Abstract
Introduction
To evaluate whether the extent of gastrectomy or the reconstruction method brings benefit of type 2 diabetes mellitus (T2DM) remission after gastrectomy in patients with gastric cancer.
Methods
PUBMED, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library were searched to find eligible studies published from inception to July 31, 2020.
Results
A total of nine studies (1424 patients) were included. At the first year and the end of follow-up time after gastrectomy, the total gastrectomy group had better T2DM remission than the subtotal gastrectomy group, and the Roux-en-Y reconstruction (R-Y) group had better T2DM remission compared with the non-R-Y group. There was no difference between R-Y and non-R-Y in terms of subtotal gastrectomy (OR 1.08, 95% CI 0.63–1.84, P = 0.78). However, total gastrectomy with R-Y had better T2DM remission than subtotal gastrectomy with R-Y (OR 2.75, 95% CI 1.19–6.35, P = 0.02).
Conclusion
Total gastrectomy with R-Y had better T2DM remission. The extent of gastrectomy rather than the reconstruction method might play an important role in T2DM remission after gastrectomy in patients with gastric cancer.
Keywords: Gastrectomy, Gastric cancer, Remission, Roux-en-Y reconstruction, Type 2 diabetes mellitus
Key Summary Points
| Why carry out the study? |
| To evaluate whether the extent of gastrectomy or the reconstruction method brings benefit of T2MD remission after gastrectomy in patients with gastric cancer. |
| What was learned from the study? |
| Total gastrectomy with R-Y reconstruction had an advantage over other surgical methods in terms of T2MD remission, but there was no difference between subtotal gastrectomy with R-Y and with non-R-Y (Billroth I and Billroth II) reconstruction in terms of T2MD remission. |
| Total gastrectomy with R-Y had better T2MD remission. |
| The extent of gastrectomy rather than the reconstruction method might play an important role in T2MD remission after gastrectomy in patients with gastric cancer. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to 10.6084/m9.figshare.12958199.
Introduction
Type 2 diabetes mellitus (T2DM) is a global epidemic disease that affects more than 200 million people, and it is expected to reach over 500 million by 2030 [1, 2]. T2DM is also a chronic metabolic disease, and its complications can involve multiple systems [3]. T2DM is related to obesity and the development of cardiovascular disease [4, 5], and it may be related to the occurrence and prognosis of cancers [6, 7].
Gastric cancer is the fourth most common malignant tumor and the third leading cause of cancer death worldwide [8]. Surgery is the main method of curative treatment [9]. For most gastric cancer located in the lower two-thirds of the stomach, distal gastrectomy is recommended, for which the reconstruction methods are Billroth I reconstruction (BI), Billroth II reconstruction (BII), and Roux-en-Y reconstruction (R-Y). For cancers located in the proximal stomach and esophagogastric junction (Siewert type II and III), total gastrectomy plus R-Y is commonly used [10–12].
For patients with concurrent gastric cancer and T2DM, gastrectomy can lead to remission of T2DM [13]. Previous literature on factors which affect T2DM remission is controversial. Many studies reported that total gastrectomy combined with R-Y was the key factor for postoperative remission of T2DM [14, 15]. Some studies reported that the extent of gastrectomy was the cause of T2DM remission; however, other studies reported that the R-Y was the main reason for the remission of T2DM [16]. Therefore, the purpose of this study is to evaluate whether the extent of gastrectomy or the reconstruction method brings benefit of T2DM remission after gastrectomy.
Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement relevant to health care [17].
Search Strategy
We searched databases in PUBMED, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library on July 31, 2020. In order to maximize the literature search, we focused on three keywords: gastric cancer, T2DM, gastrectomy. For gastric cancer, the search item was (((((stomach tumor) OR (stomach neoplasm)) OR (stomach cancer)) OR (cancer of the stomach)) OR (gastric neoplasm)) OR (gastric cancer). For T2DM, the search item was ((diabetes) OR (type 2 diabetes)) OR (diabetes mellitus). For gastrectomy, the search item was ((((gastrectomy) OR (reconstruction)) OR (operation)) OR (resection)) OR (surgery). After that, we used “AND” to combine the items of the three keywords.
Article Selection
The selected data was screened by two reviewers independently. Preliminary screening by title and abstract excluded irrelevant studies. Full texts were required for further screening. We excluded conference abstracts, case report, non-human studies, and studies with incomplete data. All included studies were checked for duplicate medical records from the same or overlapping cohort of patients. All the procedures were then cross-checked by the two reviewers.
Data Extraction
T2DM remission was defined as patients with partial or complete remission. Patients who required no medication after gastrectomy were regarded as complete remission, and patients who experienced reduced medications after gastrectomy were regarded as partial remission. Two reviewers extracted the data including the first author, year of publication, country, study design, sample size, type and time of gastrectomy, follow-up time, and number of patients with T2DM remission. The type of gastrectomy was total gastrectomy or subtotal gastrectomy according to the extent of resection. There were four types of reconstruction: Billroth I (BI), Billroth II (BII), Roux-en-Y gastrojejunostomy (RYGJ), and Roux-en-Y esophagojejunostomy (RYEJ). Disagreements were resolved by consensus or consultation with the senior author.
Quality Assessment
The Newcastle–Ottawa scale was used to assess the quality of non-randomized studies by two reviewers independently [18]. The scale awarded a maximum of nine points. Studies of high quality received a score of the maximum nine points, studies of medium quality scored seven to eight points, and studies of low quality scored less than seven points [19].
Statistical Analysis
T2DM remission was a dichotomous variable expressed as proportions. Odds ratios (ORs) and mean differences (MDs) were calculated. A 95% confidence interval (CI) was reported for T2DM remission. The value of I2 and the result of the chi-squared test were used to assess the statistical heterogeneity. The heterogeneity was considered high when I2 > 50%; for such studies, the random effect model was used, and p < 0.1 was considered statistically significant. The fixed effect model was used for the studies with I2 ≤ 50%, and p < 0.05 was considered statistically significant. The meta-analysis was performed using RevMan 5.3 (The Cochrane Collaboration, London, UK).
Compliance with Ethics Guidelines
This study was based on previously conducted studies and did not contain any studies with human participants or animals performed by any of the authors. Ethical approval was unnecessary in this study because it was a systematic review and meta-analysis of existing published articles.
Results
Study Selection
A total of 2441 studies were identified from the literature search, and 49 studies were left after screening the title and abstract. Conference abstracts, case report, studies without comparison of surgical methods, a follow-up time less than 1 year, and repeated published data from the same center were excluded. The remaining nine studies [13–16, 20–24] were included in the meta-analysis (Fig. 1).
Fig. 1.

Flowchart of study selection
Patient Characteristics
The nine studies included in this meta-analysis comprised a total of 1424 patients. The studies were published in 2012–2020; six were from Korea and three were from China. Eight were retrospective studies and one was prospective. The surgical methods in all nine studies included total gastrectomy and subtotal gastrectomy. Although the definitions of T2DM complete remission in the articles were not the same, they all declared that patients who experienced reduced medication after gastrectomy were regarded as partial remission. All nine studies contained a follow-up time of at least 1 year after gastrectomy. Details of the nine studies are shown in Table 1.
Table 1.
Characteristics of the studies included in the meta-analysis
| Author | Year | Country | Study design | Surgery type | Diabetes partial remission definition | Diabetes complete remission definition | Sample size | Follow-up (months) | NOS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c (%) | FBS (mg/dl) | HbA1c (%) | FBS (mg/dl) | ||||||||
| Kim et al. [20] | 2012 | Korea | Retrospective | BI, BII, RYEJ | Improved FBS level and either a lower required dose of oral medication | < 6 | Unknown | 385 | 33.7 | 7 | |
| Lee et al. [13] | 2012 | Korea | Retrospective | BI, BII, RYGJ, RYEJ | Unknown | < 100 | 229 | Unknown | 8 | ||
| An et al. [21] | 2013 | Korea | Retrospective | BI, BII, RYEJ | < 6 | < 126 | 64 | 12 | 8 | ||
| Wang et al. [22] | 2014 | China | Retrospective | BI, BII, RYGJ, RYEJ | Unknown | Normal | 69 | Unknown | 7 | ||
| Zhu et al. [14] | 2015 | China | Retrospective | BI, BII, RYEJ | Unknown | < 100 | 292 | 24 | 7 | ||
| Wei et al. [15] | 2014 | China | Retrospective | BII, RYEJ | 6–6.5 | 100–125 | < 6 | < 100 | 67 | 57.4 | 7 |
| Choi et al. [16] | 2017 | Korea | Prospective | BI, RYEJ | < 6.5 | < 126 | < 6 | < 126 | 40 | 12 | 8 |
| Park et al. [24] | 2017 | Korea | Retrospective | BI, BII, RYGJ, RYEJ | < 6.5 | 100–125 | Normal | < 100 | 52 | 12 | 8 |
| Kim et al. [23] | 2020 | Korea | Retrospective | BII, RYEJ | 6–6.4 | 100–125 | < 6 | < 100 | 226 | 24 | 7 |
BI Billroth I reconstruction, BII Billroth II reconstruction, RYGJ Roux-en-Y gastrojejunostomy reconstruction, RYEJ Roux-en-Y esophagojejunostomy reconstruction, HbA1c hemoglobin A1c, FBS fasting blood sugar, NOS Newcastle–Ottawa scale
Quality Assessment of Included Studies
All nine studies were assessed by the Newcastle–Ottawa scale, and the scores of each study are shown in Table 1.
T2DM Remission at First Year After Gastrectomy
Five studies [13, 14, 16, 21, 23] reported the remission of T2DM at the first year after gastrectomy. We explored the extent of residual stomach on T2DM remission, and compared total gastrectomy with subtotal gastrectomy. After pooling all the data, a significant difference was found between the two groups (OR 2.70, 95% CI 1.20–6.10, P = 0.02) (Fig. 2a). The reconstruction type was also compared between the R-Y group and non-R-Y (BI and BII) group; a significant difference was also found between the two groups (OR 2.35, 95% CI 1.08–5.08, P = 0.03) (Fig. 2b).
Fig. 2.
Forest plot showing the outcomes of T2DM remission at the first year after gastrectomy. a Total gastrectomy versus subtotal gastrectomy; b R-Y versus non-R-Y (BI and BII) reconstruction
T2DM Remission at End of Follow-Up Time After Gastrectomy
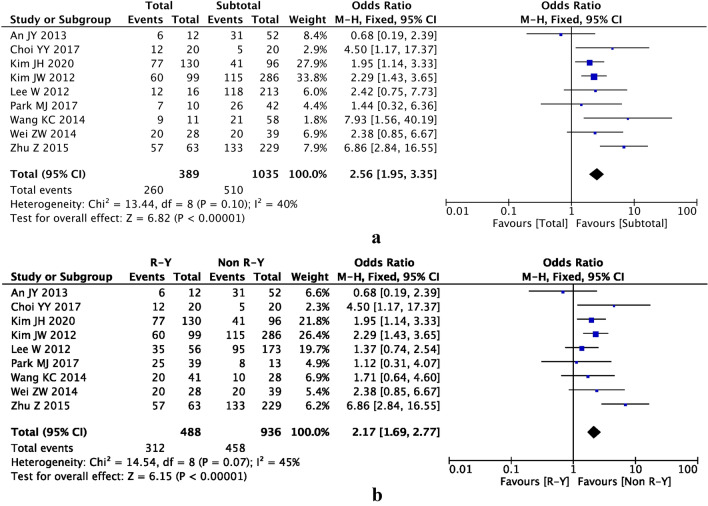
As the follow-up time was different among the nine studies, we selected the end of follow-up time to analyze the data. There was a significant difference between total gastrectomy and subtotal gastrectomy groups (OR 2.56, 95% CI 1.95–3.35, P < 0.00001) (Fig. 3a) and between R-Y and non-R-Y groups (OR 2.17, 95% CI 1.69–2.77, P < 0.00001) (Fig. 3b).
Fig. 3.
Forest plot showing the outcomes of T2DM remission at the end of follow-up time after gastrectomy. a Total gastrectomy versus subtotal gastrectomy; b R-Y versus non-R-Y (BI and BII) reconstruction
Comparing R-Y Between Total Gastrectomy and Subtotal Gastrectomy
To explore the extent of residual stomach on T2DM remission, we compared the same reconstruction method (R-Y) between total gastrectomy and subtotal gastrectomy. Three studies [13, 22, 24] were included, and a significant difference was found between the two groups (OR 2.75, 95% CI 1.19–6.35, P = 0.02) (Fig. 4a).
Fig. 4.
Forest plot showing the outcomes of T2DM remission at the end of follow-up time after gastrectomy. a Comparing R-Y between total gastrectomy and subtotal gastrectomy; b comparing R-Y and non-R-Y (BI and BII) in subtotal gastrectomy
Comparing R-Y and Non-R-Y (BI and BII) in Subtotal Gastrectomy
To explore different reconstruction methods on T2DM remission, we compared the same extent of residual stomach (subtotal gastrectomy). Three studies [13, 22, 24] were included, and no difference was found between the two groups (OR 1.08, 95% CI 0.63–1.84, P = 0.78) (Fig. 4b).
Sensitivity, Consistency I2, and Publication Bias
Repeated meta-analysis was performed by excluding one study in turn, and sensitivity analysis was performed to evaluate the impact of each individual study on the pooled OR; exclusion of any one study did not significantly alter the overall meta-estimate. Consistency was measured by estimating the degree of inconsistency in the results of the studies [25]. Publication bias for the included studies was based on a visual inspection of the funnel plots (Fig. 5).
Fig. 5.
Funnel plot of total gastrectomy versus subtotal gastrectomy at the end of follow-up time after gastrectomy
Discussion
In this meta-analysis, a total of nine studies (1424 patients) were included. We compared T2DM remission between total and subtotal gastrectomy groups at the first year and the end of follow-up time after gastrectomy. Furthermore, total gastrectomy with R-Y reconstruction had an advantage over other surgical methods, but there was no difference between subtotal gastrectomy with R-Y and with non-R-Y (BI and BII) in terms of T2DM remission. As for the same reconstruction of R-Y, total gastrectomy showed better T2DM remission than subtotal gastrectomy.
Bariatric surgery could reduce the weight and also decrease the occurrence of metabolic diseases in patients with obesity [26]. Similarly, patients with gastric cancer also had part or all of their stomach resected. Many studies found that the surgery of gastric cancer was not only a radical tumor resection surgery but also a metabolic surgery [24, 27]. Many patients with gastric cancer and T2DM could achieve partial or even complete remission after gastrectomy, and the remission rate reached 42.5–64.3% [13–16, 20–24].
Similarly, it was reported that gastrectomy could lead to remission of T2DM for patients with concurrent gastric cancer and T2DM. The mechanism for the remission of T2DM remained unclear, but it might be similar to bariatric surgery, including postprandial glucagon-like peptide 1 (GLP-1), gastroinhibitory peptide (GIP), ghrelin, glucagon, and insulin sensitivity [28–32]. In addition to the type of gastrectomy, weight loss and T2DM duration might also affect the remission of patients with concurrent gastric cancer and T2DM [20, 21]. With the extension of follow-up time, the number of patients with T2DM remission would increase [16, 22].
However, whether the extent of gastrectomy or the reconstruction method brought benefit of T2DM remission after gastrectomy was controversial [21–23]. After total gastrectomy, R-Y was the only choice, but after subtotal gastrectomy BI, BII, and R-Y were available. In previous studies, total gastrectomy with R-Y was always compared with BI, BII, or R-Y in subtotal gastrectomy [14–16]. After that, it was concluded that total gastrectomy with R-Y had better T2DM remission than other surgical methods. Some studies even reported that the key point leading to T2DM remission was the R-Y [16]. However, they ignored that the extent of total gastrectomy was larger than that of the subtotal gastrectomy, and one could therefore not draw a conclusion about the benefit of T2DM remission of R-Y from the comparison between R-Y in total gastrectomy with BI, BII, or R-Y in subtotal gastrectomy. It should be done by comparing a similar extent of stomach (subtotal gastrectomy). Therefore, in this meta-analysis, data for different reconstruction methods were extracted and related to the remission of T2DM. We compared a similar extent of gastrectomy (subtotal gastrectomy), and found that there was no difference between R-Y and non-R-Y reconstruction. Furthermore, when we compared the same reconstruction method (R-Y), we found that a larger extent of gastrectomy (total gastrectomy) was better than a smaller extent of gastrectomy (subtotal gastrectomy).
However, there were some limitations in this meta-analysis. Firstly, nine studies were included, eight of which were retrospective analysis, and the quality of the articles was generally low compared with prospective studies. Secondly, because of the limitation of the data in the studies included, we only analyzed the impact of different surgical methods and different reconstruction methods in patients with gastric cancer on the remission of T2DM. Other key factors, such as BMI change, weight loss, and the duration of T2DM were not applicable in this meta-analysis. Thirdly, there were differences in the definition of complete and partial remissions in some studies, so the accuracy of the results might be affected. Finally, the sample size of the studies included was relatively small, and all nine studies came from China and Korea; more large-scale studies are required from more countries to confirm our results in the future.
Conclusion
Total gastrectomy with R-Y had better T2DM remission. The extent of gastrectomy rather than the reconstruction method might play an important role in T2DM remission after gastrectomy in patients with gastric cancer.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Wei Zhang, Dong Peng and Yu-Xi Cheng declare that they have no conflict of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12958199.
References
- 1.International Diabetes Federation . IDF diabetes atlas. 9. Brussels: IDF; 2019. [PubMed] [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of T2DM for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88(11):1322–1335. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoeckli R, Keller U. Nutritional fats and the risk of type 2 diabetes and cancer. Physiol Behav. 2004;83:611–615. doi: 10.1016/j.physbeh.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association (ADA) Standards of medicalcare in diabetes-2014. Diabetes Care. 2014;37(1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 6.Yang HJ, Kang D, Chang Y, et al. Diabetes mellitus is associated with an increased risk of gastric cancer: a cohort study. Gastric Cancer. 2020;23:382–390. doi: 10.1007/s10120-019-01033-8. [DOI] [PubMed] [Google Scholar]
- 7.Baglia ML, Cui Y, Zheng T, et al. Diabetes medication use in association with survival among patients of breast, colorectal, lung, or gastric cancer. Cancer Res Treat. 2019;51:538–546. doi: 10.4143/crt.2017.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Dicato M, Geva R, et al. The diagnosis and management of gastric cancer: expert discussion and recommendations from the 12th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2010. Ann Oncol. 2011;22(suppl 5):v1–9. doi: 10.1093/annonc/mdr284. [DOI] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 11.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20(1):1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad SA, Xia BT, Bailey CE, et al. An update on gastric cancer. Curr Probl Surg. 2016;53(10):449–490. doi: 10.1067/j.cpsurg.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Lee W, Ahn SH, Lee JH, et al. Comparative study of diabetes mellitus resolution according to reconstruction type after gastrectomy in gastric cancer patients with diabetes mellitus. Obes Surg. 2012;22:1238–1243. doi: 10.1007/s11695-011-0580-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Z, Shan X, Cheng Y, et al. Clinical course of diabetes after gastrectomy according to type of reconstruction in patients with concurrent gastric cancer and type 2 diabetes. Obes Surg. 2015;25:673–679. doi: 10.1007/s11695-014-1426-4. [DOI] [PubMed] [Google Scholar]
- 15.Wei ZW, Li JL, Wu Y, et al. Impact of pre-existing type-2 diabetes on patient outcomes after radical resection for gastric cancer: a retrospective cohort study. Dig Dis Sci. 2014;59:1017–1024. doi: 10.1007/s10620-013-2965-6. [DOI] [PubMed] [Google Scholar]
- 16.Choi YY, Noh SH, An JY. A randomized controlled trial of Roux-en-Y gastrojejunostomy vs. gastroduodenostomy with respect to the improvement of type 2 diabetes mellitus after distal gastrectomy in gastric cancer patients. PLoS One. 2017;12:e0188904. doi: 10.1371/journal.pone.0188904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39:91–92. doi: 10.1016/j.jcms.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Wells GA, O’Connell D, Peterson J, Welch V, Lossos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 1 Nov 2011.
- 19.Admiraal WM, Dallal RM, Celik F, et al. Ethnic differences in weight loss and diabetes remission after bariatric surgery. Diabetes Care. 2012;35:1951–1958. doi: 10.2337/dc12-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JW, Cheong JH, Hyung WJ, et al. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J Gastroenterol. 2012;18:49–54. doi: 10.3748/wjg.v18.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An JY, Kim YM, Yun MA, et al. Improvement of type 2 diabetes mellitus after gastric cancer surgery: short-term outcome analysis after gastrectomy. World J Gastroenterol. 2013;19:9410–9417. doi: 10.3748/wjg.v19.i48.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang KC, Huang KH, Lan YT, et al. Outcome after curative surgery for gastric cancer patients with type 2 diabetes. World J Surg. 2014;38:431–438. doi: 10.1007/s00268-013-2291-3. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Huh YJ, Park S, et al. Multicenter results of long-limb bypass reconstruction after gastrectomy in patients with gastric cancer and type II diabetes. Asian J Surg. 2020;43:297–303. doi: 10.1016/j.asjsur.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Park MJ, Kim DH, Park BJ, et al. Impact of preoperative visceral fat proportion on type 2 diabetes in patients with low body mass index after gastrectomy. Surg Obes Relat Dis. 2017;13:1361–1368. doi: 10.1016/j.soard.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheng B, Truong K, Spitler H, et al. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg. 2017;27:2724–2732. doi: 10.1007/s11695-017-2866-4. [DOI] [PubMed] [Google Scholar]
- 27.Tae-Hoon L, Min LC, Sungsoo P, et al. Long-term follow-up for type 2 diabetes mellitus after gastrectomy in non-morbidly obese patients with gastric cancer: the legitimacy of onco-metabolic surgery. J Gastric Cancer. 2017;17(4):283–294. doi: 10.5230/jgc.2017.17.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nosso G, Griffo E, Cotugno M, et al. Comparative effects of Roux-en-Y gastric bypass and sleeve gastrectomy on glucose homeostasis and incretin hormones in obese type 2 diabetic patients: a one-year prospective study. Horm Metab Res. 2016;48:312–317. doi: 10.1055/s-0041-111505. [DOI] [PubMed] [Google Scholar]
- 29.Fellici AC, Lambert G, Lima MMO, et al. Surgical treatment of type 2 diabetes in subjects with mild obesity: mechanisms underlying metabolic improvements. Obes Surg. 2015;25:36–44. doi: 10.1007/s11695-014-1377-9. [DOI] [PubMed] [Google Scholar]
- 30.Khoo CM, Muehlbauer MJ, Stevens RD, et al. Postprandial metabolite profiles reveal differential nutrient handling after bariatric surgery compared with matched caloric restriction. Ann Surg. 2014;259:687–693. doi: 10.1097/SLA.0b013e318296633f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Mingrone G, Manco M. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32:375–380. doi: 10.2337/dc08-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradnova O, Kyrou I, Hainer V, et al. Laparoscopic greater curvature plication in morbidly obese women with type 2 diabetes: effects on glucose homeostasis, postprandial triglyceridemia and selected gut hormones. Obes Surg. 2014;24:718–726. doi: 10.1007/s11695-013-1143-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.