the most distinct serum lipid pattern.
As a heterogeneous cohort, although all GCNEI tumors express NE markers, their composition is complex. In the 2019 WHO classification, NE differentiation (NED) is defined by the presence of both morphological and immunohistochemical phenotype of NE, and it would not change the designation of an adenocarcinoma, only if the NE differentiation reached 30% [1]. However, a growing amount of evidence supported that a <30% NEC component or a component only with NE immunophenotypes in GC could contribute to more malignant biological behaviors and worse prognosis [14,23]. To help clarify this controversy, we investigated them from the aspect of patients’ serum lipid metabolism and enrolled GC with any amount of NE differentiation (GC-NEC) and GC with NE immunophenotype but no NEM (GC-NENM), as well as MANEC and NEC. The results demonstrated that mixed GCNEI with NEM (GC-NEC) was indeed an entity associated with a distinct serum lipid patterns from that of GC-NENM, especially at higher pTNM stages, however, the serum lipid patterns of GC-NENM still differed from that of PAC by lower TG, TCHO, and non-HDL-C levels. These data support the demarcation in WHO classification between GCNEI with or without NEM to some degree, but GC-NENM was still not the same as PAC from the aspect of serum lipid patterns.
The close relationship between neuroendocrine neoplasms (NENs) and serum lipid levels has been reported. Bai et al. [2] found that a higher serum LDL-C level was associated with a better survival rate and median survival time of NENs in the digestive system (G1, G2, G3 NET, and a few MANEC). In Pereira's research (Pereira, [15]), gastrointestinal NET patients with a low serum HDL-C level showed significantly higher peritumoral expression of IL-6 which was associated with systemic inflammatory status and tumor progression. Benslama et al. [3] revealed that serum cholesterol level was a predictor of the response to everolimus in metastatic NET patients, and the occurrence of hypercholesterolemia was associated with longer progression-free survival, which implied that the NET sensitive to treatment might be related with some specific characteristics of lipid metabolism. However, rare studies focused on the whole spectrum of GCNEI, especially mixed ones. The present study showed that TG and HDL-C levels were negatively associated with tumor size and(or) tumor progression of GC-NENM and GC-NEC, whereas a higher LDL-C level could increase the risk of progressing to late tumor stages in GC-NENM patients. These results accorded with Pereira's finding (Pereira, [15]) of the protective role of HDL-C but were inconsistent partially with Bai's conclusion [2] that a higher LDL-C level was accompanied by a better prognosis. The discrepancy might be caused by the different cohorts in the studies, as pure NETs (97.6%) was the majority of Bai's research while mixed carcinomas (76.6%) were predominant in our study. Furthermore, Bai's conclusion was based on the overall entity of NEN, but ours were specific to a certain subtype of GCNEI.
In this study, LDL-C showed significantly different levels among GCNEI. The LDL-C levels of GCNEI with NEM (GC-NEC and NEC) were higher than that of the matched PAC, which was not present between GCNEI without NEM (GC-NENM) and its matched PAC, meanwhile, inside GCNEI, the LDL-C level was also significantly higher in the overall entity of GCNEI with NEM than that of GC-NENM (0.515 vs −0.036, P<0.001). These differences divided GCNEI into two parts from the aspect of serum lipids and were consistent with their divergent outcomes that the prognosis of GCNEI with NEM was significantly worse than those without NEM, but no survival difference existed inside the former (GC-NEC vs NEC) [14]. This phenomenon implied a correlation between a high LDL-C level and the worse prognosis of GCNEI with NEM, and in our study, another significance of LDL-C on the risk of late stages was also found in GC-NENM, which showed a higher LDL-C level might also cause a worse prognosis inside GCNEI without NEM. Linstedt et.al [11] found that Syn-bearing intracellular vesicles were closely related to LDL receptors (LDL-R) in either prostate NEC cells or Syn-transfected cells. In Loeper's study [12], the enhanced LDL endocytosis mediated by the increased activity of LDL-R could promote the formation of secretory granule prominently in NE cells. [11]. And the decreased expression of LDL-R could inhibit the cholesterol endocytosis of NET and lead to significant tumor regression [3]. Hence, a chain from LDL-R mediated cholesterol uptake to cholesterol-related regulated exocytosis [19] might exist in NEC cells and be stimulated by a high LDL-C level, resulting in increased plasma membrane replenishment, cell proliferation, and malignant behaviors. This hypothesis might explain the relationship between LDL-C and NE phenotypes or NEC cell activity partially, but the underlying mechanism of how cholesterol metabolism affected the formation of NEM still stays unclear.
Serum lipid data of a background population were also collected and compared with the patients in the present study. To minimized the confounding factors which would affect serum lipid levels significantly, PSM was used to select the background population matched to each GCNEI subtype. Compared with them, significantly lower TG, TCHO, and HDL-C levels, and a higher LDL-C level were observed in different GCNEI subtypes, and TG, HDL-C, and(or) non-HDL-C, LDL-C levels were associated with different GCNEI subtypes independently. In two previous larger-scale studies, a lower HDL-C level [8] and a higher TCHO [16] or TG [10] level were identified as the independent risk factors of rectal NET. And in Bai's research [2], a lower LDL-C was associated with a mixed entity of NEN of the digestive system. Our finding that a lower HDL-C increased the risk of every subtype of GCNEI was consistent with the aforementioned reports about rectal NET, but the role of TG and LDL-C was on the opposite side in our GCNEI cohort, which indicated the potential different lipid metabolic manners between NET and NEC.
There were several limitations in our study. Firstly, the lipid profile identified to represent a risk for GCNEI is the typical atherogenic profile observed in patients with metabolic syndrome, thus suggesting that this cancer population was enriched in metabolic syndrome/disorders as compared to the background population. However, other metabolic parameters were out of this study focus. Meanwhile, this lipid profile often indicated a systemic inflammatory status which has an impact on tissue inflammation. This being said implies that lipid profile could be a surrogate marker of an inflammatory condition, which in turn is a recognized carcinogenic factor for several tumors. Secondly, due to the rarity of GCNEI, the sample size of each subtype was still too small, so that subgroup analyses were limited. thirdly, the percentage of NEC components in GC-NEC was not recorded, which hindered the discussion of the relationship between the amount of NEC components and serum lipid levels. Lastly, as a multicenter study, the serum lipid data from different centers were normalized before statistical analyses, so that the exact level corresponding to certain risk strength could not be determined.
In summary, the present study firstly reported the distinct and heterogeneous serum lipid patterns of the whole spectrum of GCNEI and found the associations between serum lipid levels and the clinicopathological features of each GCNEI subtype. However, due to the lack of data, the causality between lipids and GCNEI was not yet demonstrated well. Further studies are needed to evaluate the direct effect of lipids on GCNEI and to explore the underlying mechanism, which might help develop potential anticancer drugs or therapies targeting metabolism for GCNEI patients.
CRediT authorship contribution statement
Yi Zou: Conceptualization, Methodology, Software, Validation, Resources, Data curation, Writing - original draft, Visualization. Long Wu: Investigation, Resources, Data curation. Yubin Yang: Investigation, Resources, Data curation. Zonghui Ding: Writing - review & editing. Jiaming Huang: Resources, Data curation. Peng Li: Investigation. Chunpeng Zhu: Conceptualization, Validation, Formal analysis, Writing - review & editing, Project administration, Funding acquisition. Ying Yuan: Conceptualization, Validation, Supervision, Supervision, Supervision.
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
Acknowledgments
Acknowledgements
Grateful acknowledgement is made to the teams of Second Affiliated Hospital of Zhejiang University, Union Hospital of Fujian Medical University and Second Affiliated Hospital of Fujian Medical University for their support in this work.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province [Grant Nos. LY16H160031 and LY20H160031] and Key Disciplines of Chinese Medicine (Integrated Traditional Chinese and Western Medicine) in Zhejiang Province during the Thirteenth Five-year Plan period [Grant No. 2017-XK-A40].
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.100925.
Contributor Information
Chunpeng Zhu, Email: zhuchunpeng@zju.edu.cn.
Ying Yuan, Email: yuanying1999@zju.edu.cn.
Appendix. Supplementary materials
References
- 1.Assarzadegan N., Montgomery E. What is new in 2019 World Health Organization (WHO) Classification of tumors of the digestive system: review of selected updates on neuroendocrine neoplasms, appendiceal tumors, and molecular testing. Arch. Pathol. Lab. Med. 2020 doi: 10.5858/arpa.2019-0665-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai J. A retrospective study of NENs and miR-224 promotes apoptosis of BON-1 cells by targeting PCSK9 inhibition. Oncotarget. 2017;8(4):6929–6939. doi: 10.18632/oncotarget.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benslama N. Prediction of response to everolimus in neuroendocrine tumors: evaluation of clinical, biological and histological factors. Invest. N. Drugs. 2016;34(5):654–662. doi: 10.1007/s10637-016-0363-6. [DOI] [PubMed] [Google Scholar]
- 4.Brunner F.J. Application of non-HDL cholesterol for population-based cardiovascular risk stratification: results from the multinational cardiovascular risk consortium. Lancet. 2019;394(10215):2173–2183. doi: 10.1016/S0140-6736(19)32519-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carneiro, F., et al. (2019). WHO classification of tumours of the digestive system. 5th ed. [DOI] [PMC free article] [PubMed]
- 6.Ghahremanfard F. The valuable role of measuring serum lipid profile in cancer progression. Oman Med. J. 2015;30(5):353–357. doi: 10.5001/omj.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han J., Kamber M., Pei J. 3rd ed. Morgan Kaufmann; Waltham: 2012. Data Mining: Concept and Techniques. [Google Scholar]
- 8.Jung Y.S. Risk factors associated with rectal neuroendocrine tumors: a cross-sectional study. Cancer Epidemiol. Biomark. Prev. 2014;23(7):1406–1413. doi: 10.1158/1055-9965.EPI-14-0132. [DOI] [PubMed] [Google Scholar]
- 9.Kim B.S. Comparison of relapse-free survival in gastric neuroendocrine carcinoma (WHO grade 3) and gastric carcinoma. Ther. Adv. Gastroenterol. 2017;10(5):407–415. doi: 10.1177/1756283X17697870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko S.H. Clinical characteristics, risk factors and outcomes of asymptomatic rectal neuroendocrine tumors. Surg. Endosc. 2017;31(10):3864–3871. doi: 10.1007/s00464-016-5413-9. [DOI] [PubMed] [Google Scholar]
- 11.Linstedt A.D., Kelly R.B. Synaptophysin is sorted from endocytotic markers in neuroendocrine PC12 cells but not transfected fibroblasts. Neuron. 1991;7(2):309–317. doi: 10.1016/0896-6273(91)90269-6. [DOI] [PubMed] [Google Scholar]
- 12.Loeper S., Asa S.L., Ezzat S. Ikaros modulates cholesterol uptake: a link between tumor suppression and differentiation. Cancer Res. 2008;68(10):3715–3723. doi: 10.1158/0008-5472.CAN-08-0103. [DOI] [PubMed] [Google Scholar]
- 13.Nam S.Y. Effect of Helicobacter pylori eradication and high-density lipoprotein on the risk of de novo gastric cancer development. Gastrointest. Endosc. 2019;90(3):448–456. doi: 10.1016/j.gie.2019.04.232. e441. [DOI] [PubMed] [Google Scholar]
- 14.Park J.Y. Prognostic significance of neuroendocrine components in gastric carcinomas. Eur. J. Cancer. 2014;50(16):2802–2809. doi: 10.1016/j.ejca.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Pereira S.S. Higher IL-6 peri-tumoural expression is associated with gastro-intestinal neuroendocrine tumour progression. Pathology. 2019;51(6):593–599. doi: 10.1016/j.pathol.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Pyo J.H. Evaluation of the risk factors associated with rectal neuroendocrine tumors: a big data analytic study from a health screening center. J. Gastroenterol. 2016;51(12):1112–1121. doi: 10.1007/s00535-016-1198-9. [DOI] [PubMed] [Google Scholar]
- 17.Rajyaguru D.J. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: analysis of the national cancer database. J. Clin. Oncol. 2018;36(6):600–608. doi: 10.1200/JCO.2017.75.3228. [DOI] [PubMed] [Google Scholar]
- 18.Ren H. The significant influence of the neuroendocrine component on the survival of patients with gastric carcinoma characterized by coexisting exocrine and neuroendocrine components. J. Oncol. 2019;2019 doi: 10.1155/2019/3671268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rituper B. Cholesterol-mediated membrane surface area dynamics in neuroendocrine cells. Biochim. Biophys. Acta. 2013;1831(7):1228–1238. doi: 10.1016/j.bbalip.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Santos A.P. Visceral obesity and metabolic syndrome are associated with well-differentiated gastroenteropancreatic neuroendocrine tumors. Cancers (Basel) 2018;10(9) doi: 10.3390/cancers10090293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura T. Evaluation of serum high-density lipoprotein cholesterol levels as a prognostic factor in gastric cancer patients. J. Gastroenterol. Hepatol. 2012;27(10):1635–1640. doi: 10.1111/j.1440-1746.2012.07189.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J.-.C. New utility of an old marker: serum low-density lipoprotein predicts histopathological response of neoadjuvant chemotherapy in locally advanced gastric cancer. Onco Targets Ther. 2016;9:5041–5047. doi: 10.2147/OTT.S97061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou Y. Prognostic threshold of neuroendocrine differentiation in gastric carcinoma: a clinicopathological study of 945 cases. J. Gastric Cancer. 2019;19(1):121–131. doi: 10.5230/jgc.2019.19.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.