Abstract
Organic solvent-tolerant lipase-producing microorganisms were isolated from petrol spilled soil. From ten morphologically distinct lipase-producing bacterial isolates, highest amount of lipase-producing isolate UBT1 was identified as Acinetobacter sp. using 16S rRNA gene sequencing (NCBI Accession No: MH879815). An increase in lipase production from 42 U/mL to 243 U/mL was obtained when different deoiled seed cakes were supplemented instead of olive oil in the medium. Further optimization of media components by the statistical approach assisted in discerning the main influencing media components and their optimum concentrations. Nine components glucose, castor seedcake, potassium nitrate, gum arabic, calcium chloride, magnesium sulphate, potassium di-hydrogen phosphate, dipotassium hydrogen phosphate, and ferric chloride were selected for Plackett–Burman design. The optimum concentrations of three significant selected components for the lipase production were found to be 0.025 gm% glucose, 0.002 gm% calcium chloride, and 0.2 gm% potassium di-hydrogen phosphate as determined by Response Surface Methodology. Increase in lipase production with 292.29 U/mL was achieved in the media containing optimized components and 2 gm% deoiled castor seed cake. Purification studies with ammonium sulphate precipitation, dialysis, and gel permeation chromatography resulted in 77.54% recovery with 5.77-fold partially purified lipase. The residual activity of lipase in 50 and 75% concentration of n-hexane among other solvents after 24 h was 105.05 and 90.42%, respectively, indicating its solvent tolerance. The present study reports the isolation of organic solvent-tolerant lipase-producing Acinetobacter sp. UBT1, optimization of the culture media for lipase production using the deoiled castor seed cake, and its partial purification.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02501-0) contains supplementary material, which is available to authorized users.
Keywords: Deoiled seed cakes, Response surface methodology, Central composite design, Partial purification, Solvent-tolerant lipase
Introduction
Lipases (3.1.1.3) are important enzymes of the class hydrolases. The enzymes in this class perform only hydrolysis, but lipase is an exception which also carries out the synthesis reactions such as esterification, transesterification, inter-esterification, aminolysis, and acidolysis (Jaeger and Reetz 1998). The lipases are distinguished from the esterases mostly on the basis of their interfacial activation, i.e., they catalyse a reaction only at oil/water interface, they also differ in their substrate specificity where the preference of lipases is for long-chain fatty acids, whereas the esterases prefer short-chain fatty acids (Kapoor and Gupta 2012; Casas-Godoy et al. 2012). There has always been a continuous search for this enzyme from novel organisms and its economical production. One approach for economical production is the use of food and agricultural waste products and the other is optimizing media components for enzyme production.
Oilseed cakes are the spin-offs of the oil mills, after the oil extraction. Every year, a vast amount of these is generated as agro-industrial by-products’ residues. Most of these being less utilized and untreated ends up with either dumping or burning, resulting in serious environmental issues. Biotransformation of these residues through fermentation may help in overcoming the environmental problems (Sadh et al. 2018; Sahoo et al. 2018). Use of other media ingredients and their optimization is very important while utilizing such agro-industrial waste materials as all media components play a crucial role in the growth of microorganisms as well as for enzyme production. The major outcome of applying the statistical methods in the sequence of screening the significant media components by Plackett–Burman Design followed by the Response Surface Methodology is a better understanding of the required optimum concentrations of significant components along with their individual and interactive effect on the fermentation process (Gupta et al. 2007; Ruchi et al. 2008).
The property of lipases to carry out hydrolysis, as well as synthetic reactions in the presence of organic solvents has resulted in more interest for their application in the areas like detergent, paper and pulp, pharmaceutical, food and dairy, cosmetics, energy, biosensors, and leather processing (Sarmah et al. 2018; Goncalves et al. 2019). The presence of solvents during the synthesis reactions may affect the activity and stability of the enzyme (Kumar et al. 2016), and hence, solvent-tolerant lipase is a requirement for such industrial applications.
Materials and methods
Isolation and identification of solvent-tolerant lipase-producing organisms
Soil samples collected from a petrol pump at Anand (22.5645° N, 72.9289° E), Gujarat, India, were serially diluted and plated on the Nutrient Agar plates using the spreading technique. The plates were incubated at 37 °C, till the colonies were visible. Morphologically distinct colonies obtained on Nutrient agar plates were screened on Tributyrin agar [(g/L) peptone 5.0; yeast extract 3.0; tributyrin 1.0 (v/v); agar 2.0] plates for the lipase producers. Ten isolates showing zone of clearance on tributyrin agar plates indicating lipase production were further screened on solid and liquid media containing Bushnell Haas Medium (BHM) [g/L) MgSO4 0.2; CaCl2 0.02; KH2PO4 1.0; K2HPO4 1.0; NH4NO3 1.0; FeCl3 0.05; pH 7.0] with 10% olive oil (Prajapati et al. 2014). Isolate UBT1 showing the growth on olive oil agar plate and highest enzyme activity in the broth was selected for further studies and maintained on Nutrient agar slants with frequent transfers. Identification of the selected isolate was performed through amplification of the 16S rRNA using Sanger sequencing. The DNA isolation was carried out by following the manual of HiPurA™ Bacterial Genomic DNA Purification Kit (Himedia). The sequencing was performed by ABI PRISM® 310 Genetic Analyzer (Applied Biosystems, USA), using BigDye® Terminator v3.1 Cycle sequencing kit (Applied Biosystems, USA). The 16S rRNA amplification was carried out using 8F-5′AGAGTTTGATCCTGGCTCAG-3′, 1492R-5′-TACGGYTACCTTGTTACGACTT-3′ primers. The amplification was carried out under following PCR reaction conditions: initial denaturation at 95 °C for 2:45 min, followed by 35 cycles of 98 °C for 10 s, 60 °C for 15 s, 72 °C for 20 s, and a final extension step at 72 °C for 1 min. The sequencing data obtained from the two end sequences were used to assemble the full-length 16S rRNA using the Clone Manager software. Sequence analysis of the two assembled end sequences of the gene was carried out by comparing with the available sequences at National Centre for Biotechnology Information (NCBI) GenBank. The phylogenetic relationships of the isolate identified and designated in the present study as Acinetobacter sp. UBT1 were inferred using MEGA7 software after performing alignment using CLUSTAL. The phylogenetic tree visualisation was improved using Interactive Tree Of Life (iTOL) v.5.
Inoculum preparation
A loopful of culture from the Nutrient agar slant was inoculated into 5 mL of Nutrient broth and incubated under shaking condition at 150 rpm, 30 °C for 24 h. For inoculum development, 1 mL of this culture (O.D600 ~1.0) was inoculated into 250 mL Erlenmeyer flask having 100 mL BHM broth with 10% olive oil and incubated under similar conditions. This inoculum was used during the further lipase production studies.
Lipase assay
The enzyme assay was performed by modified Winkler and Stuckmann (1979) method with para-nitrophenyl palmitate as the substrate. 4-Nitrophenol was used as standard. The substrate was prepared by adding (mg%): p-nitrophenyl palmitate 30, bile salts 207, and gum arabic 100 into 0.05 M sodium phosphate buffer, pH 7.0. The reaction system contained 2.4 mL freshly prepared substrate and 0.1 mL of the enzyme, which was incubated for 10 min at 55 °C, and the product released was measured at O.D410 against the control. One enzyme unit is defined as 1 nmol of para nitrophenol released per mL of enzyme per min.
Optimization of physical parameters and media components using one factor at a time (OFAT) approach
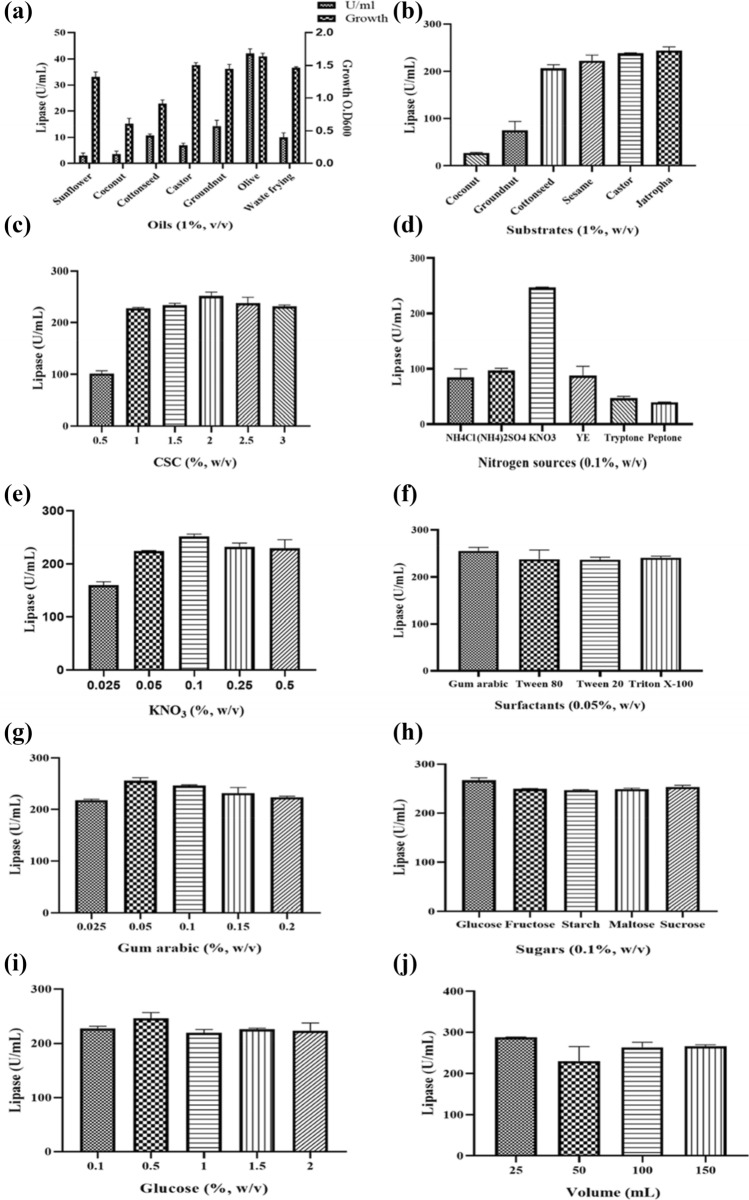
The physical parameters such as time (24, 48, 72, 96, 120, and 144 h), temperature (25, 30, and 37 °C), pH (5, 6, 7, 8, 9, and 10), and inoculum volume (0.5, 1, 2, 3, 4, and 5%) were optimized in medium containing olive oil as mentioned earlier. Keeping the BHM components constant, the effect of oils (1%, v/v; sunflower, coconut, cottonseed, castor, groundnut, olive, and waste frying), agro-industrial wastes (1%, w/v; deoiled seedcakes of coconut, groundnut, cottonseed, sesame, castor, and jatropha), nitrogen sources (0.1%, w/v; NH4Cl, (NH4)2SO4, KNO3, yeast extract, tryptone, and peptone), sugars (0.1%, w/v; glucose, fructose, starch, maltose, and sucrose), surfactants (0.05%, w/v; gum arabic, tween 80, tween 20, and triton X-100), and the effect of media volume (25, 50, 100, and 150 mL media in 250 mL flask) on lipase production were studied. All the data are mean values of triplicate experimental sets with the Standard Deviation (± SD).
Screening of significant variables using Plackett–Burman design
The significant media components were screened using the multifactorial design given by Plackett and Burman (1946). The maximum (+) and minimum (−) ranges of the components are shown in Table 1. The nine selected components were glucose, castor seedcake, potassium nitrate, gum arabic, calcium chloride, magnesium sulphate, potassium di-hydrogen phosphate, dipotassium hydrogen phosphate, and ferric chloride. The N = 12 design was selected (N = number of experiments), with two dummies and nine components (Table 2). The factors and their range in this design were selected based on the highest lipase production obtained in the OFAT approach. All the experiments for the design sets were carried out in a 25 mL system in 250 mL Erlenmeyer flask. The flasks were incubated for 72 h at 30 °C. After incubation, enzyme activity from the supernatant was determined as U/mL.
Table 1.
The minimum (−) and maximum (+) range parameters used in the Plackett–Burman design
| Variables | Components (gm %) | + | − |
|---|---|---|---|
| X1 | Glucose | 0.56 | 0.056 |
| X2 | Castor seed cake (CSC) | 10 | 1 |
| X3 | Potassium nitrate | 0.56 | 0.056 |
| X4 | Gum arabic | 0.13 | 0.013 |
| X5 | Calcium chloride | 0.06 | 0.006 |
| X6 | Magnesium sulphate | 0.04 | 0.004 |
| X7 | Potassium di-hydrogen phosphate | 0.2 | 0.02 |
| X8 | Dipotassium hydrogen phosphate | 0.2 | 0.02 |
| X9 | Ferric chloride | 0.01 | 0.001 |
Table 2.
Plackett–Burman design for screening of media components for lipase production by Acinetobacter sp. UBT1
| RUN | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | D1 | D2 | Lipase activity (U/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | − | + | + | + | − | − | − | + | − | 170.385 |
| 2 | − | + | + | − | + | + | + | − | − | − | + | 281.080 |
| 3 | + | − | + | + | − | + | + | + | − | − | − | 268.960 |
| 4 | − | + | − | + | + | − | + | + | + | − | − | 264.819 |
| 5 | − | − | + | − | + | + | − | + | + | + | − | 271.384 |
| 6 | − | − | − | + | − | + | + | − | + | + | + | 263.506 |
| 7 | + | − | − | − | + | − | + | + | − | + | + | 186.242 |
| 8 | + | + | − | − | − | + | − | + | + | − | + | 233.712 |
| 9 | + | + | + | − | − | − | + | − | + | + | − | 274.919 |
| 10 | − | + | + | + | − | − | − | + | − | + | + | 275.828 |
| 11 | + | − | + | + | + | − | − | − | + | − | + | 136.146 |
| 12 | − | − | − | − | − | − | − | − | − | − | − | 265.021 |
The Plackett–Burman (PB) design is of the first-order reaction and the effect of each variable was determined by the following:
| 1 |
where ∑ (xi) is the concentration effect of the tested variable. Mi + and Mi– are the lipase production from the trials where the variable (Xi) measured was present at the high and low concentration, respectively, and N is the number of experiment (12 experiments). The Standard Error (SE) of the concentration effect was the square root of the variance of an effect, and the significance level (p value) of each concentration effect was determined using student’s t-test:
| 2 |
where E (xi) is the effect of variable xi. The variables with confidence levels greater than 90% were considered to influence the enzyme production significantly.
Response surface methodology (RSM)–Central composite design (CCD)
Central Composite Design (CCD) is employed to observe the interactive effects of one or more components found significant in the PB Design. It is a factorial design where the optimal response of the system is depicted in the form of contour plots depending on linear or quadratic effects of the key components and also found by the model equation derived. There are two F factorial points, 2 k axial points (± α) and natural centre points. The minimum and maximum values for the CCD were decided based on PB results. The experiments were designed using Design Expert Software, Trial v12, Stat- Ease Inc, USA. Total of 20 sets of experiments were designed, and in each experiment, 250 mL Erlenmeyer flask contained 25 mL of media with keeping the castor seed cake, potassium nitrate, gum arabic, magnesium sulphate, dipotassium hydrogen phosphate, and ferric chloride concentration constant at optimum pH and temperature with varying amount of glucose, calcium chloride, and potassium di-hydrogen phosphate (Table 3).
Table 3.
Coded and actual values of the factors used in CCD for lipase production by Acinetobacter sp. UBT1
| Factors | Symbol | Actual factor levels at coded levels | ||||
|---|---|---|---|---|---|---|
| − 1 | − α | 0 | + α | + 1 | ||
| Glucose (gm %) | X1 | 0.025 | − 0.051 | 0.137 | 0.326 | 0.250 |
| CaCl2 (gm %) | X2 | 0.002 | − 0.004 | 0.011 | 0.026 | 0.020 |
| KH2PO4 (gm %) | X3 | 0.200 | − 0.413 | 1.100 | 0.261 | 2.000 |
Partial purification and solvent tolerance of lipase enzyme
The organism was allowed to grow under optimized media conditions for 72 h and supernatant was obtained by centrifugation at 10,000 g for 40 min at 4 °C. From the supernatant, enzyme was precipitated using ammonium sulphate (0–60%). The protein precipitates were collected by centrifugation at 10,000 g for 30 min at 4 °C. The pellet was resuspended in 0.05 M sodium phosphate buffer (pH 7) for protein solubilization and was dialysed against the same buffer for 24 h to eliminate excess salt. The dialysed enzyme was subjected to Gel Permeation Chromatography using Sephadex G-50 column. The column was pre-equilibrated with the 0.05 M sodium phosphate buffer (pH 7). Fractions of 0.5 mL were collected with a flow rate of 0.1 mL/min and were subjected to enzyme and protein assay (Lowry et al. 1951).
The solvent tolerance property of partially purified lipase was tested by incubating the enzyme with the solvents (Iso-octane, n-heptane, n-hexane, xylene, toluene, benzene, chloroform, and methanol) in two final concentration 50 and 75% (v/v) for 24 and 48 h on shaker at 150 rpm, 30 °C. The residual activity of the lipase enzyme was then assayed.
Results and discussion
Isolation and identification of the strain
The bacterial isolate UBT1 showed zone of clearance of 1.1 cm and the culture diameter of 0.3 cm on Tributyrin agar plate after 48 h (Supplementary Fig. 1). It showed growth on olive oil agar plate and highest enzyme production 42 U/mL in BHM broth with olive oil and hence selected for further studies. The data obtained for 16S rRNA sequence of the isolate UBT1 when subjected to the NCBI gene database search BLASTn showed very close similarity with the Acinetobacter indicus. The isolated culture has been thus identified and referred to as Acinetobacter sp. UBT1 and its relatedness with another lipase-producing Acinetobacter spp. is shown in the phylogenetic tree in Fig. 1. The gene sequence has been deposited in the NCBI GenBank (Accession No: MH879815).
Fig. 1.
The evolutionary history was inferred using the neighbor-joining method. The optimal tree with the sum of branch length = 3.23813284 is shown. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. The analysis involved 26 nucleotide sequences. Codon positions included were 1st + 2nd + 3rd + Noncoding. All positions containing gaps and missing data were eliminated. Evolutionary analyses were conducted in MEGA7
Optimization using OFAT strategy
Optimization of physical parameters
As depicted in Fig. 2a for the time-course study, lipase production was increased with increase in time with maximum growth and lipase production at 72 h. The production of lipases during late log phase, i.e., 48 h or thereafter, has been reported for most of the extracellular lipases (Jagtap et al. 2010). Some organisms like Bacillus sp. ZR-5 showed highest lipase production at 24 h of incubation time (Soleymani et al. 2017). Our results are in accordance with other studies for lipase production by Acinetobacter haemolyticus and Pseudomonas sp. which showed maximum lipase production at 72 h (Jagtap et al. 2010; Tembhurkar et al. 2012).
Fig. 2.
Effect of physical parameters on lipase production by Acinetobacter sp. UBT1 using one factor at a time approach: (a) Time course, (b) Temperature, (c) pH, and (d) Inoculum volume
Temperature is a crucial parameter, as it influences the extracellular enzyme secretion by changing the cell membrane properties and its effect also varies from one organism to other (Veerapagu et al. 2013). The highest growth and lipase production (25 U/mL) by our strain was at 30 °C (Fig. 2b). Our isolate is showing similar results for optimum temperature requirement of 30 °C with Acinetobacter sp. AUO7 and Acinetobacter radioresistens isolated from the distillery waste and sludge wastewater, respectively (Gururaj et al. 2016; Chen et al. 1998).
pH plays a crucial role in the growth of microorganisms and for enzyme production. In our studies, maximum growth and lipase production were at pH 7, while minimum was observed at pH 5. The lipase activity was recorded in the range of pH 6–10 (Fig. 2c). Most lipase-producing microorganisms and especially bacteria have been reported to have pH 7 as optimum requirement (llesanmi et al. 2020). Similar results have also been reported for lipase production by Staphylococcus sp. and Serratia sp. (Sirisha et al. 2010; Zheng 2018). Proximate to our finding, Acinetobacter radioresistens CMC-1 and Staphylococcus aureus have been reported for maximum lipase production at pH 7.5 (Hong and Chang 1998; Khatape et al. 2015).
The inoculum volume from 0.5 to 5% were studied and the highest lipase production (51 U/mL) was observed when the production media was inoculated with 2% of inoculum (Fig. 2d). Appropriate inoculum volume is required for rapid and maximum enzyme production. Burkholderia pyrrocinia B1213 and Serratia marcescens VITSD2 have been reported to produce maximum lipase with 4% inoculum (Li et al. 2018; Mohanasrinivasan et al. 2018). In contrast to the latter, the maximum inoculum volume required by the Acinetobacter sp. K5b4 was 0.4% (Allimoun et al. 2015).
Media component optimization
The maximum lipase production by Acinetobacter sp. UBT1 (51 U/mL) was obtained when olive oil was as carbon source (Fig. 3a). It was observed to produce low amount of lipase with other oils as substrates. During lipase production studies, Pseudomonas sp., Acinetobacter baylyi, and Staphylococcus aureus showed 12.5 U/mL, 0.358 U/mL, and 6.45 U/mL lipase activity, respectively, by utilizing olive oil, which is less than the Acinetobacter sp. UBT1 lipase activity (Khatape et al. 2015; Furini et al. 2018; Habibollahi and Salehzadeh 2018). Our result was close to the Acinetobacter haemolyticus TA 106 lipase production studies, where 42 U/mL enzyme production utilizing olive oil has been reported (Jagtap et al. 2010).
Fig. 3.
The graphical representation of effects of different media components for lipase production by Acinetobacter sp. UBT1: (a) effect of oils, (b) effect of different agro-wastes, (c) effect of different percentage of castor seed cake (CSC), (d) effect of different nitrogen sources, (e) different percentages of KNO3, (f) effect of surfactants, (g) different percentage of gum arabic, (h) effect of sugars, (i) different percentage of glucose, and (j) effect of media volume-to-surface ratio
Agricultural wastes such as oil seed cakes are an alternative and cheap source which can serve as substrate for the lipase production. These oil cake wastes are produced in huge amounts, and apart from their use as a fodder for cattles, many studies have reported the use of different oil cakes for microbial production of enzymes. Using six different agro-industrial wastes as substrates a drastic increase in enzyme production by Acinetobacter sp. UBT1 was observed. In the liquid media, 243 U/mL and 238 U/mL lipase production was obtained using jatropha (JSC) and castor seed cake (CSC), respectively (Fig. 3b). As CSC showed good enzyme production and is easily available, different percentage of this substrate were checked for the optimization (Fig. 3c). In a study by Jain and Naik (2018), the maximum lipase yield of 25 U/g was obtained using deoiled CSC as a substrate during solid-state fermentation using Aspergillus japonicas.
Different organic and inorganic nitrogen were supplemented in the media. The nitrogen sources at concentration 0.1% potassium nitrate showed maximum lipase production (251.28 U/mL) among all the nitrogen sources (Fig. 3d). Furthermore, the KNO3 concentration was optimized in the range of 0.025–0.5%, and it was observed that, at 0.1% concentration, maximum lipase activity was obtained (Fig. 3e). Quite similar to our results, maximum lipase production was observed when inorganic nitrogen sources like ammonium nitrate and potassium nitrate were used for enzyme production by Pseudomonas otitidis G5 and Pseudomonas aeruginosa AAU2, respectively (Haque et al. 2019; Bose and Keharia 2013).
Four different surfactants were considered for their effect on lipase production. Results showed that all four surfactants were effective in increasing the lipase production (Fig. 3f). Gum arabic supplementation resulted in highest lipase production (256 U/mL) at 0.05% concentration (Fig. 3g). Addition of gum arabic has also been reported to produce maximum lipase at 25 mg/L concentration (Noor et al. 2003).
The media supplemented with glucose produced highest lipase (260 U/mL) (Fig. 3h). There is marginal difference in the lipase activity between other sugars used. Glucose may help to initiate the growth as it is the simplest sugar utilized first. The glucose concentration of 0.5% was found to be optimum for lipase production (Fig. 3i). In contrast to our result, A. haemolyticus TA 106, S. aureus, and Acinetobacter sp. K5b4 showed maximum lipase production by utilizing 3% sucrose, 1% glycerol, and 0.2% glycerol, respectively, as non-lipidic carbon sources (Jagtap et al. 2010; Khatape et al. 2015; Allimoun et al. 2015). To check whether good production could be obtained by reducing the volume of production medium which would save the utilization of excessive resources, different media volumes were taken in the 250 mL flasks. The lower volume of media in larger flask also increases aeration. The maximum activity was found in the flask containing 25 mL medium volume in 250 mL flask. Other studies have also reported similar results where lower volumes of the media like 50 mL and 30 mL have been found effective (Zheng 2018; Masomian et al. 2010; Kumar et al. 2005).
Plackett–Burman design
Plackett–Burman design was used as a statistical platform for screening the most effective components and their concentrations to attain highest lipase production by Acinetobacter sp. UBT1. Though, OFAT strategy has helped in increasing the lipase production, experiments using Plackett–Burman Design would be expected to give more precise result. The design was made to run in 12 sets, where X1–X9 were variables and X10–X11 were dummy. The variables chosen were with respect to the results obtained from one factor at a time strategy, where other are the individual components taken from the basal salt medium. The positive and negative sign of Exi (concentration effect of tested variables) shows the influence of the variable on lipase yield. Positive indicates that the influence of variable is greater at high concentration and negative indicates influence of variable is greater at low concentration. Three variables are selected by getting the high percentage of confidence, mostly > 95%. Four variables tested, i.e., X1 (Glucose), X4 (Gum arabic), X5 (CaCl2), and X9 (Ferric chloride), were influencing for the high yield at lower concentrations, while X2 (Castor seed cake), X3 (KNO3), X6 (MgCl2), X7 (KH2PO4), and X8 (K2HPO4) were found effective at their higher concentrations. The effect, txi, p, and confidence level of each component are shown in Table 4. Kumar et al. (2011) screened 12 different variables using PB Design and found that significant role of olive oil, tween 80, and KH2PO4. Enhanced lipase production has been achieved from Bacillus cereus ASSCRC-P1 by optimizing the media components using PB Design with waste frying oil, triton X-100, and MnSO4 as significant variables (Awad et al. 2015). Similarly, Ruchi et al. (2008) and Gupta et al. (2007) have also reported the significant components like tryptone, gum arabic, MgSO4, yeast extract, glucose, and oil using the PB statistical analysis which contributes for maximum lipase production.
Table 4.
Statistical analysis (Plackett–Burman design) of components for lipase production by Acinetobacter sp. UBT1
| ANOVA analysis | ||||
|---|---|---|---|---|
| Exi | txi | P | % Confidence | |
| Glucose | − 58.545 | − 5.13 | 0.04 | 96.41 |
| CSC | 18.245 | 1.6 | 0.25 | 74.91 |
| KNO3 | 2.771 | 0.24 | 0.83 | 16.93 |
| GA | − 40.121 | − 3.52 | 0.07 | 92.78 |
| CaCl2 | − 45.315 | − 3.97 | 0.06 | 94.21 |
| MgSO4 | 14.345 | 1.26 | 0.34 | 66.44 |
| KH2PO4 | 49.175 | 4.31 | 0.05 | 95.02 |
| K2HPO4 | 36.315 | 3.18 | 0.09 | 91.38 |
| FeCl3 | − 18.5083 | − 1.62 | 0.25 | 75.38 |
Response surface methodology–Central composite design
To assess the combined effects of the factors found significant in the PB design, the CCD was executed, in which 20 experimental sets showing the actual and predicted response value are shown in Table 5. Three factors, viz., glucose, calcium chloride, and KH2PO4, were optimized using CCD of RSM for lipase production. The polynomial equation was used for obtaining the maximum lipase production:
Table 5.
Full experimental setup for Central Composite Design showing the actual values, coded values, and the response
| Std | A: Glucose (gm %) | B: Calcium chloride (gm %) | C: KH2PO4 (gm %) | Response: R1 (U/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| Actual level | Coded level | Actual level | Coded level | Actual level | Coded level | Actual value | Predicted value | |
| 1 | 0.025 | − 1 | 0.002 | − 1 | 0.2 | − 1 | 291.29 | 283.63 |
| 2 | 0.25 | 1 | 0.002 | − 1 | 0.2 | − 1 | 281.39 | 282.87 |
| 3 | 0.025 | − 1 | 0.02 | 1 | 0.2 | − 1 | 281.59 | 283.63 |
| 4 | 0.25 | 1 | 0.02 | 1 | 0.2 | − 1 | 272.98 | 282.45 |
| 5 | 0.025 | − 1 | 0.002 | − 1 | 2 | 1 | 278.16 | 276.77 |
| 6 | 0.25 | 1 | 0.002 | − 1 | 2 | 1 | 283.21 | 290.32 |
| 7 | 0.025 | − 1 | 0.02 | 1 | 2 | 1 | 279.17 | 283.63 |
| 8 | 0.25 | 1 | 0.02 | 1 | 2 | 1 | 282.00 | 284.46 |
| 9 | − 0.0517 | − α | 0.011 | 0 | 1.1 | 0 | 284.82 | 283.63 |
| 10 | 0.32670 | + α | 0.011 | 0 | 1.1 | 0 | 276.54 | 282.34 |
| 11 | 0.1375 | 0 | − 0.00414 | − α | 1.1 | 0 | 280.99 | 278.06 |
| 12 | 0.1375 | 0 | 0.02613 | + α | 1.1 | 0 | 277.15 | 279.14 |
| 13 | 0.1375 | 0 | 0.011 | 0 | − 0.41361 | − α | 281.59 | 283.63 |
| 14 | 0.1375 | 0 | 0.011 | 0 | 2.61361 | + α | 279.57 | 278.41 |
| 15 | 0.1375 | 0 | 0.011 | 0 | 1.1 | 0 | 281.23 | 280.34 |
| 16 | 0.1375 | 0 | 0.011 | 0 | 1.1 | 0 | 284.22 | 272.03 |
| 17 | 0.1375 | 0 | 0.011 | 0 | 1.1 | 0 | 283.01 | 283.63 |
| 18 | 0.1375 | 0 | 0.011 | 0 | 1.1 | 0 | 289.98 | 281.91 |
| 19 | 0.1375 | 0 | 0.011 | 0 | 1.1 | 0 | 280.38 | 280.36 |
| 20 | 0.1375 | 0 | 0.011 | 0 | 1.1 | 0 | 283.21 | 281.3 |
Y = 283.631 + − 1.79834 * A + − 1.81314 * B + − 0.594443 * C + − 0.116875 * AB + 3.29837 * AC + 2.23787 * BC + − 0.775398 * A2 + − 1.34674 * B2 + − 0.811106 * C2,
where Y is the response factor, A is glucose (gm%), B is calcium chloride (gm%), and C is KH2PO4 (gm%).
As per the data shown in Table 6, the Fisher’s F test value of the model 3.92 from analysis of variance (ANOVA) implies that this regression is statistically highly significant for maximum lipase production. There is only a 2.21% chance that an F value could be large due to noise. The value of probability (Prob > F) observed was < 0.05, i.e., 0.0224. The values of p < 0.05 indicate that the model terms are significant. In this study, A, B, AC, and BC are significant model terms. The Lack of Fit F-value of 0.27 implies the Lack of Fit is not significant relative to pure error. There is 90.88% chance that this large Lack of Fit F-value could occur due to noise. Non-significant Lack of Fit is good for the model. The multiple correlation coefficient (R2) value near to 1 indicates better correlation between the predicted and observed values. By determining the coefficient of variance (CV), the degree of precision with which the experiments are compared is obtained. Our results showing a low CV (0.96) denotes that the experiments carried out are with high precision. Adequate precision measures the signal-to-noise ratio. A ratio greater than 4 is desirable. Here, the ratio of 9.557 indicates an adequate signal. This model can be used to navigate the design space.
Table 6.
Analysis of variance (ANOVA) for the response surface quadratic model for lipase production from Acinetobacter sp. UBT1
| Source | Sum of squares | df | Mean square | F value | p value | |
|---|---|---|---|---|---|---|
| Model | 258.86 | 9 | 28.76 | 3.92 | 0.0221 | Significant |
| A-Glucose | 44.17 | 1 | 44.17 | 6.03 | 0.034 | Significant |
| B-CaCl2 | 44.9 | 1 | 44.9 | 6.13 | 0.0328 | Significant |
| C-KH2PO4 | 4.83 | 1 | 4.83 | 0.6584 | 0.436 | |
| AB | 0.1093 | 1 | 0.1093 | 0.0149 | 0.9052 | |
| AC | 87.03 | 1 | 87.03 | 11.87 | 0.0063 | Significant |
| BC | 40.06 | 1 | 40.06 | 5.47 | 0.0415 | Significant |
| A2 | 8.66 | 1 | 8.66 | 1.18 | 0.3024 | |
| B2 | 26.14 | 1 | 26.14 | 3.57 | 0.0883 | |
| C2 | 9.48 | 1 | 9.48 | 1.29 | 0.2819 | |
| Residual | 73.3 | 10 | 7.33 | |||
| Lack of fit | 15.79 | 5 | 3.16 | 0.2745 | 0.9088 | Not significant |
| Pure error | 57.51 | 5 | 11.5 | |||
| Cor Total | 332.16 | 19 |
The contour plots and response surface plots have helped in predicting the optimal values for the lipase production and also the interactions between three factors glucose, CaCl2, and KH2PO4 (Fig. 4). The lipase production, main effect, squared effect, and interaction effect of glucose and CaCl2 at different concentration when analysed (Fig. 4a (1), (2)) indicated that at high and low concentrations of both components glucose and CaCl2, there is no significant increase in the lipase production. As the CaCl2 concentration increases, the lipase production also increases up to a certain level. However, high glucose and low CaCl2 concentration lead to high lipase production. Figure 4b (1), (2) shows the interaction between the glucose and KH2PO4. It shows increase in lipase production with increasing concentration of glucose and KH2PO4. Although both increasing concentrations give more lipase production, glucose in comparison gives higher production. The high and low concentration of both components leads to decrease in lipase production. Figure 4c (1), (2) depicts the contour plot and three- dimensional curve of CaCl2 and KH2PO4 interaction, where highest lipase production is observed at high CaCl2 and low KH2PO4 concentration. The enzyme yield was decreased with simultaneous increase and decrease in concentration of both. The Bacillus pumilus RK31 was found to produce maximum lipase by utilizing the three screened components from the Plackett Burman Design. These were then subjected to further optimization of parameters using Response Surface Model, in which the maximum lipase yield was obtained at 10 mL/L, 5 mL/L, and 8 g/L of olive oil, tween 80, and KH2PO4, respectively (Kumar et al. 2011). Four components screened by PB Design (tryptone, yeast extract, gum arabic, and MgSO4) were further analysed by Response Surface Methodology to find the interactive effects in the lipase production media by Pseudomonas aeruginosa (Ruchi et al. 2008).
Fig. 4.
Response surface graph and contour plots showing the interactions between (a) glucose and CaCl2, (b) glucose and KH2PO4, and (c) CaCl2 and KH2PO4, during lipase production using CCD by Acinetobacter sp. UBT1
Validation of the quadratic model
Validation of the above-mentioned components predicted by the response surface model in the production medium was carried out. The optimum concentrations were glucose 0.025 gm%, CaCl2 0.002 gm%, and KH2PO4 0.2 gm%. The predicted value of lipase production obtained from the model using these optimum concentrations of the three components was 283.63 U/mL. The actual lipase production obtained was 291.29 U/mL which is higher than the predicted value. Therefore, this Response Surface Model was reliable for predicting higher lipase production by Acinetobacter sp. UBT 1.
Partial purification and solvent tolerance of lipase
Acinetobacter sp. UBT1 lipase was purified to 5.77-fold through ammonium sulphate precipitation followed by dialysis and Gel Permeation Chromatography (Table 7). The specific activity of lipase obtained was 267.39 U/mg with 77.54% yield. The enzyme yields up to 18.3% and 8.4% have been reported for Acinetobacter sp. k5b4 and A. haemolyticus NS02-30 lipase (Al-Limoun et al. 2019; Sarc and Ugur 2016).
Table 7.
Purification of lipase from Acinetobacter sp. UBT1
| Purification steps | Activity (U/mL) |
Protein (mg/mL) |
Specific activity (U/mg) | Yield (%) |
Purification (Fold) |
|---|---|---|---|---|---|
| Crude extract | 304 | 6.56 | 46.28 | 100 | 1 |
| Ammonium sulphate precipitation (0–60%) | 243 | 4.29 | 56.54 | 79.93 | 1.22 |
| Gel permeation chromatography | 235.73 | 0.88 | 267.39 | 77.54 | 5.77 |
As the solvent toxicity is measured by its log P value, different solvents were selected in the present study with range of log P values (Table 8). Acinetobacter sp. UBT1 lipase showed stability in the presence of organic solvents, both polar (hydrophilic) and non-polar (hydrophobic), i.e., methanol, chloroform, n-heptane, n-hexane, xylene, toluene, and benzene, at 50% and 75% concentration for 48 h. A sharp increase in the relative activity was observed in the presence of 50% n-hexane (105.05%) when enzyme was incubated for 24 h retaining considerable activity retained when incubated with 75% n-hexane (90.42%). High lipase activity was observed in the presence of non-polar solvents as these solvents may provide better microenvironment for the lipase to catalyse the reaction (Sharma and Kanwar 2014). Lipases from different strains of Acinetobacter sp. show difference in tolerance to the solvents. Lipase from Acinetobacter sp. has shown tolerance to all the solvents tested, i.e., both water miscible and non-miscible (methanol (114.7%), acetonitrile (105.9%), ethanol (153.4%), acetone (133.9%), 2- propanol (135.9%), ethyl acetate (111.4%), and hexane (114.0%)) (Khoramnia et al. 2011). Highest activity was reported by them in ethanol (polar solvent), which is in contrast to our result. In accordance with our result, Acinetobacter sp. EH28 lipase showed increased residual activity (112%) in 30 mM concentration of n-Hexane (Ahmed et al. 2010). This solvent tolerance capacity of the Acinetobacter sp. UBT1 lipase may be useful in its biotechnological applications in various fields which may include its use in synthesis reactions in the presence of organic solvents.
Table 8.
Organic solvent stability of purified Acinetobacter sp. UBT1 lipase
| Solvent | Log p | 50% | 75% | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | ||
| Control | – | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Iso-Octane | 4.5 | 72.53 ± 0.99 | 63.61 ± 1.27 | 20.68 ± 0.18 | 36.13 ± 1.98 |
| n-Heptane | 4.3 | 71.41 ± 2.06 | 56.47 ± 1.63 | 35.93 ± 2.86 | 16.59 ± 2.61 |
| n-Hexane | 3.5 | 105.05 ± 0.76 | 64.96 ± 1.63 | 90.42 ± 2.32 | 37.00 ± 0.63 |
| Xylene | 3.1 | 17.92 ± 2.14 | 9.92 ± 1.85 | 14.51 ± 1.52 | 7.49 ± 0.39 |
| Toluene | 2.5 | 32.98 ± 1.57 | 2.02 ± 0.15 | 4.82 ± 3.08 | 0.62 ± 0.06 |
| Benzene | 2.1 | 19.19 ± 3.32 | 7.67 ± 0.80 | 12.36 ± 1.49 | 7.87 ± 0.26 |
| Chloroform | 2.0 | 71.80 ± 0.83 | 62.86 ± 1.64 | 43.27 ± 0.19 | 35.80 ± 2.69 |
| Methanol | − 0.76 | 14.75 ± 1.03 | 6.42 ± 0.44 | 13.69 ± 0.56 | 9.89 ± 1.58 |
Conclusion
The Acinetobacter sp. UBT1 identified by 16S rRNA nucleotide sequence analysis is a potential solvent-tolerant lipase-producing strain. The use of deoiled seedcakes has helped in increasing lipase production. In the present study, the use of OFAT strategy and the PB-RS methodology has helped in optimizing lipase production using deoiled castor seedcake as a potential low-cost medium component. The use of ammonium sulphate precipitation, dialysis, and Gel Permeation Chromatography during partial purification of Acinetobacter sp. UBT1 lipase has resulted in 77.54% yield with 5.77-fold purification. The enzyme has been found tolerant to range of solvents at 50 and 75% concentrations promising its use for synthesis reactions in organic solvents.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Lipase producing Acinetobacter sp. UBT1 showing the zone of clearance onTributyrin agar plate (DOCX 205 KB)
Acknowledgements
Accession number: The 16S rRNA of the Acinetobacter sp. UBT1 have been submitted to the NCBI gene bank under accession number MH879815.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Ahmed EH, Raghavendra T, Madamwar D (2010). An alkaline lipase from organic solvent tolerant Acinetobacter sp. EH28: application for ethyl caprylate synthesis. Bioresour Technol, 101(10), 3628–3634. https://doi.org/10.1016/j.biortech.2009.12.107 [DOI] [PubMed]
- Allimoun MO, Ananzeh MR, Khleifat KM (2015). Screening selection and optimization of extracellular methanol and ethanol tolerant lipase from Acinetobacter sp. K5b4. Int J Biosci, 6(10), 44–56. http://dx.doi.org/10.12692/ijb/6.10.44-56
- Al-Limoun MO, Khleifat KM, Alsharafa KY, Qaralleh HN, Alrawashdeh SA (2019). Purification and characterization of a mesophilic organic solvent tolerant lipase produced by Acinetobacter sp. K5b4. Biocatal Biotranfor, 37(2), 139–151. https://doi.org/10.1080/10242422.2018.1506445
- Awad GE, Mostafa H, Danial EN, Abdelwahed NA, Awad HM. Enhanced production of thermostable lipase from Bacillus cereus ASSCRC-P1 in waste frying oil based medium using statistical experimental design. J Appl Pharm Sci. 2015;5:7–15. doi: 10.7324/JAPS.2015.50902. [DOI] [Google Scholar]
- Bose A, Keharia H. Production, characterization and applications of organic solvent tolerant lipase by Pseudomonas aeruginosa AAU2. Biocatal Agri Biotechnol. 2013;2(3):255–266. doi: 10.1016/j.bcab.2013.03.009. [DOI] [Google Scholar]
- Casas-Godoy L, Duquesne S, Bordes F, Sandoval G, Marty A (2012). Lipases: An overview. In Lipases and phospholipases (pp. 3–30). Humana Press. https://doi.org/10.1007/978-1-61779-600-5_1
- Chen SJ, Cheng CY, Chen TL. Production of an alkaline lipase by Acinetobacter radioresistens. J Ferment Bioeng. 1998;86(3):308–312. doi: 10.1016/S0922-338X(98)80135-9. [DOI] [Google Scholar]
- Furini G, Berger JS, Campos JA, SAND ST, Germani JC, Production of lipolytic enzymes by bacteria isolated from biological effluent treatment systems. An Acad Bras Ciênc. 2018;90(3):2955–2965. doi: 10.1590/0001-3765201820170952. [DOI] [PubMed] [Google Scholar]
- Gonçalves Filho D, Silva AG, Guidini CZ. Lipases: Sources, immobilization methods, and industrial applications. Appl Microbiol Biotechnol. 2019;103(18):7399–7423. doi: 10.1007/s00253-019-10027-6. [DOI] [PubMed] [Google Scholar]
- Gupta N, Sahai V, Gupta R. Alkaline lipase from a novel strain Burkholderia multivorans: Statistical medium optimization and production in a bioreactor. Process Biochem. 2007;42(4):518–526. doi: 10.1016/j.procbio.2006.10.006. [DOI] [Google Scholar]
- Gururaj P, Ramalingam S, Devi GN, Gautam P (2016). Process optimization for production and purification of a thermostable, organic solvent tolerant lipase from Acinetobacter sp. AU07. Braz J Microbiol, 47(3), 647–657. https://doi.org/10.1016/j.bjm.2015.04.002 [DOI] [PMC free article] [PubMed]
- Habibollahi H, Salehzadeh A (2018). Isolation, optimization, and molecular characterization of a lipase producing bacterium from oil contaminated soils. Pollut, 4(1), 119–128. https://doi.org/10.22059/POLL.2017.238410.297
- Haque E, Velmurugane J, Nagarajan J (2019). Media optimization for lipase production from Pseudomonas otitidis G5. J Adv Sci Res Manag, 4(7).
- Hong MC, Chang MC. Purification and characterization of an alkaline lipase from a newly isolated Acinetobacter radioresistens CMC-1. Biotechnol Lett. 1998;20(11):1027–1029. doi: 10.1023/A:1005407005371. [DOI] [Google Scholar]
- Ilesanmi OI, Adekunle AE, Omolaiye JA, Olorode EM, Ogunkanmi AL. Isolation, optimization and molecular characterization of lipase producing bacteria from contaminated soil. Sci Afr. 2020;8:e00279. [Google Scholar]
- Jaeger KE, Reetz MT. Microbial lipases form versatile tools for biotechnology. Trends Biotechnol. 1998;16(9):396–403. doi: 10.1016/S0167-7799(98)01195-0. [DOI] [PubMed] [Google Scholar]
- Jagtap S, Gore S, Yavankar S, Pardesi K, Chopade B. Optimization of medium for lipase production by Acinetobacter haemolyticus from healthy human skin. Indian J Exp Biol. 2010;48(9):936–941. [PubMed] [Google Scholar]
- Jain R, Naik SN. Adding value to the oil cake as a waste from oil processing industry: Production of lipase in solid state fermentation. Biocatal Agri Biotechnol. 2018;15:181–184. doi: 10.1016/j.bcab.2018.06.010. [DOI] [Google Scholar]
- Kapoor M, Gupta MN. Lipase promiscuity and its biochemical applications. Process Biochem. 2012;47(4):555–569. doi: 10.1016/j.procbio.2012.01.011. [DOI] [Google Scholar]
- Khatape A, Chavan S, Khade S, Doiphode N. Isolation and identification of lipid degrading microorganisms, optimization of medium and partial purification of the lipase enzyme. Int J Life Sci Res. 2015;3(1):99–103. [Google Scholar]
- Khoramnia A, Ebrahimpour A, Beh BK, Lai OM (2011). Production of a solvent, detergent, and thermotolerant lipase by a newly isolated Acinetobacter sp. in submerged and solid-state fermentations. J Biomed Biotechnol, 2011. [DOI] [PMC free article] [PubMed]
- Kumar S, Kikon K, Upadhyay A, Kanwar SS, Gupta R. Production, purification, and characterization of lipase from thermophilic and alkaliphilic Bacillus coagulans BTS-3. Protein Expr Purif. 2005;41(1):38–44. doi: 10.1016/j.pep.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Kumar R, Mahajan S, Kumar A, Singh D. Identification of variables and value optimization for optimum lipase production by Bacillus pumilus RK31 using statistical methodology. New Biotechnol. 2011;28(1):65–71. doi: 10.1016/j.nbt.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dhar K, Kanwar SS, Arora PK. Lipase catalysis in organic solvents: advantages and applications. Biol Proced Online. 2016;18(1):2. doi: 10.1186/s12575-016-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Shen W, Fan G, Li X (2018). Screening, purification and characterization of lipase from Burkholderia pyrrocinia B1213. 3 Biotech, 8(9), 387. https://doi.org/10.1007/s13205-018-1414-9 [DOI] [PMC free article] [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Masomian M, Rahman RNZRA, Salleh AB, Basri M. A unique thermostable and organic solvent tolerant lipase from newly isolated Aneurinibacillus thermoaerophilus strain HZ: Physical factor studies. World J Microbiol Biotechnol. 2010;26(9):1693–1701. doi: 10.1007/s11274-010-0347-1. [DOI] [Google Scholar]
- Mohanasrinivasan V, Devi CS, Jayasmita D, Selvarajan E, Naine SJ. Purification and characterization of extracellular lipase from Serratia marcescens VITSD2. Proc Natl Acad Sci India Sect B Biol Sci. 2018;88(1):373–381. doi: 10.1007/s40011-016-0763-6. [DOI] [Google Scholar]
- Noor I, Hasan M, Ramachandran K. Effect of operating variables on the hydrolysis rate of palm oil by lipase. Process Biochem. 2003;39(1):13–20. doi: 10.1016/S0032-9592(02)00263-7. [DOI] [Google Scholar]
- Plackett RL, Burman JP. The design of optimum multifactorial experiments. Biometrika. 1946;33(4):305–325. doi: 10.2307/2332195. [DOI] [Google Scholar]
- Prajapati V, Patel H, Trivedi U, Patel K. Kinetic and thermodynamic characterization of lipase produced by Cellulomonas flavigena UNP3. J Basic Microbol. 2014;54(9):976–983. doi: 10.1002/jobm.201300065. [DOI] [PubMed] [Google Scholar]
- Ruchi G, Anshu G, Khare SK. Lipase from solvent tolerant Pseudomonas aeruginosa strain: Production optimization by response surface methodology and application. Bioresour Technol. 2008;99(11):4796–4802. doi: 10.1016/j.biortech.2007.09.053. [DOI] [PubMed] [Google Scholar]
- Sadh PK, Duhan S, Duhan JS. Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess. 2018;5(1):1. doi: 10.1186/s40643-017-0187-z. [DOI] [Google Scholar]
- Sahoo RK, Kumar M, Mohanty S, Sawyer M, Rahman PK, Sukla LB, Subudhi E. Statistical optimization for lipase production from solid waste of vegetable oil industry. Prep Biochem Biotechnol. 2018;48(4):321–326. doi: 10.1080/10826068.2018.1431785. [DOI] [PubMed] [Google Scholar]
- Sarac N, Ugur A. A green alternative for oily wastewater treatment: lipase from Acinetobacter haemolyticus NS02-30. Desalin Water Treat. 2016;57(42):19750–19759. doi: 10.1080/19443994.2015.1106346. [DOI] [Google Scholar]
- Sarmah N, Revathi D, Sheelu G, Rani KY, Sridhar S, Mehtab V, Sumana C. Recent advances on sources and industrial applications of lipases. Biotechnol Prog. 2018;34(1):5–28. doi: 10.1002/btpr.2581. [DOI] [PubMed] [Google Scholar]
- Sharma S. Kanwar SS (2014) The Sci World J: Organic solvent tolerant lipases and applications; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirisha E, Rajasekar N, Narasu ML. Isolation and optimization of lipase producing bacteria from oil contaminated soils. Adv Biol Res. 2010;4(5):249–252. [Google Scholar]
- Soleymani S, Alizadeh H, Mohammadian H, Rabbani E, Moazen F, Sadeghi HM, Rabbani M. Efficient media for high lipase production: One variable at a time approach. Avicenna J Med Biotechnol. 2017;9(2):82. [PMC free article] [PubMed] [Google Scholar]
- Tembhurkar VR, Kulkarni MB, Peshwe SA (2012). Optimization of lipase production by Pseudomonas spp. in submerged batch process in shake flask culture. Sci Res Rep, 2(1), 46–50.
- Veerapagu M, Narayanan AS, Ponmurugan K, Jeya KR. Screening selection identification production and optimization of bacterial lipase from oil spilled soil. Asian J Pharm Clin Res. 2013;6(3):62–67. [Google Scholar]
- Winkler UK, Stuckmann MARTINA. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J Bacteriol. 1979;138(3):663–670. doi: 10.1128/JB.138.3.663-670.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C (2018). Growth characteristics and enzyme production optimization of lipase producing strain. IOP Publishing. In IOP Conference Series: Earth and Environmental Science 108(4), 042087.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lipase producing Acinetobacter sp. UBT1 showing the zone of clearance onTributyrin agar plate (DOCX 205 KB)