Summary
Transition metal catalyzed [3 + 2] annulation of imines with double bonds via directed C-H activation offers a direct access to amino cyclic motifs. However, owing to weak coordination and steric hindrance, trifluoromethylated ketimines have been an unaddressed challenge for TM-catalyzed annulations. Here, a rhenium-catalyzed [3 + 2] annulation of trifluoromethylated ketimines with isocyanates via C(sp2)-H activation has been disclosed. This approach provides an efficient platform for rapid access to a privileged library of CF3-containing iminoisoindolinones and polyamides by utilizing challenging CF3-ketimines as the annulation component. The capability of gram scale synthesis, the post-functionalization of the cyclization adduct, the derivation of complex natural molecules and the facile synthesis of polyamides highlight a diversity of synthetic potential of the current methodology.
Subject Areas: Chemistry, Molecular Inorganic Chemistry, Catalysis, Organic Synthesis
Graphical Abstract

Highlights
-
•
Challenging sp2 C-H activation
-
•
Valuable CF3-containing heterocycles
-
•
Variant synthetic applications
-
•
Interesting fluorinated polyamides
Chemistry; Molecular Inorganic Chemistry; Catalysis; Organic Synthesis
Introduction
Besides serving as versatile and important organic intermediates for the synthesis of molecules with amine functionality (Layer, 1963; Bloch, 1998; Kobayashi and Ishitani, 1999; Martin, 2009; Blicke, 2011), imines are also eminent for the directing role in transition metal-catalyzed C-H functionalizations (Zhang et al., 2014a, 2014b; Chen et al., 2015; Sambiagio et al., 2018; Li and Shi, 2012; Rouquet and Chatani, 2013; Jiao et al., 2016; Gensch et al., 2016; Leitch and Frost, 2017; He et al., 2017; Yang et al., 2017; Hummel et al., 2017; Park et al., 2017; Dong et al., 2017; Gandeepan, et al., 2019). In particular, the directed ortho C(sp2)-H bond transformation of imines with an unsaturated bonds (Zhang et al., 2014a, 2014b; Yang and Huang, 2015) and the following cyclization process, a formal [3 + 2] annulation, which was first reported by Kuninobu (Kuninobu et al., 2005, 2006), has become a powerful route for the construction of amino carbon (hetero) cycles (Scheme 1A) (Kuninobu et al., 2010; Tran and Cramer, 2010, 2011; Zhang et al., 2013; Liu et al., 2015; Liang et al., 2017). However, trifluoromethylated ketimines remain a major challenge and have not been engaged in such an appealing procedure, which would give rise to biologically interesting and highly valuable CF3-containing amino carbon or hetero cyclic motifs with a quaternary carbon center (Figure 1) (Sani et al., 2007; Petrov, 2009; Satoshi et al., 2008; Hurley et al., 2009). The probable challenges could be ascribed to two aspects: (1) the weakened coordination potential of nitrogen atom by the strong electron withdrawing effect of CF3 group; (2) the increased steric hindrance by CF3 group compared with a common alkyl group (such as Me and Et) (Meanwell, 2018). Considering the fact that the electron-withdrawing character of CF3 group might enhance the reactivity of ketimines (Wang et al., 2006), the nucleophilic cyclization step would be likely assisted by CF3 group. To this end, we envisaged that, if the insertion of unsaturated bond into the C-H bond of CF3-ketimines could be accomplished by certain transition metal catalysis, it would also allow for the subsequent nucleophilic cyclization (Scheme 1B: route a). The proposed challenging [3 + 2] strategy would be more straightforward compared with the post-functionalization of a cyclic imine precursor with CF3-nucleophiles to deliver CF3-containing amino cycles (Scheme 1B: route b), and the latter approach may suffer from tedious synthetic steps of cyclic imine precursor and site selectivity issue during nucleophilic trifluoromethylation if other unsaturated bond presented in the imine structure.
Scheme 1.
The Progresses of the [3 + 2] Annulation of Imines, the Strategies on the Synthesis of CF3-Containing Cycles from Imine and Current Work
Figure 1.
Selected Biologically Active CF3-Containing Amino (Hetero) Cycles
Since the pioneering works of several groups (Chen and Hartwig, 1999; Kuninobu et al., 2005, 2006), rhenium-catalyzed C-H functionalization has become a promising implement for the rapid construction of new C-C and C-X bonds in an atom economic and environmentally benign manner (Kuninobu et al., 2008; Fukumoto et al., 2012; Sueki et al., 2013; Wang et al., 2013; Tang et al., 2013; Jin et al., 2013). Among these reactions, rhenium often exhibited exclusive catalytic activities over other transition metals, owing to the special properties of rhenium (Kuninobu and Takai, 2011). However, merging rhenium-catalyzed C-H activation with the synthesis of fluorinated molecules has been relatively underexploited. Herein, we describe an effective rhenium-catalyzed insertion of isocyanate into the C-H bond of CF3-ketimine/nucleophilic cyclization sequence for the rapid access CF3-substituted iminoisoindolinones (Scheme 1C). In this reaction, the imino group has been preserved in the absence of leaving group.
Results and Discussion
Reaction Optimization
We commenced our studies by using 2,2,2-trifluoro-N,1-diphenylethan-1-imine (1a) as the mode substrate to react with p-tolyl isocyanate (2a). After systematic optimization of the various reaction parameters, the expected 3-amino-3-(trifluoromethyl)isoindolin-1-one was obtained in 82% yield (Table 1, entry 1). In a control reaction that Re2(CO)10 was omitted, no desired product was detected (entry 2). Re salts other than Re2(CO)10 also mediated the formation of the product albeit in slightly lower yields (entries 3–4). A range of other metal carbonyls showed no catalytic activities on this annulation (entries 5–11). In addition, the use of common Pd, Cu, or Rh catalysts, which were successfully implemented in C-H functionalization of imines, resulted in no conversion of the staring material (entries 12–15). The nature of solvent also plays a key role in reaction efficiency. After the replacement of o-xylene with PhCl or toluene, the comparable results were achieved (entries 16–17). The use of polar solvents gave low conversions (entries 18–20), whereas very polar solvents such as DMSO totally impeded the formation of the desired product (entry 21). At last, no reaction occurred at lower temperature (entry 22).
Table 1.
Influence of Reaction Parameters on [3 + 2] Annulation
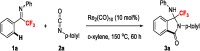 | ||
|---|---|---|
| Entry | Change from the Standard Conditions | Yield (%)a |
| 1 | None | 82 |
| 2 | In the absence of Re2(CO)10 | _ |
| 3 | ReBr(CO)5 instead of Re2(CO)10 | 47 |
| 4 | ReCl(CO)5 instead of Re2(CO)10 | 66 |
| 5 | Ru3(CO)10 instead of Re2(CO)10 | _ |
| 6 | Cr(CO)6 instead of Re2(CO)10 | _ |
| 7 | Fe2(CO)9 instead of Re2(CO)10 | _ |
| 8 | Co2(CO)8 instead of Re2(CO)10 | _ |
| 9 | Mn2(CO)10 instead of Re2(CO)10 | _ |
| 10 | W(CO)6 instead of Re2(CO)10 | _ |
| 11 | Mo(CO)6 instead of Re2(CO)10 | _ |
| 12 | Pd(OAc)2 instead of Re2(CO)10 | _ |
| 13 | Cu(OAc)2 instead of Re2(CO)10 | _ |
| 14 | [Rh(OAc)2]2 instead of Re2(CO)10 | _ |
| 15 | Rh(PPh3)3Cl instead of Re2(CO)10 | _ |
| 16 | PhCl instead of o-xylene | 75 |
| 17 | Toluene instead of o-xylene | 78 |
| 18 | THF instead of o-xylene | 18 |
| 19 | EtOAc instead of o-xylene | 20 |
| 20 | DCE instead of o-xylene | 16 |
| 21 | DMSO instead of o-xylene | _ |
| 22 | At 130°C | _ |
Reaction conditions: Ketimine 1a (0.3 mmol, 1 equiv.), isocyanate 2a (0.6 mmol, 2 equiv.), catalyst (0.03 mmol, 0.1 equiv.), in solvent (3 mL) under Ar at 150 °C for 60 h.
Isolated yield.
Substrate Scope regarding CF3-Ketimines
The Re-catalyzed insertion/cyclization process can be applied to a series of CF3-ketimines with different substitution patterns on amines or ketones (Scheme 2). For the amine part, the reactions of imines with either electron-deficient or electron-rich groups on para, meta, or ortho position on anilines were conducted to furnish 3 in decent to excellent yields. In general, more electron-deficient aniline derived substrates gave slightly lower yields, and the ortho-substituted ones were converted in low to moderate yields probably owing to the steric hindrance during the cyclization step. A series of functional groups such as F, Cl, Br, methyl ethers, dioxolyl, and CF3 on anilines were compatible under the optimal conditions. It is noticed that 2-naphthylamine and amylamine were well tolerated to give corresponding cyclization products in good yields (3s, 3t). In regard of substitutions on ketone part, there was no obvious electronic effect on reaction outcomes in which substrates with either electron-deficient or electron-rich substituents on aromatic ring all afforded desired products in moderate to good yields. In addition, owing to regioselectivity, a mixture of two regioisomers was obtained from the meta-substituted ketone (3z, 3ac, 3ad). Furthermore, the reaction of 2-thienyl ketone derived CF3-ketimine 1af proceeded to afford the expected product 3af in low yield, probably owing to the instability of the substrate under standard conditions. Diimine 1ag gave the mono-annulation adduct in 72% yield, while delivering the di-annulation product in trace amount. Gratefully, vinylic CF3-ketimine 1ah smoothly underwent the [3 + 2] sequence to give the desired cyclization product in moderate yield (3ah).
Scheme 2.
Scope of the Ketimines
Reaction conditions: Ketimine 1 (0.3 mmol, 1 equiv.), isocyanate 2 (0.6 mmol, 2 equiv.), catalyst (0.03 mmol, 0.1 equiv.), in o-xylene (3 mL), under Ar at 150 °C for 60 h, isolated yield. ain PhCl for 48 h. bat 160 °C. cat 140 °C. dat 130 °C. eat 110 °C. fon 0.15 mmol scale, at 160 °C for 72 h. gon 0.2 mmol scale.
Substrate Scope regarding Isocyanates
The reaction scope was then tested on the isocyanate side (Scheme 3). Aryl isocyanates with either an electron-donating or electron-withdrawing group smoothly underwent the cyclization reaction to give the desired product in moderate to excellent yield, implying no significant electronic effect on the aromatic ring of isocyanate. Interestingly, in the case of the ortho-CH3 substituted isocyanate, the reaction outcome was moderate (3aq). Functional groups such as F, Cl, Br, methyl ethers, phenyl ether, and CF3 were amenable to the optimal reaction conditions. In addition, naphthalenyl isocyanate was transformed to corresponding product in good yield (3ax). Furthermore, aliphatic isocyanates with linear or cyclic chains smoothly proceeded to generate the desired products in good to high yield (3ay-bb). The vinylic C-H bond of ketimine 1ah could be further functionalized with substituted isocyanate (2d), affording the desired annulation adduct in moderate yield. Upon the treatment of diimine 1ag with the highly electron-deficient isocyanate 2c, the di-annulation adduct was isolated as the main product.
Scheme 3.
Scope of the Isocyanates
Reaction conditions: Ketimine 1 (0.3 mmol, 1 equiv.), isocyanate 2 (0.6 mmol, 2 equiv.), catalyst (0.03 mmol, 0.1 equiv.), in o-xylene (3 mL), under Ar at 150 °C for 60 h, isolated yield. aIn PhCl for 48 h. bOn 0.2 mmol scale. cOn 0.15 mmol scale, at 160 °C for 72 h.
With aromatic sp2 and benzylic sp3 C-H bonds installed in the same molecule, the sp2 C-H bond activation was preferred over sp3 C-H bond when 1ai was subjected to standard reaction conditions, indicating the highly selective profile of the protocol (Figure 2).
Figure 2.
Intramolecular Competitive sp2 and sp3 C-H Bond Activation
Mechanistic Investigations
To gain some mechanistic information on this reaction, the deuterium-labeling experiments were conducted (Scheme 4). With the deuterium-labeled compound [D]5-1a, D-H exchange was observed with neither the substrate nor the product (Scheme 4, top). Based on the initial rates of parallel reactions, the intermolecular kinetic isotope effect (KIE) of the rhenium-catalyzed C-H functionalization was measured to be kH/kD = 0.8, demonstrating that the C–H bond cleavage of imine 1a might not be the kinetically relevant step (Scheme 4, bottom).
Scheme 4.
The D–H Exchange Experiment and the Kinetic Isotope Effect Experiments
Several control experiments were performed for further mechanistic studies (Scheme 5). Under O2, no desired annulations product was observed, suggesting low-valent Re catalyst is essential for the reaction since rhenium could be oxidized to a high oxidation state by O2 (Ivin and Mol, 1997). In the presence of radical scavenger TEMPO or under CO, the annulation was impeded, indicating CO dissociation and Re(CO)5 radicals formation likely involved in annulations process (Reynolds et al., 2001). In the presence of catalytic amount of base, the reaction efficiency varied. It was observed that Et3N gave slightly higher yield, whereas inorganic bases such as Na2CO3 and NaOAc exhibited reduced performance on reaction outcomes. Interestingly, after replacement of CF3 with CH3 on imine structure, the deaminative annulation product 3bf was generated in good yield (80%) under standard reaction conditions (Hou et al., 2013), indicating the role of CF3 group for stabilizing the resulting amino heterocycles.
Scheme 5.
Control Experiments
On the basis of these mechanistic investigations and previous reports (Chen and Hartwig, 1999; Kuninobu et al., 2005, 2006, 2008; Fukumoto et al., 2012; Sueki et al., 2013; Wang et al., 2013; Tang et al., 2013; Jin et al., 2013; Reynolds et al., 2001; Lapointe and Fagnou, 2010; Ackermann, 2011; Engle et al., 2012), we then presumed two catalytic pathways for the annulation of CF3-ketimines with isocyanates (Scheme 6). In pathway I, with the assistance of base, the initial C-H metalation of CF3-ketimine 1 formed rhenacycle A after the coordination of imine with rhenium catalyst, which was followed by the insertion of isocyanate to generate the aminated rhenium species B. The further intramolecular nucleophilic amination and proto-demetalation of the aminated rhenium species C furnished the desired cyclization product and regenerated the active rhenium catalyst. In pathway II, the homolytic Re-Re bond cleavage of Re2(CO)10 produced Re(CO)5 radicals, which reacted with CF3-ketimine 1 to form dinuclear Re complexes A′ through CO dissociation and C-H bond cleavage. The followed insertion of isocyanate generated the aminated dinuclear rhenium species B′, which further underwent intramolecular nucleophilic amination to give species C’. The reductive elimination of C′ furnished the desired cyclization product and regenerated the active rhenium catalyst.
Scheme 6.
Plausible Reaction Mechanism
Synthetic Applications
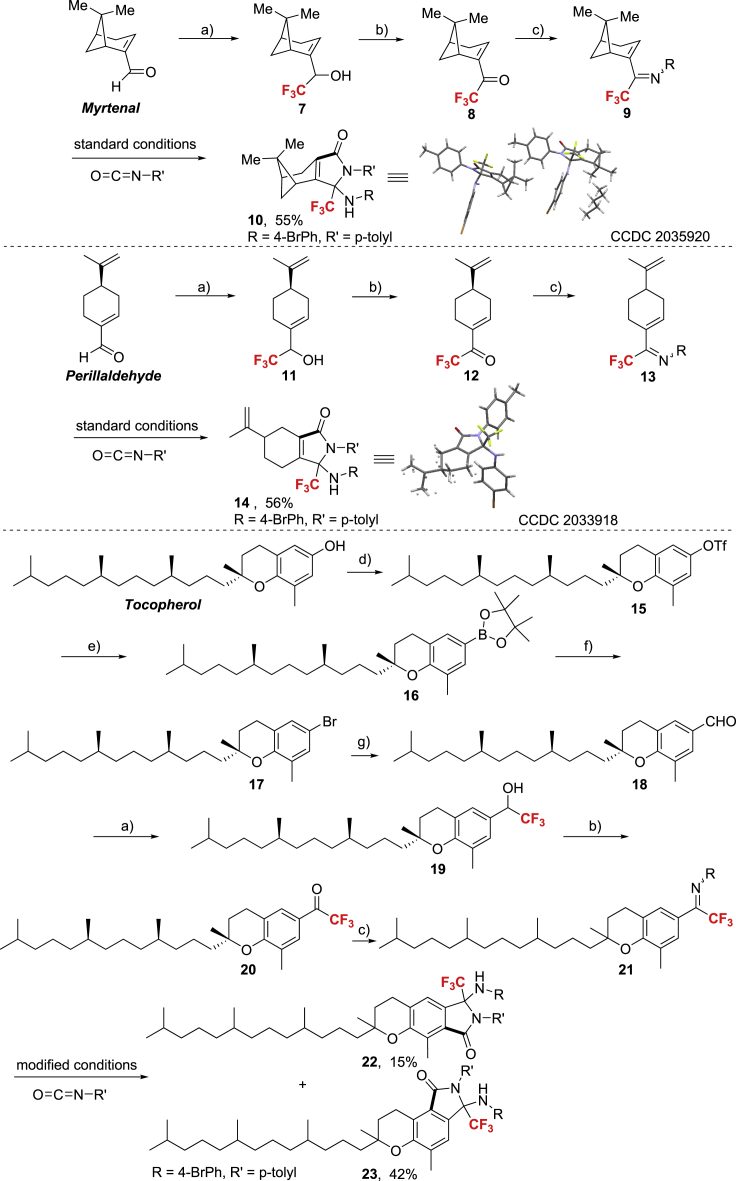
Next, the synthetic utility of this approach was explored (Scheme 7). The annulation of 1a and 2a was conducted on 4.5 mmol scale with reduced catalyst loading (8 mol%) and prolonged reaction time, furnishing 3a in 81% yield (1.39 g), which is comparable with the aforementioned result on small scale. The methylation of 3a with MeI as the alkylation reagent smoothly afforded 4 in 86% yield. Surprisingly, the treatment of 3a with PIDA as the oxidant in TFE delivered trifluoroethyl ketal moiety 5 in 59% yield, arising from the oxidation of the aniline part of 3a. Upon the treatment of 3a with stoichiometric amount of strong Lewis acid BF3·Et2O, the unexpected C-N bond cleavage was observed to give CF3-tertiary alcohol and the OH group likely came from trace water. Notably, the structures of 5 and 6 were further unambiguously confirmed by X-ray crystallography and the latter two transformations constitute novel discoveries that relevant processes have been scarcely reported in the literature.
Scheme 7.
Synthetic Applications
Reaction conditions: (a) LiHMDS (8.0 equiv.), MeI (8.0 equiv.), in THF under Ar, refluxing for 24 h; (b) PIDA (4.0 equiv.), Cs2CO3 (1.5 equiv.), in TFE, at 70 °C for 6 h; (c) BF3⋅Et2O (3.0 equiv.), in CH3CN, under Ar, at 80 °C for 24 h.
The applicability of this Re-catalyzed annulations was further examined by selected derivation of several natural products (Scheme 8). For example, by nucleophilic trifluoromethylation of Myrtenal or Perillaldehyde with TMSCF3/TBAF system, the subsequent oxidation with DMP, and the followed condensation with aniline, the desired Myrtenal or Perillaldehyde derived CF3-ketimine 9 or 13 was formed in decent yield, which was converted to the corresponding CF3-containing amino hetero cycles in good yield under standard conditions. It was established that the strained four-member ring in 9 was intact during the annulations process and the structures of 10 and 14 were further unambiguously confirmed by X-ray crystallography. Following the similar procedure after conversion of Tocopherol to its aldehyde moiety through a subsequent four-step procedure, the expected CF3-ketimine 21 was generated in decent yield, which was subjected to slightly modified annulations conditions to furnish two regioisomers 22 and 23 as the cyclization adduct.
Scheme 8.
Derivation of Complex Molecules
Reaction conditions: (a) TMSCF3 (2.2 equiv.), TBAF (0.5 equiv.), in THF under Ar, -40 °C to rt; (b) DMP (1.2 equiv.), in DCM, rt for 30 min; (c) 4-Br-aniline (2.0 equiv.), TsOH⋅H2O (0.2 equiv.), in toluene, at 140 °C for 48 h; (d) Tf2O (1.3 equiv.), Et3N (2.5 equiv.), in DCM, at 0 °C for 30 min; (e) B2pin2 (2.0 equiv.), PdCl2(dppf) (10 mol%), Et3N (3.0 equiv.), in dioxane, at 100 °C for 4 h; (f) CuBr2 (3.0 equiv.), in MeOH, at 90 °C for 72 h; (g) n-BuLi (2.0 equiv.), DMF (5.0 equiv.) in THF, at -78°C.
Inspired by the success of double annulation reactions of the CF3-diimine (1ag) and Kuninobu's leading work in the synthesis of polyimides (Sueki et al., 2013), we then attempted to explore the possibility for the synthesis of important trifluoromethylated polyamides bearing potential unique properties such as enhanced stability, solubility, and low surface energy (Wang, et al., 2004; Tsuchiya et al., 2006) through Re-catalyzed [3 + 2] annulations via C-H activation (Suraru et al., 2016; Yang et al., 2018; Blaskovits and Leclerc, 2019). Finally, trifluoromethylateddiimines with a diphenyl backbone were proved to be suitable annulation partners with phenyl diisocyanate, affording the trifluoromethylated polyamides in moderate yields with Mw/Mn from 1.6 to 1.8 and good solubility in organic solvents such as dichloromethane, chloroform, and THF (Figure 3, top). The preliminary study of optical properties of these polymers was performed. The UV spectra of 24a−d display maximum absorption bands at 255–265 nm in CH2Cl2, which is ascribed to the π−π∗ transition of arenes (see Supplementary Information). The 24a-d solutions show strong blue emissions with the emission peaks around 438 nm (Figure 3, bottom), which make them suitable as host materials for blue organic light-emitting devices.
Figure 3.
The Synthesis of CF3-Containing Polyamides and Optical Fluorescence Spectra of 24a-d in DCM Solution (1 mg/mL) (Insets Show the Respective Photographs under UV Illumination)
Conclusion
In conclusion, we have presented an unprecedented [3 + 2] annulation of CF3-ketimines with isocyanates via rhenium-catalyzed C-H activation. This approach demonstrated good functional group tolerance and broad substrate scope both on ketimines and isocyanates. A series of novel CF3-containing iminoisoindolinones were constructed in decent to excellent yield. This is the first example on functionalization of unactivated sp2 C-H bonds of CF3-ketimines, leading to the simultaneous formation of new C-C and C-N bonds by one simple operation. The imino group being intact during the annulations process in the absence of leaving group highlights the ability for trifluoromethylated amine synthesis of the catalytic protocol. The preliminary mechanistic studies indicated that Re(CO)5 radicals and dinuclear rhenium species were likely involved in the annulation process. Furthermore, the capability for gram scale synthesis, the diverse transformations of the annulation adduct, the derivation of the natural products and the ability for the construction of polyamides show the cases for synthetic applications of current strategy. Further employment of CF3-ketimines as the annulations partner with other unsaturated bonds and the systematic mechanistic study are ongoing in our laboratory.
Limitations of the Study
The catalyst loading was a little high compared with previously reported Re systems, and lower catalyst loading (<10 mol%) was detrimental for reaction efficiency.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Guangwu Zhang (gw.zhangchem@hotmail.com).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact without restriction.
Data and Code Availability
The structure of 3-((4,4-bis(2,2,2-trifluoroethoxy)cyclohexa-2,5-dien-1-ylidene)amino)-2-(p-tolyl)-3-(trifluoromethyl)isoindolin-1-one (5, CCDC, 2016466), 3-hydroxy-2-(p-tolyl)-3-(trifluoromethyl)isoindolin-1-one (6, CCDC, 2016473), 3-((4-bromophenyl)amino)-5,5-dimethyl-2-(p-tolyl)-3-(trifluoromethyl)-2,3,4,5,6,7-hexahydro-1H-4,6-methanoisoindol-1-one (10, CCDC, 2035920), 3-((4-bromophenyl)amino)-6-(prop-1-en-2-yl)-2-(p-tolyl)-3-(trifluoromethyl)-2,3,4,5,6,7-hexahydro-1H-isoindol-1-one (14, CCDC, 2033918) in this article have been deposited in the Cambridge Crystallographic Data Center.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This research was supported by Henan University, the National Natural Science Foundation of China (21801061), the Natural Science Foundation of Shandong Province (ZR2019MEM026, ZR2019BB026). Professor Pengtao Ma is thanked for the X-ray structure.
Author Contributions
G.Z. directed and coordinated the project. S.Z., X.-Y.L., Z.C., X.Q., and H.-Y.X. performed the experiments, analyzed the data, and prepared the Supplementary Information. G.Z., S.Z., X.-Y.L., Z.C., X.Q., and H.-Y.X. wrote the paper.
Declaration of Interests
The authors declare no competing financial interests.
Published: November 20, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101705.
Contributor Information
Heng-Ying Xiong, Email: xionghengying@vip.henu.edu.cn.
Guangwu Zhang, Email: gw.zhangchem@hotmail.com.
Supplemental Information
References
- Ackermann L. Carboxylate-assisted transition-metal-catalyzed C−H bond functionalizations: mechanism and scope. Chem. Rev. 2011;111:1315–1345. doi: 10.1021/cr100412j. [DOI] [PubMed] [Google Scholar]
- Blaskovits J.T., Leclerc M. C−H activation as a shortcut to conjugated polymer synthesis. Macromol. Rapid Commun. 2019;40:1800512. doi: 10.1002/marc.201800512. [DOI] [PubMed] [Google Scholar]
- Blicke F.F. John Wiley and Sons, Inc.; 2011. The Mannich Reaction. Organic Reactions; p. 303. [Google Scholar]
- Bloch R. Additions of organometallic reagents to C=N Bonds: reactivity and selectivity. Chem. Rev. 1998;98:1407–1438. doi: 10.1021/cr940474e. [DOI] [PubMed] [Google Scholar]
- Chen H., Hartwig J.F. Catalytic, regiospecific end-functionalization of alkanes: rhenium-catalyzed borylation under photochemical conditions. Angew. Chem. Int. Ed. 1999;38:3391–3393. doi: 10.1002/(sici)1521-3773(19991115)38:22<3391::aid-anie3391>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Chen Z., Wang B., Zhang J., Yu W., Liu Z., Zhang Y. Transition metal-catalyzed C–H bond functionalizations by the use of diverse directing groups. Org. Chem. Front. 2015;2:1107–1295. [Google Scholar]
- Dong Z., Ren Z., Thompson S.J., Xu Y., Dong G. Transition-metal-catalyzed C–H alkylation using alkenes. Chem. Rev. 2017;117:9333–9403. doi: 10.1021/acs.chemrev.6b00574. [DOI] [PubMed] [Google Scholar]
- Engle K.M., Mei T.-S., Wasa M., Yu J.-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 2012;45:788–802. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto Y., Daijo M., Chatani N. Rhenium-catalyzed regio- and stereoselective addition of imines to terminal alkynes leading to N-alkylideneallylamines. J. Am. Chem. Soc. 2012;134:8762–8765. doi: 10.1021/ja3022818. [DOI] [PubMed] [Google Scholar]
- Gandeepan P., Muller T., Zell D., Cera G., Warratz S., Ackermann L. 3d transition metals for C–H activation. Chem. Rev. 2019;119:2192–2452. doi: 10.1021/acs.chemrev.8b00507. [DOI] [PubMed] [Google Scholar]
- Gensch T., Hopkinson M.N., Glorius F., Wencel-Delord J. Mild metal-catalyzed C–H activation: examples and concepts. Chem. Soc. Rev. 2016;45:2900–2936. doi: 10.1039/c6cs00075d. [DOI] [PubMed] [Google Scholar]
- He J., Wasa M., Chan K.S.L., Shao Q., Yu J.-Q. Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev. 2017;117:8754–8786. doi: 10.1021/acs.chemrev.6b00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W., Zhou B., Yang Y., Feng H., Li Y. Rh(III)-Catalyzed addition of alkenyl C-H bond to isocyanates and intramolecular cyclization: direct synthesis 5-ylidenepyrrol-2(5H)-ones. Org. Lett. 2013;15:1814–1817. doi: 10.1021/ol4003674. [DOI] [PubMed] [Google Scholar]
- Hummel J.R., Boerth J.A., Ellman J.A. Transition-metal-catalyzed C–H bond addition to carbonyls, imines, and related polarized π bonds. Chem. Rev. 2017;117:9163–9227. doi: 10.1021/acs.chemrev.6b00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley T.B., Peukert S., Wattanasin S. Novartis Institutes for Biomedical Research, Inc.; 2009. Inhibitors of Undecaprenyl Pyrophosphate Synthase. [Google Scholar]
- Ivin K.J., Mol J.C. Academic Press; 1997. Olefin Metathesis and Metathesis Polymerisation. [Google Scholar]
- Jiao J., Murakami K., Itami K. Catalytic methods for aromatic C–H amination: an ideal strategy for nitrogen-based functional molecules. ACS Catal. 2016;6:610–633. [Google Scholar]
- Jin H., Xie J., Pan C., Zhu Z., Cheng Y., Zhu C. Rhenium-catalyzed acceptorless dehydrogenative coupling via dual activation of alcohols and carbonyl compounds. ACS Catal. 2013;3:2195–2198. [Google Scholar]
- Kobayashi S., Ishitani H. Catalytic enantioselective addition to imines. Chem. Rev. 1999;99:1069–1094. doi: 10.1021/cr980414z. [DOI] [PubMed] [Google Scholar]
- Kuninobu Y., Takai K. Organic reactions catalyzed by rhenium carbonyl complexes. Chem. Rev. 2011;111:1938–1953. doi: 10.1021/cr100241u. [DOI] [PubMed] [Google Scholar]
- Kuninobu Y., Kawata A., Takai K. Rhenium-catalyzed formation of indene frameworks via C−H bond Activation: [3+2] annulation of aromatic aldimines and acetylenes. J. Am. Chem. Soc. 2005;127:13498–13499. doi: 10.1021/ja0528174. [DOI] [PubMed] [Google Scholar]
- Kuninobu Y., Tokunaga Y., Kawata A., Takai K. Insertion of polar and nonpolar unsaturated molecules into carbon-rhenium bonds generated by C-H bond activation: synthesis of phthalimidine and indene derivatives. J. Am. Chem. Soc. 2006;128:202–209. doi: 10.1021/ja054216i. [DOI] [PubMed] [Google Scholar]
- Kuninobu Y., Nishina Y., Matsuki T., Takai K. Synthesis of Cp−Re complexes via olefinic C−H activation and successive formation of cyclopentadienes. J. Am. Chem. Soc. 2008;130:14062–14063. doi: 10.1021/ja805921f. [DOI] [PubMed] [Google Scholar]
- Kuninobu Y., Yu P., Takai K. Rhenium-catalyzed diastereoselective synthesis of aminoindanes via the insertion of allenes into a C−H bond. Org. Lett. 2010;12:4274–4276. doi: 10.1021/ol101627x. [DOI] [PubMed] [Google Scholar]
- Lapointe D., Fagnou K. Overview of the mechanistic work on the concerted metallation–deprotonation pathway. Chem. Lett. 2010;39:1118–1126. [Google Scholar]
- Layer R.W. The chemistry of imines. Chem. Rev. 1963;63:489–510. [Google Scholar]
- Leitch J.A., Frost C.G. Ruthenium-catalysed σ-activation for remote meta-selective C–H functionalization. Chem. Soc. Rev. 2017;46:7145–7153. doi: 10.1039/c7cs00496f. [DOI] [PubMed] [Google Scholar]
- Li B., Shi Z. From C(sp2)–H to C(sp3)–H: systematic studies on transition metal-catalyzed oxidative C–C formation. Chem. Soc. Rev. 2012;41:5588–5598. doi: 10.1039/c2cs35096c. [DOI] [PubMed] [Google Scholar]
- Liang Y.-F., Mgller V., Liu W., Mgnch A., Stalke D., Ackermann L. Methylenecyclopropane annulation by manganese(I)-Catalyzed stereoselective C−H/C−C activation. Angew. Chem. Int. Ed. 2017;56:9415–9419. doi: 10.1002/anie.201704767. [DOI] [PubMed] [Google Scholar]
- Liu W., Zell D., John M., Ackermann L. Manganese-catalyzed synthesis of cis-β-Amino acid esters through organometallic C-H activation of ketimines. Angew. Chem. Int. Ed. 2015;54:4092–4096. doi: 10.1002/anie.201411808. [DOI] [PubMed] [Google Scholar]
- Martin S.F. Recent applications of imines as key intermediates in the synthesis of alkaloids and novel nitrogen heterocycles. Pure Appl. Chem. 2009;81:195–204. doi: 10.1351/PAC-CON-08-07-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meanwell N.A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design, of bioisosteres for drug design. J. Med. Chem. 2018;61:5822–5880. doi: 10.1021/acs.jmedchem.7b01788. [DOI] [PubMed] [Google Scholar]
- Park Y., Kim Y., Chang S. Transition metal-catalyzed C–H amination: scope, mechanism, and applications. Chem. Rev. 2017;117:9247–9301. doi: 10.1021/acs.chemrev.6b00644. [DOI] [PubMed] [Google Scholar]
- Petrov V.A. DuPont Central Research and Development; 2009. Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications. [Google Scholar]
- Reynolds M.A., Guzei I.A., Angelici R.J. Re2(CO)10-Mediated carbon-hydrogen and carbon-sulfur bond cleavage of dibenzothiophene and 2,5-dimethylthiophene. Organometallics. 2001;20:1071–1078. [Google Scholar]
- Rouquet G., Chatani N. Catalytic functionalization of C(sp2)-H and C(sp3)-H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 2013;52:11726–11743. doi: 10.1002/anie.201301451. [DOI] [PubMed] [Google Scholar]
- Sambiagio C., Schonbauer D., Blieck R., DaoHuy T., Pototschnig G., Schaaf P., Wiesinger T., Zia M.F., WencelDelord J., Besset T. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 2018;47:6603–6743. doi: 10.1039/c8cs00201k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sani M., Volonterio A., Zanda M. The trifluoroethylamine function as peptide bond replacement. ChemMedChem. 2007;2:1693–1700. doi: 10.1002/cmdc.200700156. [DOI] [PubMed] [Google Scholar]
- Satoshi H., Shingo Y., Kazushi W., Nobuyuki S., Daisuke S., Hiroaki H., Junya O., Takatoshi K. Astellas Pharma Inc.; 2008. AminoindanDerivative or Salt Thereof. [Google Scholar]
- Sueki S., Guo Y., Kanai M., Kuninobu Y. Rhenium-catalyzed synthesis of 3-imino-1-isoindolinones by C-H bond activation: application to the synthesis of polyimide derivatives. Angew. Chem. Int. Ed. 2013;52:11879–11883. doi: 10.1002/anie.201306360. [DOI] [PubMed] [Google Scholar]
- Suraru S.-L., Lee J.A., Luscombe C.K. C–H arylation in the synthesis of π-conjugated polymers. ACS Macro Lett. 2016;5:724–729. doi: 10.1021/acsmacrolett.6b00279. [DOI] [PubMed] [Google Scholar]
- Tang Q., Xia D., Jin X., Zhang Q., Sun X.-Q., Wang C. Re/Mg bimetallic tandem catalysis for [4+2] annulation of benzamides and alkynes via C-H/N-H functionalization. J. Am. Chem. Soc. 2013;135:4628–4631. doi: 10.1021/ja400020e. [DOI] [PubMed] [Google Scholar]
- Tran D.N., Cramer N. syn-Selective rhodium(I)-Catalyzed allylations of ketimines proceeding through a directed C-H activation/allene addition sequence. Angew. Chem. Int. Ed. 2010;49:8181–8184. doi: 10.1002/anie.201004179. [DOI] [PubMed] [Google Scholar]
- Tran D.N., Cramer N. Enantioselective rhodium(I)-Catalyzed [3+2] annulations of aromatic ketimines induced by directed C-H activations. Angew. Chem. Int. Ed. 2011;50:11098–11102. doi: 10.1002/anie.201105766. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K., Shibasaki Y., Aoyagi M., Ueda M. Synthesis of a novel poly(binaphthylene ether) containing trifluoromethyl groups with a low dielectric constant. Macromolecules. 2006;39:3964–3966. [Google Scholar]
- Wang W.-C., Vora R.H., Kang E.-T., Neoh K.-G., Ong C.-K., Chen L.-F. Nanoporous ultra-low-κ films prepared from fluorinated polyimide with grafted poly(acrylic acid) side chains. Adv. Mater. 2004;16:54–57. [Google Scholar]
- Wang H., Zhao X., Li Y., Lu L. Solvent-controlled asymmetric strecker Reaction: stereoselective synthesis of α-trifluoromethylatedα-amino acids. Org. Lett. 2006;8:1379–1381. doi: 10.1021/ol0601186. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang L., Yang Y., Zhang P., Du Z., Wang C. Alkene oxyalkylation enabled by merging rhenium catalysis with hypervalent iodine(III) reagents via decarboxylation. J. Am. Chem. Soc. 2013;135:18048–18051. doi: 10.1021/ja410195j. [DOI] [PubMed] [Google Scholar]
- Yang L., Huang H. Transition-metal-catalyzed direct addition of unactivated C–H bonds to polar unsaturated bonds. Chem. Rev. 2015;115:3468–3517. doi: 10.1021/cr500610p. [DOI] [PubMed] [Google Scholar]
- Yang Y., Lan J., You J. Oxidative C–H/C–H coupling reactions between two (Hetero)arenes. Chem. Rev. 2017;117:8787–8863. doi: 10.1021/acs.chemrev.6b00567. [DOI] [PubMed] [Google Scholar]
- Yang Y., Nishiura M., Wang H., Hou Z. Metal-catalyzed C−H activation for polymer synthesis and functionalization. Coord. Chem. Rev. 2018;376:506–532. [Google Scholar]
- Zhang J., Ugrinov A., Zhao P. Ruthenium(II)/N-Heterocyclic carbene catalyzed [3+2]Carbocyclization with aromatic N-H ketimines and internal alkynes. Angew. Chem. Int. Ed. 2013;52:6681–6684. doi: 10.1002/anie.201209031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Zhang Y., Jie X., Zhao H., Li G., Su W. Recent advances in directed C–H functionalizations using monodentate nitrogen-based directing groups. Org. Chem. Front. 2014;1:843–895. [Google Scholar]
- Zhang X.-S., Chen K., Shi Z.-J. Transition metal-catalyzed direct nucleophilic addition of C–H bonds to carbon–heteroatom double bonds. Chem. Sci. 2014;5:2146–2159. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The structure of 3-((4,4-bis(2,2,2-trifluoroethoxy)cyclohexa-2,5-dien-1-ylidene)amino)-2-(p-tolyl)-3-(trifluoromethyl)isoindolin-1-one (5, CCDC, 2016466), 3-hydroxy-2-(p-tolyl)-3-(trifluoromethyl)isoindolin-1-one (6, CCDC, 2016473), 3-((4-bromophenyl)amino)-5,5-dimethyl-2-(p-tolyl)-3-(trifluoromethyl)-2,3,4,5,6,7-hexahydro-1H-4,6-methanoisoindol-1-one (10, CCDC, 2035920), 3-((4-bromophenyl)amino)-6-(prop-1-en-2-yl)-2-(p-tolyl)-3-(trifluoromethyl)-2,3,4,5,6,7-hexahydro-1H-isoindol-1-one (14, CCDC, 2033918) in this article have been deposited in the Cambridge Crystallographic Data Center.