Abstract
Diabetic kidney disease (DKD) occurs in approximately 20–40% of patients with type 2 diabetes mellitus. Patients with DKD have a higher risk of cardiovascular and all-cause mortality. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and antihyperglycemic drugs form the mainstay of DKD management and aim to restrict progression to more severe stages of DKD. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) control hyperglycemia by blocking renal glucose reabsorption in addition to preventing inflammation, thereby improving endothelial function and reducing oxidative stress; consequently, this class of prescription medicines is emerging as an important addition to the therapeutic armamentarium. The EMPA-REG OUTCOME, DECLARE TIMI 58, and CANVAS trials demonstrated the renoprotective effects of SGLT2i, such as restricting decline in glomerular filtration rate, in the progression of albuminuria, and in death due to renal causes. The renoprotection provided by SGLT2i was further confirmed in the CREDENCE study, which showed a 30% reduction in progression of chronic kidney disease, and in the DELIGHT study, which demonstrated a reduction in albuminuria with dapagliflozin compared with placebo (− 21.0%, confidence interval [CI] − 34.1 to − 5.2, p = 0.011). Furthermore, a meta-analysis demonstrated a reduced risk of dialysis, transplantation, or death due to kidney disease (relative risk 0.67; 95% CI 0.52–0.86; p = 0.0019) and a 45% risk reduction in worsening of renal function, end-stage renal disease, or renal death (hazard ratio 0.55, CI 0.48–0.64, p < 0.0001) with SGLT2i, irrespective of baseline estimated glomerular filtration rate. Thus, there is emerging evidence that SGLT2i may be used to curb the mortality and improve the quality of life in patients with DKD. However, clinicians need to effectively select candidates for SGLT2i therapy. In this consensus statement, we have qualitatively synthesized evidence demonstrating the renal effects of SGLT2i and proposed recommendations for optimal use of SGLT2i to effectively manage and delay progression of DKD.
Keywords: Delay, Diabetic kidney disease, Progression, Renoprotection, SGLT2 inhibitors
Key Summary Points
| Diabetic kidney disease (DKD) occurs in approximately 20–40% of patients with type 2 diabetes mellitus. |
| Patients with DKD have a higher risk of cardiovascular and all-cause mortality. |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blocker and antihyperglycemic drugs form the mainstay of DKD management and aim to restrict progression to more severe stages of DKD. |
| The sodium-glucose cotransporter 2 inhibitors (SGLT2i) control hyperglycemia by blocking renal glucose reabsorption in addition to preventing inflammation, thereby improving endothelial function and reducing oxidative stress, and hence are emerging as an important addition to the therapeutic armamentarium. |
| The EMPA-REG OUTCOME, DECLARE TIMI 58, and CANVAS trials demonstrated the renoprotective effects of SGLT2i, which were further confirmed by the CREDENCE study and DELIGHT study. In addition, a meta-analysis demonstrated a reduced risk of dialysis, transplantation, or death due to kidney disease and 45% risk reduction in worsening of renal function, end-stage renal disease, or renal death with SGLT2i, irrespective of baseline estimated glomerular filtration rate level. Thus, there is emerging evidence that SGLT2i may be used to curb mortality and improve quality of life in patients with DKD, although clinicians need to effectively select candidates for SGLT2i therapy. |
| In this consensus statement, we have qualitatively synthesized evidence demonstrating the renal effects of SGLT2i and have proposed recommendations for optimal use of SGLT2i to effectively manage and delay progression of DKD. |
Summary of Recommendations
| SGLT2i can be used as add-on second-line therapy in patients with T2DM having mild-to-moderate DKD with the aim to delay the progression of DKD1 |
| Initial reduction in estimated glomerular filtration rate (eGFR) is expected after initiation of SGLT2i, hence close monitoring of patients is recommended. |
| Patients with eGFR < 60 mL/min/1.73 m2 should be closely monitored as per local practice for renal function parameters. |
| SGLT2i can be used if eGFR is > 45 mL/min/1.73 m2. |
| SGLT2i can be continued if eGFR is between 30 and 45 mL/min/1.73 m2. |
| SGLT2i should be discontinued if eGFR falls below 30 mL/min/1.73 m2. |
| SGLT2i should not be used in patients with end-stage renal disease or on dialysis. |
| Patient selection and education should be an integral part of management when initiating SGLT2i. |
| Caution should be taken when prescribing SGLT2i to patients with a history of prior amputation, severe peripheral vascular disease, neuropathy, foot ulcers, hypersensitivity reactions, euglycemic DKA, and those on diuretic therapy. |
| Patients should be educated on perineal and genital hygiene and signs and symptoms of mycotic infections; early diagnosis and treatment should be encouraged. |
Digital Features
This article is published with digital features to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12871889
Introduction
Diabetes mellitus is a global pandemic affecting approximately 463 million adults (20–79 years); in 2019 it accounted for a health expenditure of approximately USD 760 billion, 10% of the total spending on adult healthcare [1, 2]. Sustained hyperglycemia resulting from type 2 diabetes mellitus (T2DM) causes microvascular injury and long-term diabetic complications such as nephropathy, now known as diabetic kidney disease (DKD) [3, 4]. In the third National Health and Nutrition Examination Survey conducted in the USA, 42.3% of the individuals with diabetes had chronic kidney disease (CKD) (N = 15,046), and the 10-year cumulative mortality, standardized to population age, sex, and race, in this patient population was 31.1% (95% confidence interval [CI] 24.7–37.5%) [5]. Of 5097 patients with T2DM in the UK Prospective Diabetes Study (UKPDS), 24.9% developed microalbuminuria and 0.8% required renal replacement therapy (RRT) within 10 years following the diagnosis [6].
India ranks highest in the prevalence of CKD (39.8%, 95% CI 38.3–41.4%) in the Joint Asia Diabetes Evaluation Registry [7]. An observational analysis from START-India study in 1500 Indian patients with T2DM reported the prevalence of CKD to be 46.5% [8]. Based on data from the Screening and Early Evaluation of Kidney Disease (SEEK)-India community-based cohort, hypertension and diabetes are the most common risk factors for CKD. The prevalence of diabetes among subjects with CKD in this study was approximately double (31.6%) that of those not having CKD (16.1%) [9]. A study of US electronic medical records demonstrated that CKD was associated with the highest incremental risk of all-cause mortality (ACM) in people newly diagnosed with T2DM [10]. Evaluation of the Singapore National Healthcare Group CKD Registry revealed a high annual mortality rate of 64.1 per 1000 patients with DKD (95% CI 60.2–68.3), with the mortality rate increasing with increasing severity of CKD (37, 57.5, 98.3, and 198.5% in patients with CKD stages 3A, 3B, 4, and 5, respectively) [11]. Diabetes is the leading cause of end-stage renal disease (ESRD), and individuals with diabetes are tenfold more likely to progress to ESRD than those without diabetes [12], as evidence from the rising prevalence of diabetes among deaths from renal failure in India: from 26% (257/1151) in 2001–2003 to 34% (937/2943) in 2010 [13].
Moreover, CKD contributes to a 1.4- to 2.9-fold increase in cardiovascular (CV) mortality in individuals with diabetes. Approximately 44% of all ESRD cases are due to diabetes, with this patient group reported to have up to an 18-fold increase in mortality [11, 12, 14]. A subnormal estimated glomerular filtration rate (eGFR) of between 61 and 90 mL/min/1.73 m2 has been reported to be a strong predictor of major CV events in diabetic patients even with normoalbuminuria [15]. In contrast, the risk for ACM, CV mortality, coronary heart disease, and stroke in this population was found to be reduced after controlling multiple risk factors, such as glycated hemoglobin (HbA1c), blood pressure, cholesterol, and smoking [14]. Thus, control of all associated risk factors is important to curb the risk of mortality in people with T2DM having CKD.
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are glucose-lowering drugs that have beneficial effects on blood pressure and bodyweight, indirectly exerting renoprotection. They also reduce albuminuria and are postulated to have direct hemodynamic effects on the kidney. Large-scale CV outcome trials (CVOTs) of SGLT2i, conducted to meet regulatory requirements and ensure CV safety, have shown promising effects on a range of albuminuria and kidney outcomes in the populations studied [90].
With this background, in this consensus statement, we discuss the treatment approaches and the role of SGLT2i in managing as well as delaying the progression of DKD in people with T2DM.
Methodology
The therapeutic strategies for managing CKD in people with T2DM using SGLT2i are presented in this consensus statement in the form of recommendations.
In order to generate these recommendations, we performed a literature search of the MEDLINE and EMBASE databases using combinations of the following key terms: “SGLT2i,” “composite renal outcomes,” “T2DM,” “chronic kidney disease,” “CKD,” “Diabetes,” “renoprotection,” “eGFR,” “ACR,” “creatinine,” “death,” “mortality,” and “nephropathy”. Key experts in the field of endocrinology and nephrology critically reviewed the results presented in the literature, and the recommendations were developed. The type of source evidence, such as randomized controlled trials (RCTs), meta-analysis of RCTs, and nonrandomized controlled trials, were mapped for each of the recommendations. The American Association of Clinical Endocrinologists and American College of Endocrinology Protocol guidelines were used to assess the quality of evidence [16]. The recommendations were reviewed once again and modified to incorporate the clinical practice experience to generate the consensus recommendations presented here.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Diagnosis and Prognosis of Chronic Kidney Disease
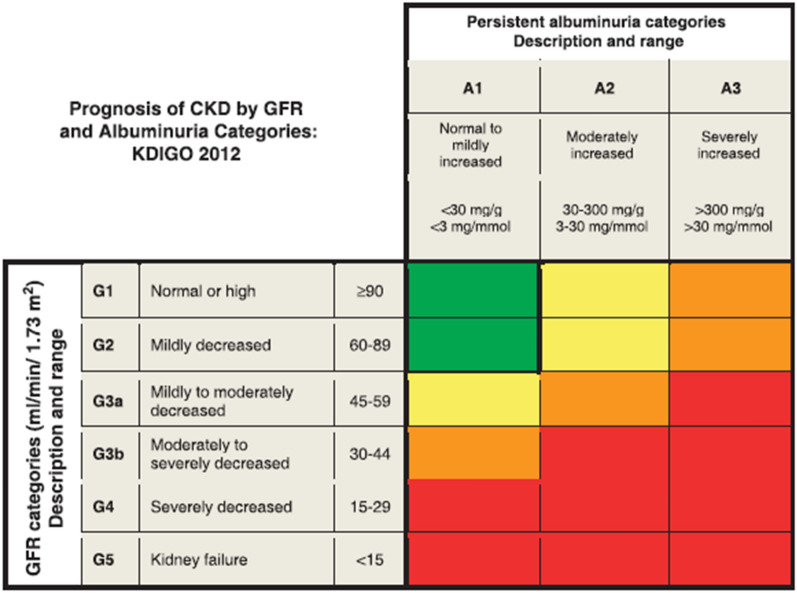
The National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guideline defines CKD as the presence of either one or both of the following two criteria for at least 3 months: (1) GFR < 60 mL/min/1.73 m2; (2) markers of kidney damage (≥ 1), such as albuminuria (albumin: creatinine ratio [ACR] ≥ 30 mg/g), urinary sediment abnormality, electrolyte, or other abnormality due to tubular disorder, abnormalities on histology, structural abnormalities detected by imaging, or history of kidney transplantation [17–19]. The prognosis of CKD is determined by assessing the GFR category together with the albuminuria category. The color-coding in Fig. 1 explains the level of risk associated with the GFR and albuminuria categories [20].
Fig. 1.
Diagnostic and prognostic criteria for chronic kidney disease. The GFR and albuminuria grid depicts the risk of progression, morbidity, and mortality by color, from best (low risk; green) to worst (red; very high risk) (green → yellow [moderately increased risk] → orange [high risk] → red). CKD Chronic kidney disease, GFR glomerular filtration rate, KDIGO Kidney Disease: Improving Global Outcomes. (Reproduced with permission from KDIGO 2012 [19])
Pathophysiology and Therapeutic Targets of DKD
Pathophysiology of DKD
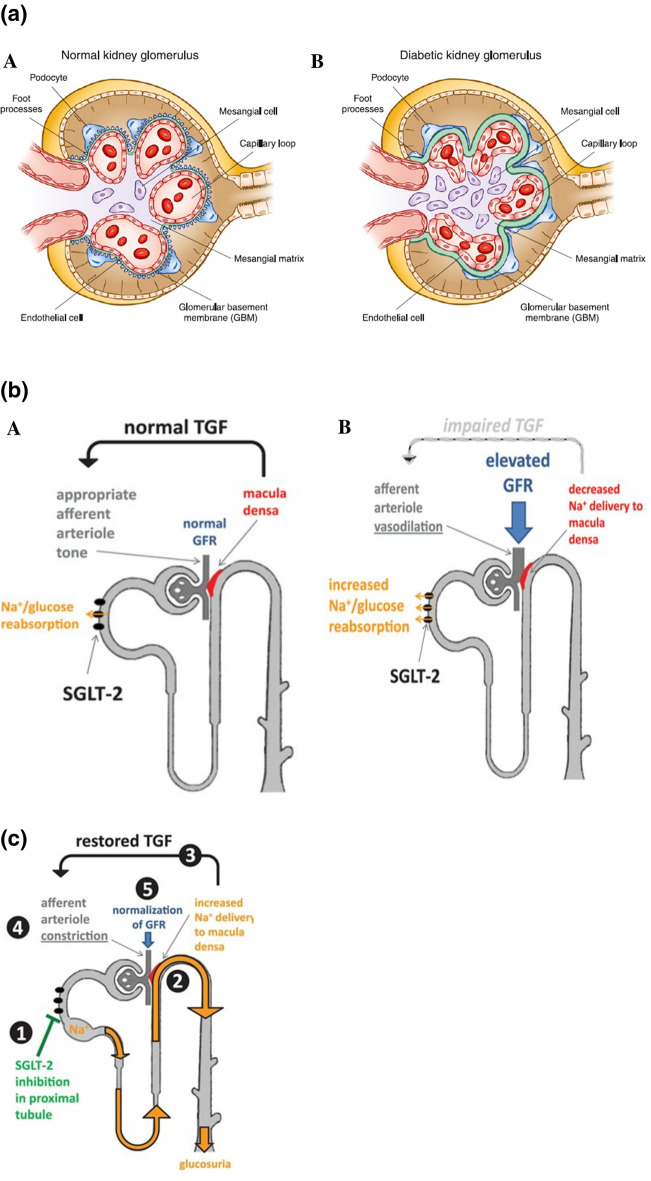
In persons with normoglycemia and normal GFR, kidneys filter up to approximately 180 g of glucose per day, which equals approximately 30% of the daily energy expenditure. Glucose is reabsorbed in the proximal convoluted tubule (PCT) through the expression of SGLT2 and SGLT1, which substantially prevents glucose loss into the urine and contributes to intrarenal and systemic metabolic control. Chronic hyperglycemia in T2DM leads to metabolic dysregulation due to increased glycolysis, which then upregulates multiple distinct pathways, such as the polyol, hexosamine, advanced glycation end products, and protein kinase C pathways, leading to glomerular hyperfiltration and proteinuria. Additionally, hyperglycemia causes renal hemodynamic changes, oxidative stress, inflammation, hypoxia, and an overactive renin–angiotensin–aldosterone system (RAAS), which mediates adverse changes in the vasculature [21–24]. These changes include thickening of the glomerular basement membrane, loss of endothelial fenestrations, mesangial matrix expansion, and loss of podocytes with effacement of foot processes (Fig. 2a) [25].
Fig. 2.
a Structural changes in kidney morphology with diabetic kidney disease. (Reproduced with permission from Alicicet al. [25]). b Hemodynamic changes in diabetic kidney disease. Na+ Sodium, SGLT2 sodium-glucose co-transporter 2, TGF tubuloglomerular feedback. (Reproduced with permission from Cherney et al. [30]). c Restoration of tubuloglomerular feedback by SGLT2 inhibitors. (Reproduced with permission from Cherney et al. [30])
Chronic hyperglycemia causes an increase in proximal tubular glucose delivery and leads to excessive glucose reabsorption by the SGLT2 receptors. As a cascading effect, there is reduced fluid delivery to the distal tubule, lower tubular back pressure in the Bowman space, and higher effective glomerular filtration pressure accounting for up to 50% of the hyperfiltration seen in DKD [26]. Hyperfiltration increases tubular transport load since all of the salt and fluid that is filtered by the kidneys is reabsorbed by the tubular system. The increased tubular transport load enhances oxygen consumption, increases release of inflammatory cytokines, and decreases erythropoietin production. The resultant hypoxia stimulates renal interstitial fibrosis. This entire cascading sequence is considered to be a major pathway in the progression of DKD (Fig. 2b) [27–30].
Therapeutic Targets and Management of DKD
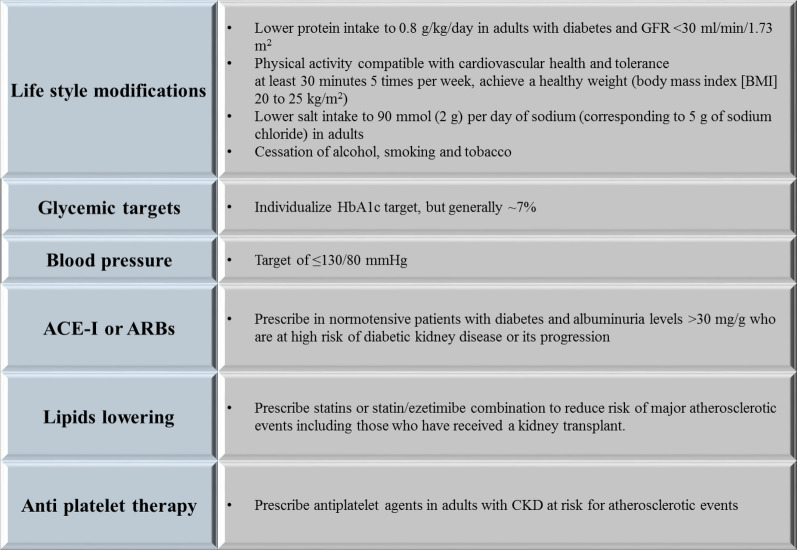
The American Diabetes Association (ADA) 2020 guideline recommends a patient-centered approach to guide the choice of pharmacologic agents. DKD is an important comorbidity to be considered in addition to factors such as cost, patient preferences, and possible side effects (e.g., hypoglycemia and impact on weight) [31]. Various guidelines emphasize a multipronged approach to manage DKD in T2DM [23, 31–33]. Figure 3 shows the components of a multipronged approach to the management of DKD in T2DM.
Fig. 3.
Multipronged approach to manage diabetic kidney disease in type 2 diabetes mellitus. ACE-I Angiotensin-converting enzyme inhibitor, ARBs angiotensin II receptor blockers, BMI body mass index, DKD diabetic kidney disease, HbA1c glycated hemoglobin
The Diabetes Control and Complications Trial was the first to demonstrate the renoprotective effects of intensive glycemic control through a reduction in the incident micro- and macro-albuminuria in persons with insulin-dependent diabetes mellitus by 39 and 54%, respectively.[34]. Similar results with significant reductions in the risk of ESRD by 65%, microalbuminuria by 9%, and macroalbuminuria by 30% were obtained with intensive glycemic control in the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial with gliclazide [35]. These were the earliest two landmark trials in type 1 DM and T2DM, respectively, that demonstrated the renoprotective effects of good glycemic control. Renoprotective effects of angiotensin II receptor blockers (ARBs) were proven by a delay in the onset of microalbuminuria and doubling of serum creatinine in the Randomized Olmesartan And Diabetes Microalbuminuria Prevention (ROADMAP) [36], Irbesartan Diabetic Nephropathy Trial (IDNT) [37], and Reduction of Endpoints in T2DM with the Angiotensin II Antagonist Losartan (RENAAL) trials [38–40]. On the other hand, a combination of ARBs and angiotensin-converting enzyme inhibitors (ACE-Is) was associated with an increased risk of dialysis, doubling of serum creatinine and death (hazard ratio [HR] 1.09, 95% confidence interval [CI] 1.01–1.18, p ≤ 0.037) [41]. Little data exist on comparative effectiveness of ARBs versus ACE-Is in terms of reducing the risk of CV outcomes. According to the general consensus, they can be administered interchangeably [40]. The Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints (ALTITUDE), which studied the addition of aliskiren (direct renin inhibitor) to RAAS blockade in people with T2DM with high CV and renal risk, was prematurely halted because of an increase in adverse events (AEs), with no reduction in CV or renal outcomes [42]. Novel therapies like sulodexide (SUN-MACRO trial) [43] and bardoxolone (Bardoxolone Methyl Evaluation in Patients with CKD and Type 2 Diabetes [BEACON] trial) have not shown renoprotective benefits or a reduction in risk of ESRD and CV death [40, 44].
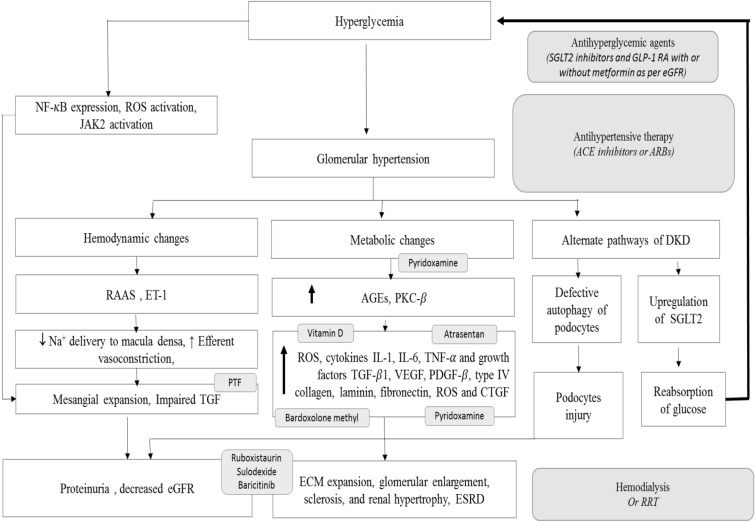
ACE-Is or ARBs as monotherapy and antihyperglycemic drugs form the mainstay of DKD management and aim to restrict progression to ESRD. The robust renoprotection results from the recent landmark CREDENCE trial [45] and recent revision to the prescribing information for dapagliflozin have led to the increasing recognition of role of SGLT2i in patients with DKD with eGFR ≥ 30 to < 90 mL/min/1.73 m2 to reduce the risk of CKD progression and CV events [20, 46]. Figure 4 depicts the various pathways involved in DKD and the therapeutic targets.
Fig. 4.
Pathophysiology of diabetic kidney disease. AGEs Advanced glycation end products, CTGF connective tissue growth factor, ECM extracellular matrix, eGFR estimated GFR, ESRD end-stage renal disease, ET-1 endothelin-1, GLP-1RA glucagon-like peptide 1 receptor agonists, IL interleukin, JAK Janus kinase, PDGF platelet-derived growth factor, NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells, PKC-β protein kinase C-β, PTF pentoxiphylline, RAAS renin–angiotensin–aldosterone system, ROS reactive oxygen species, RRT renal replacement therapy, TGF-β1 transforming growth factor-β1, TNF-α tumor necrosis factor-α, VEGF vascular endothelial growth factor
Role of SGLT2i in DKD
Mechanism of Renoprotection by SGLT2i
Sodium-glucose cotransporter 1 inhibitor is localized to the proximal tubule and is responsible for approximately 90% of the reabsorption of the glucose filtered by the kidney. SGLT2i prevent this reabsorption of glucose, thereby reducing the renal workload. SGLT2- and SGLT1-mediated glucose reabsorption is associated with Na+ and fluid reabsorption, a process which reduces the concentrations of Na+, Cl−, and K+ in the tubular fluid as well as at the distal end. Since the coupling ratio of glucose to sodium is 1:2 with SGLT1 and 1:1 with SGLT2, SGLT2 may increase sodium reabsorption in the proximal tubule. The lower Na+, Cl−, and K+ concentrations are sensed by the macula densa cells and cause the single-nephron GFR to increase through the mechanism of tubuloglomerular feedback (TGF) [47]. The role of TGF is to stabilize the salt and fluid delivery to the distal nephron, where a fine regulation of salt and fluid balance is established. Osmotic effects of high luminal glucose concentration due to SGLT2i action increase distal tubular fluid delivery and lower GFR by increasing hydrostatic pressure in Bowman’s space. Reduction in the GFR reduces tubular transport load, mainly in the PCT, thus lowering renal cortical oxygen demand and increasing oxygen tension. The reduction in GFR may also reduce tubular growth and albuminuria, as well as the resultant kidney inflammation. The diuretic and natriuretic effects of SGLT2 inhibition also reduce effective circulating volume, blood pressure, and body weight, contributing to CV protection.
SGLT2i may selectively reduce interstitial volume with minimal change in blood volume, whereas loop diuretics may cause a reduction in both interstitial and intravascular volume. This differential volume regulation by SGLT2i is assumed to limit the aberrant reflex neurohumoral stimulation in the setting of intravascular depletion [48]. In the early stages of treatment, SGLT2i can cause polyuria and natriuresis, and subsequent reductions in extracellular volume and blood pressure can potentially activate the RAAS. In turn, RAAS activation can lead to increased angiotensin II and endothelin-1 levels, with efferent arteriolar vasoconstriction and hyperfiltration. However, this action of SGLT2i has been observed to be transient [49]. As SGLT2i do not activate intrarenal RAAS, there is no increased risk of kidney injury or hypertension in T2DM [50–53]. The interaction between SGLT2i and RAAS is not yet completely understood. Moreover, SGLT2i (empagliflozin) restores glycemic control, prevents inflammation, improves endothelial function (thoracic aorta), and reduces oxidative stress in the aorta and in the blood of diabetic rats [54, 55]. In addition, SGLT2i alter renal hemodynamics, decrease intraglomerular pressure, and attenuate diabetes-associated hyperfiltration and tubular hypertrophy, together with reducing the tubular toxicity of glucose to directly protect kidney. Thus, SGLT2i can play a vital role in delaying the progression of DKD in people with T2DM.
SGLT2i and Glycemic Control
Glycemic control is important to curb the progression of DKD. The SGLT2i have demonstrated glycemic efficacy as well as other benefits such as weight reduction, reduction in blood pressure, and an increase in high-density lipoprotein-cholesterol. In a meta-analysis comparing SGLT2i with placebo for 24 weeks, the reduction in HbA1c was 0.6% (95% CI 0.5–0.7; p < 0.001, N = 9 trials) with dapagliflozin and 0.8% (95% CI 0.7–0.8; p < 0.001, N = 3 trials) with canagliflozin [56].
Several meta-analyses and RCTs have demonstrated the glycemic efficacy of SGLT2i (empagliflozin [57, 58], canagliflozin [59, 60], and dapagliflozin [61]) to be comparable to that of other antihyperglycemic agents, with the additional benefits of reduction in body weight and blood pressure.
The glycemic efficacy (mean changes from baseline in HbA1c) of empagliflozin 10 mg (− 52%, 95% CI − 0.72 to − 0.32) and 25 mg (− 0.68%, 95% CI − 0.88 to − 0.49) (both p < 0.0001) over placebo has been demonstrated even in people with T2DM having stage 2 and 3 CKD [62].
Post 2008, a number of CVOTs were undertaken to comply with the US Food and Drug Administration (FDA) recommendation for demonstrating no unacceptable increase in CV risk with any new antidiabetic therapy [63]. The first evidence of the CV benefit and renoprotective effect of SGLT2i came in 2015–2016 with the EMPA-REG CVOT [64, 65]. Subsequent trials with SGLT2i demonstrated robust CV as well as renal protection.
Clinical Evidence of CV Benefits with SGLT2i
Progression of CKD may lead to adverse CV outcomes; hence it is important to manage CV comorbidities in persons with T2DM having CKD. Three CVOTs have demonstrated strong CV benefits with the use of SGLT2i in people with T2DM. Empagliflozin in the EMPA-REG OUTCOME study [64] and dapagliflozin in DECLARE-TIMI 58 study [66] revealed a significant reduction in hospitalization due to heart failure (HHF) (HR 0.65; 95% CI 0.50–0.85, p = 0.002 and HR 0.73, 95% CI 0.61–0.88, respectively). Dapagliflozin resulted in a lower rate of CV death or HHF compared with placebo (4.9 vs. 5.8%; HR 0.83, 95% CI 0.73–0.95, p = 0.005). Empagliflozin, compared with placebo, demonstrated significantly lower rates of death from CV causes (3.7 vs. 5.9%; HR 0.62, 95% CI 0.49–0.77) and death from any cause (5.7 and 8.3%; HR 0.68, 95% CI 0.57–0.82). In addition, the efficacy of dapagliflozin with respect to the rate of CV deaths or HHF was similar in the subgroup of people with established atherosclerotic cardiovascular disease (ASCVD) (dapagliflozin 7.8% and placebo 9.3%; HR 0.83, 95% CI 0.71–0.98) and in the subgroup of people with multiple risk factors but not having established ASCVD (dapagliflozin 2.8% and placebo 3.4%; HR 0.84, 95% CI 0.67–1.04, Pinteraction = 0.99) [66]. Similarly, the CANVAS program with canagliflozin also showed a reduction in HHF (HR 0.64, 95% CI 0.35–1.15 vs. HR 0.68; CI 0.51–0.90; Pinteraction = 0.91) in the primary (individuals ≥ 50 years of age with ≥ 2 risk factors for CV events, but with no prior CV event,) and secondary prevention cohorts (individuals ≥ 30 years of age with a prior CV event) [67]. The US FDA has approved empagliflozin for lowering CV death [68], canagliflozin for lowering the risk of major adverse cardiac event in people with T2DM having established CV disease (CVD) [69], and dapagliflozin to reduce the risk of HHF in adults with T2DM and established CVD or multiple CV risk factors [70]; and provided a fast track designation for development of dapagliflozin to delay the progression of renal failure and prevent CV and renal death in patients with CKD with/without T2DM [71].
Clinical Evidence of Renoprotective Effect with SGLT2i
Regulatory Guidance for Renal Outcome Trials
The European Renal Best Practice guideline group recently observed that high-quality studies addressing clinically relevant questions for CKD patients are lacking, despite the frequency of CKD. Inadequate reporting of outcomes is challenging for clinical decision-making and for generating evidence-based guidance. A sufficiently large change in GFR has been considered a surrogate endpoint for the CKD outcomes [72]. As the progression of CKD is slow and there are no overt symptoms until the end stage, establishing clinical parameters to define its progress is challenging. A scientific workshop sponsored by the NKF and the US FDA proposed a 30 or 40% decline in eGFR as an endpoint for clinical trials in CKD. It was also noted that an eGFR decline of 40% may be more broadly acceptable than a 30% decline across a wider range of baseline GFRs and patterns of treatment effects on GFR. Evidence was stronger for a GFR decline of 40%. The 30 and 40% reduction in eGFR correspond to a 1.3- and 1.5-fold increase in serum creatinine level, respectively. For both endpoints, a follow-up during the trial of at least 2–3 years and a sample size > 1000 to allow 90% power is recommended. The workshop concluded that more research is required for composite endpoints, including other kidney outcomes, such as the occurrence of CKD GFR category 4 or acute kidney injury (AKI) [73]. A meta-analysis of 37 randomized trials also endorsed the primary endpoint of a 30–40% reduction in eGFR [74]. The European Medical Agency and FDA endorsed the 40% reduction in eGFR as a primary endpoint [75, 76] and further recommended the collection of meaningful clinical events (ESRD/mortality) as secondary outcomes, with the aim to provide important supportive information on patient benefit [75]. More recently, major adverse renal events (MARE) have been proposed as the outcome measure for future studies. MARE includes a set of major morbidity events such as the development of new-onset DKD, reaching ESRD, starting RRT, or receiving a kidney transplant, and mortality [77].
Evidence From Phase II/III Trials
Early evidence that SGLT2i provided renal protection was seen in phase II and phase III trials. In a phase III trial comparing empagliflozin and glimepiride, preservation of renal function was reported with empagliflozin (25 mg once daily, n = 769) from baseline to week 104 compared with glimepiride (1–4 mg once daily, n = 780) as an add-on to metformin. The adjusted mean difference in eGFR (baseline to week 104) for empagliflozin versus glimepiride was 3.3 mL/min/1.73 m2 (95% CI 2.0–4.7, p < 0.0001) [78]. A post-hoc analysis of data pooled from two phase III clinical trials in patients having hypertension and T2DM, receiving stable doses of ACE-Is or ARBs and having microalbuminuria or macroalbuminuria at baseline showed that dapagliflozin was associated with greater 12-week reductions in albuminuria compared with placebo [79].
A phase III study of empagliflozin as an add-on treatment in people with T2DM and stage 2 and 3 CKD demonstrated that empagliflozin reduces albuminuria [62]. In a phase III study in individuals with T2DM already on metformin, canagliflozin slowed the decline in kidney function compared with glimepiride, independent of the glycemic benefits. In addition, canagliflozin significantly decreased albuminuria; the effects of canagliflozin on ACR were consistent irrespective of baseline RAAS blockade [80].
Evidence from CVOTs
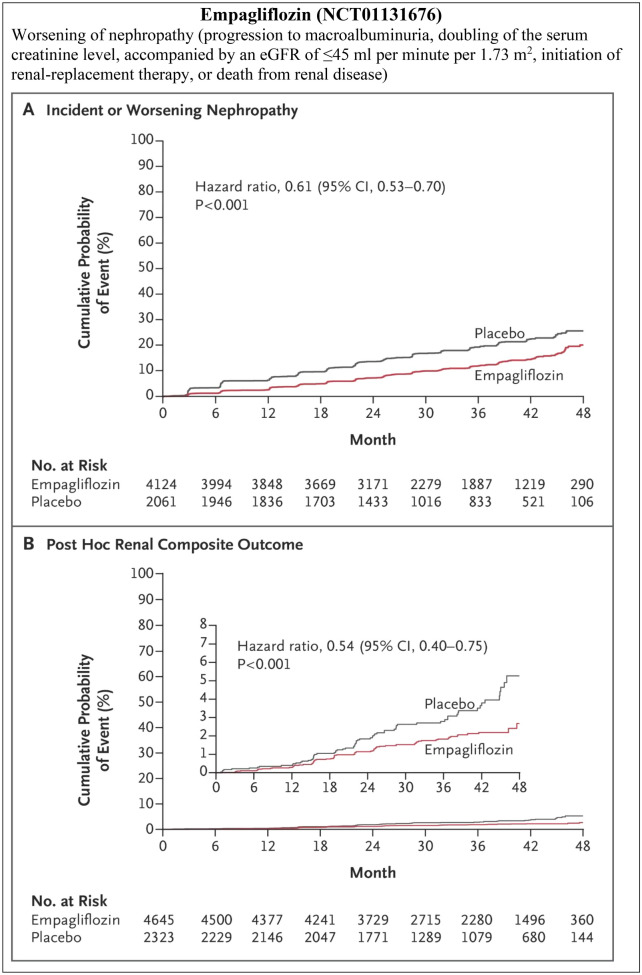
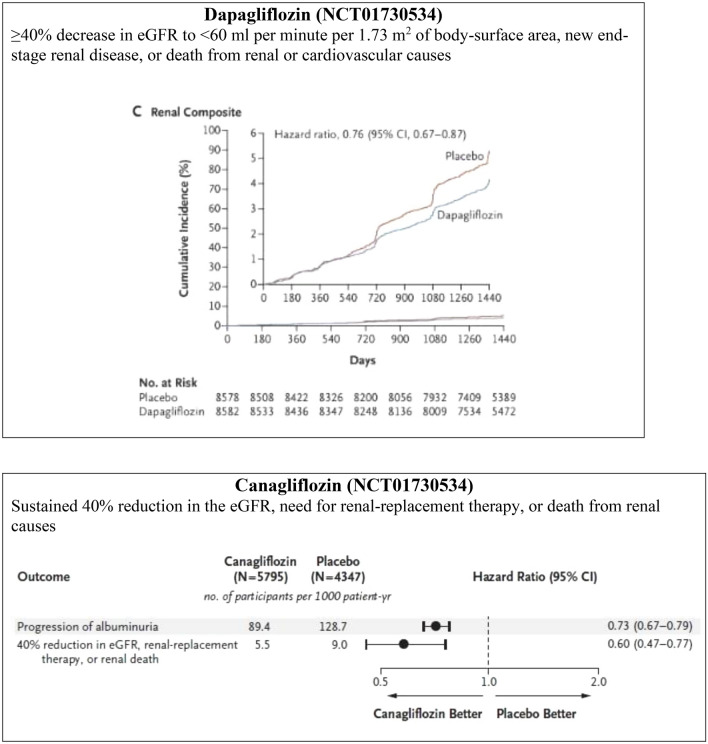
The CVOTs of SGLT2i had renal outcomes as their secondary endpoints, hence they were not sufficiently powered to establish the renoprotective effects of SGLT2i; also, they had a relatively small proportion of patients with late-stage CKD. However, despite this lack of sufficient power to assess renal outcomes, these trials have successfully demonstrated renoprotective benefits in terms of composite renal endpoints (Fig. 5a). In the EMPA-REG OUTCOME trial involving people with T2DM having established CVD, incident or worsening nephropathy occurred in 525 of 4124 patients (12.7%) in the empagliflozin group and in 388 of 2061 (18.8%) in the placebo group (HR 0.61, 95% CI 0.53–0.70, p < 0.001) [65]. The CANVAS program involving similar patient profiles, achieved better outcomes with canagliflozin in terms of the composite outcome of a sustained 40% reduction in the eGFR, the need for RRT, or death from renal causes in comparison with placebo (HR 0.60, 95% CI 0.47–0.77) [81]. The DECLARE-TIMI 58 trial in people with T2DM with and without established CVD found that patients treated with dapagliflozin were at a lower risk of composite renal outcomes compared with patients receiving placebo (3.7 vs. 7.0 events/1000 patient-years; HR 0.53, 95% CI 0.43–0.66) [66]. Renal events occurred in 4.3% in the dapagliflozin group and 5.6% in the placebo group (HR 0.76; 95% CI 0.67–0.87) and death from any cause occurred in 6.2 and 6.6% of the respective groups (HR 0.93; 95% CI 0.82–1.04) [66]. A significant risk reduction in terms of composite renal events (sustained decrease in eGFR by at least 40% to < 60 mL/min/1.73 m2, ESRD, or renal death) was achieved with dapagliflozin compared with placebo in people with T2DM with multiple risk factors and without established ASCVD (HR 0.51, CI 0.37–0.69), as well as in those with established ASCVD (HR 0.55, CI 0.41–0.75). There was no difference with respect to the renal-specific composite outcome (Pinteraction = 0.72) and for the cardiorenal composite outcome (Pinteraction = 0.67) between the established ASCVD and multiple risk factors for ASCVD cohorts. Furthermore, the benefit with dapagliflozin in terms of composite renal events was reported to be similar across groups stratified by eGFR at baseline (Pinteraction = 0.87), and greater in patients without diuretic use compared with those on diuretics at baseline (HR 0.36, 95% CI 0.26–0.50 vs. HR 0.72, 95% CI 0.54–0.95; Pinteraction = 0.0021) [86].
Fig. 5.
Composite renal outcomes in cardiovascular outcome trials and renal outcome trials. a Composite renal outcomes in cardiovascular outcome trials, b composite renal outcomes in renal outcome trials. CI Confidence interval
In the EMPA-REG OUTCOME trial (follow-up visit), the adjusted mean eGFR change (from baseline) with each of the two doses of empagliflozin versus placebo was 4.7 mL/min/1.73 m2 (95% CI 4.0–5.5, p < 0.001 for both comparisons). Patients receiving empagliflozin demonstrated a 55% lower risk of RRT, 38% reduction in progression to macroalbuminuria, and 44% lower risk in doubling of serum creatinine levels [65].
An analysis of data from the CANVAS trial demonstrated that the mean change in eGFR in the placebo arm was − 3.9 ± 0.2 mL/min/1.73 m2 compared with − 8 ± 0.2 mL/min/1.73 m2 in the canagliflozin arm (mean difference 2.0 mL/min/1.73 m2, 95% CI 1.5–2.6). Canagliflozin treatment reduced the risk of sustained loss of kidney function and mitigated the eGFR decrease [82]. Another analysis from CANVAS showed that the effect of canagliflozin on renal composite (40% decrease in eGFR, ESRD, or renal death) was significantly pronounced for preserved eGFR at baseline (60–90 mL/min/1.73 m2, HR 0.58, 95% CI 0.41–0.84 and > 90 mL/min/1.73 m2, HR 0.44, 95% CI 0.25–0.78) [83].
Additional Evidence
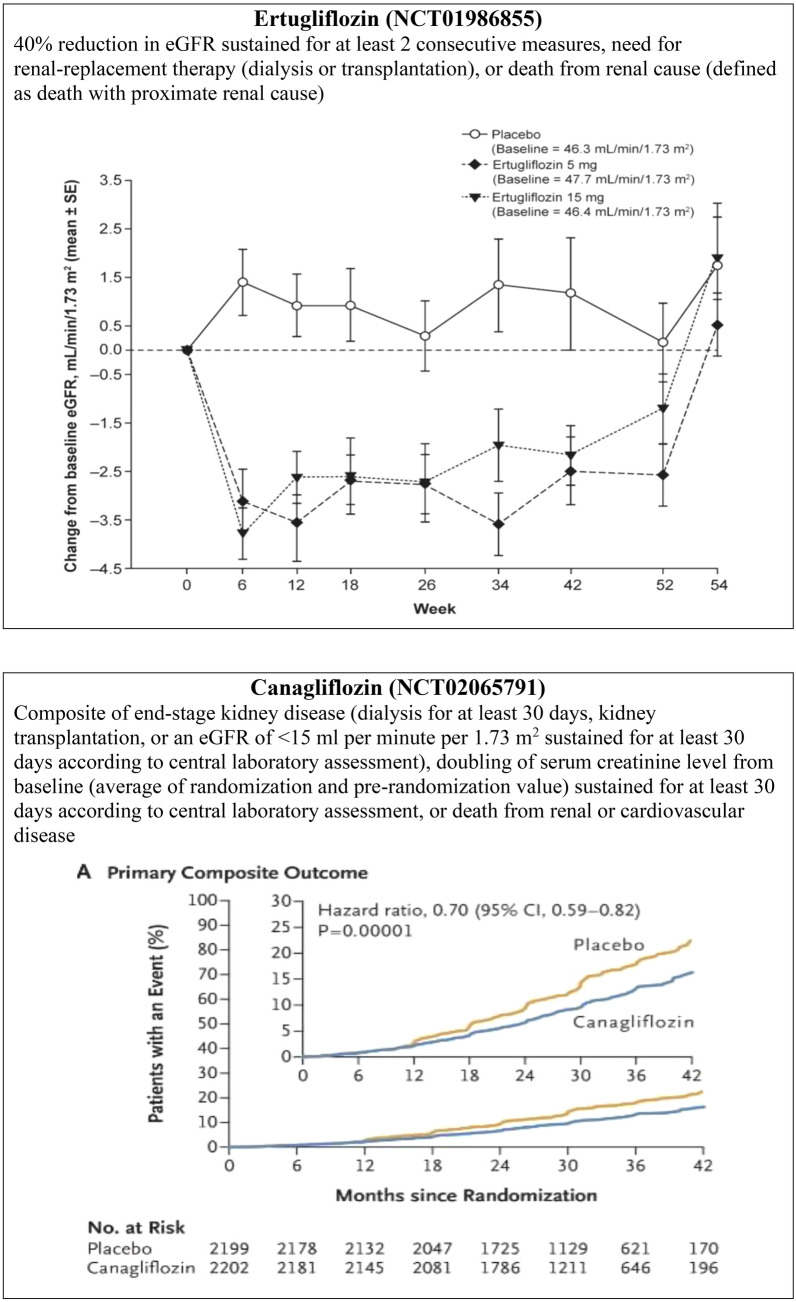
Sodium-glucose cotransporter 2 inhibitors are associated with an initial decrease and long-term preservation of eGFR. In a meta-analysis of 47 RCTs on SGLT2i, significant change in eGFR from baseline was not observed with the use of SGLT2i compared with placebo in overall patients (weighted mean difference [WMD] − 0.33 mL/min/1.73 m2, 95% CI − 0.90 to 0.23) or in patients with CKD (WMD:− 0.78 mL/min/1.73 m2, 95% CI − 2.52 to 0.97). Additionally, the investigators found that SGLT2i was associated with a reduction in the eGFR in short-term trials and preservation of the same in long-term trials [84]. Similarly, in the VERTIS-RENAL trial, conducted in people with T2DM and stage 3 CKD, the number of patients who had > 30% decrease in eGFR from baseline at weeks 26 and 52 was higher in the ertugliflozin 5 mg and ertugliflozin 15 mg groups (week 26: 10.3 and 8.7%, respectively; week 52: 13.5 and 14.0%, respectively) than in the placebo group (week 26: 2.6%; week 52: 7.3%), with eGFR reverting to baseline levels upon stopping ertugliflozin [85]. This phenomenon supports the theory that eGFR reduction with SGLT2i is related to an improvement in the dysregulated renal hemodynamics and not due to renal injury [53, 80, 86, 87]. The renal outcome trials further confirmed the renal benefits of SGLT2i.
Real-world evidence has also corroborated the findings from RCTs and CVOTs with regards to the renal benefits of SGLT2i. DARWIN-T2D was a retrospective study conducted in Italy. Changes in albumin excretion rate (AER) and eGFR were analyzed in patients receiving dapagliflozin versus other glucose-lowering agents. After 6 months, median AER declined by 37% in the dapagliflozin group and did not change in the comparator group. After adjusting for confounders, dapagliflozin was associated with an AER reduction of 26.4 ± 13.1 mg/g (p = 0.045). This study confirmed, for the first time by real-world data, the antiproteinuric effect of dapagliflozin [114].
CVD-REAL 3 was a real-world study conducted with > 65,000 patients from Israel, Italy, Japan, Taiwan, and the UK who initiated treatment for T2DM between 2013 and 2018. After propensity matching, it was observed that > 35,000 patients were started on SGLT2i. Over an average follow-up period of 14.9 months, the annual improvement in mean eGFR was 0.46 mL/min/1.73 m2 in those treated with SGLT2i, compared with a decline of 1.21 mL/min/1.73 m2 for those treated with other agents. SGLT2i use was also associated with lower risk of a confirmed decline in eGFR of 57, 50, and 40%, respectively; a 51% lower risk for the composite endpoint of a sustained reduction in eGFR of ≥ 50% or end-stage kidney disease, as well as a lower risk of HHF and all-cause mortality. Thus, this study suggests that SGLT2i are able to slow the progression of CKD in patients with T2DM, also in real-world daily clinical practice [115].
Effectiveness of SGLT2i in Ameliorating Albuminuria
In the EMPA-REG CVOT trial, 80.7% of the patients were taking ACE-Is or ARBs at baseline and had controlled blood pressure. Incident or worsening nephropathy was reduced by 39% in the empagliflozin arm compared to placebo, with the renal effects of empagliflozin evident even in patients on RAAS blockers [65]. This finding supports the potential use of empagliflozin in combination with RAAS blockers in people with T2DM and CKD. In accordance with an exploratory analysis of the EMPA-REG OUTCOME trial, patients treated with empagliflozin were more likely to experience a sustained improvement from microalbuminuria to normoalbuminuria (HR 1.43, 95% CI 1.22–1.67, p < 0.0001) or from macroalbuminuria to microalbuminuria or normoalbuminuria (HR 1.82, 95% CI 1.40–2.37, p < 0.0001) and less likely to experience a sustained deterioration from normoalbuminuria to microalbuminuria or macroalbuminuria (HR 0.84, 95% CI 0.74–0.95, p = 0.0077). Moreover, the decrease in urine ACR was evident since week 12 of the treatment [88]. In the dapagliflozin phase II/III trial, overall, 17.8, 18.9, and 7.0% of patients improved to normoalbuminuria status in the dapagliflozin 10 mg and 5 mg groups and placebo group, respectively [87]. Similarly, in a pooled analysis of phase III dapagliflozin trials, greater 12-week reductions in albuminuria (− 33.2%, 95% CI − 45.4 to − 18.2) compared with placebo was evident. The reduction in albuminuria was also present after adjusting for age, sex, changes in HbA1c, systolic blood pressure, body weight, and eGFR, i.e., − 23.5% (95% CI − 37.6 to − 6.3) [79]. A meta-analysis of 47 RCTs of SGLT2i in 22,843 people with T2DM having CKD reported a significant reduction in urine ACR (weighted mean difference − 107.35 mg/g, 95% CI − 192.53 to − 22.18) [84]. These results support the use of SGLT2i in people with T2DM and CKD, irrespective of the patients’ albuminuria status at baseline and even in patients with normoalbuminuria to prevent progression to micro- or macroalbuminuria. Canagliflozin prevented the progression of albuminuria by 27% (HR 0.73; CI 0.67–0.79) and induced regression of albuminuria more frequently in the trial participants (HR 1.70; 95% CI 1.51–1.91) [81]. Table 1 shows the renoprotective effects of SGLT2 inhibitors.
Table 1.
Renoprotective effects of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus
| Parameters | Empagliflozin [64, 65] | Empagliflozin [62] | Canagliflozin [80] | Dapagliflozin [87] | Dapagliflozin [66, 86] |
|---|---|---|---|---|---|
| NCT (ClinicalTrials.gov identifier) number | NCT01131676 | NCT01164501 | NCT00968812 | NCT00663260 | NCT01730534 |
| Number of enrolled patients | 7020 | 741 | 1450 | 166 (post hoc) | 17,190 |
| Median observation time/follow-up period | 3.1 years | 52 weeks | 24 weeks | 104 weeks | 4.2 years |
| Baseline glycemic, renal, cardiac eligibility |
eGFR ≥ 30 mL/min/1.73 m2 Established CVD HbA1c = 7–9% |
HbA1c = 7–10% eGFR ≥ 90 mL/min/1.73 m2 |
Patients with HbA1c between ≥ 7% and ≤ 9.5% at Week 2 |
HbA1c = 7–11% eGFR = 30 to 59 mL/min/1.73 m2 Stage 3 CKD Increased albuminuria (≥ 3.4 mg/mmol) |
High risk for CV events |
| Intervention | Empa 10 or 25 mg |
Stage 2 CKD: Empa 10 or 25 mg OD Stage 3 CKD: Empa 25 mg OD Stage 4 CKD: Empa 25 mg OD |
Cana 100 or 300 mg once daily + metformin | Dapa 5 or 10 mg | Dapa 10 mg |
| Control | Glimepiride (up-titrated to 6 or 8 mg/day) | ||||
| Renal outcomes | |||||
| Worsening of nephropathya |
Empa: 525/4124 (12.7%) HR 0.61 (95% CI 0.53–0.70, p < 0.001) |
Not reported | Not reported | Not reported | Not reported |
| Time to first event of renal composite endpointb | Not reported | Not reported | Not reported | Not reported |
Dapa: 127/8582 (4-year event rate: 1.5%) HR 0.53 (95% CI 0.43–0.66, p < 0.0001) |
| Annual eGFR decline | Not reported | Not reported |
Glimepiride: 3.3 mL/min/1.73 m2 (95% CI 2.8–3.8) Cana 100 mg: 0.5 mL/min/ 1.73 m2 (95% CI − 0.0 to 1.0) Cana 300 mg: 0.9 mL/min/1.73 m2 (95% CI 0.4–1.4) |
Not reported | Not reported |
| eGFR decline | Not reported | Not reported |
Overall patientsd Compared with glimepiride Cana 100 mg: 6.7% (HR 0.66, 95% CI 0.42–1.04, p = 0.07) Cana 300 mg: 9.0% (HR 0.93, 95% CI 0.62–1.42, p = 0.75) Patients with urine ACR ≥ 30 mg/g Cana 100 mg: 0.37% (95% CI 0.15–0.90, p = 0.03) Cana 300 mg: 0.69% (95% CI 0.33–1.45, p = 0.33) |
Not reported |
Dapa: 120/8582 (4-year event rate 1.4%)e HR 0.54 (95% CI 0.43–0.67, p < 0.0001) |
| Urine ACR | Not reported |
Mean difference at 52 weeks vs. placebo Stage 2 CKD: Empa 10 mg: − 184.59 (95% CI − 393.57 to 24.38, p = 0.0831) Empa 25 mg: − 235.86, (5% CI − 442.85 to − 28.86) p = 0.0257) Stage 3 CKD − 183.78 (95% CI − 305.18 to − 62.38, p = 0.0031) |
ACR decrease compared with glimepiride Overall patients Cana 100 mg: 5.7% (95% CI 22.3–13.1, p = 0.16) Cana 300 mg: 11.2% (95% CI 3.6–18.3, p < 0.01) Patients with urine ACR ≥ 30 mg/g Cana 100 mg: 31.7% (95% CI 8.6–48.9, p = 0.01) Cana 300 mg: 49.3% (95% CI 31.9–62.2, p < 0.001) |
ACR reduction compared with placebo Dapa 10 mg: − 57.2% (95% CI − 77.1 to − 20.1) Dapa 5 mg: − 43.8% (95% CI − 71.0 to 9.0) |
Not reported |
| Reduction in urine ACR | Not reported | Not reported | Not reported |
Dapa 10 mg: 33.9% Dapa 5 mg: 39.6% |
Not reported |
| Progression to macroalbuminuria |
Empa: 459/4091 (11.2%) HR 0.62 (95% CI 0.54–0.72) |
Empa 25 mg: 2% | Not reported | Not reported | Not reported |
| Improvement from macroalbuminuria at baseline to microalbuminuria | The difference in adjusted geometric mean compared with placebo − 29% (95% CI − 44 to − 10, p = 0.0048) | Empa 25 mg: 32.6% | Not reported | Not reported | Not reported |
| Improvement from microalbuminuria at baseline to albuminuria | Difference adjusted geometric mean compared with placebo − 22% (95% CI − 32 to − 11, p = 0.0003) | Empa 25 mg: 27.5% | Not reported | Not reported | Not reported |
| Doubling of serum creatininec |
Empa: 70/4645 patients (1.5%) HR 0.56 (95% CI 0.39–0.79) |
Not reported | Not reported | Not reported | Not reported |
| Initiation of RRT |
Empa: 13/4687 (0.3%) HR 0.45 (95% CI 0.21–0.97) |
Not reported | Not reported | Not reported | Not reported |
| Death from renal disease | Empa: 3/4687 (0.1%) | Not reported | Not reported | Not reported |
Dapa: 6/8582 (4-year event rate: 0.1%) HR 0.60 (95% CI 0.22–1.65, p = 0.32) |
ACR Albumin:creatinine ratio, CI confidence interval, Cana canagliflozin, CKD chronic kidney disease, CV cardiovascular, CVD cardiovascular disease, Dapa dapagliflozin, eGFR estimated glomerular filtration rate, Empa empagliflozin, ESRD end-stage renal disease, HbA1c glycated hemoglobin, HR hazards ratio, OD once a day, T2DM type 2 diabetes mellitus, RRT renal replacement therapy
aProgression to macroalbuminuria, a doubling of the serum creatinine level, accompanied by an eGFR of ≤ 45 mL/min/1.73 m2, the initiation of RRT, or death from renal disease
bConfirmed sustained ≥ 40% decrease in eGFR to < 60 mL/min/1.73 m2 and/or ESRD and/or renal or CV death
cAccompanied by an eGFR of ≤ 45 mL/min/1.73 m2
d30% eGFR decline endpoint
eSustained eGFR decrease of 40% to eGFR < 60 mL/ min/1.73 m2
Evidence From Renal Outcome Trials and Meta-Analysis
Two major trials recently reported the renal outcomes of SGLT2i as primary endpoints (Table 2). The recent CREDENCE trial demonstrated the renoprotective effect of canagliflozin in people with T2DM having albuminuric CKD (ACR > 300–5000) and who were already on stable doses of ACE-Is or ARBs (Fig. 5b). The relative risk (RR) of the primary outcome (ESRD, doubling of serum creatinine, death from renal, or CV cause) was 30% lower in the canagliflozin group than in the placebo group; the RR of the composite of ESRD, a doubling of the creatinine level, or death from renal causes was also lower in the canagliflozin group, by 34%, and the RR of ESRD was lower by 32% [45]. The DELIGHT trial also confirmed the renoprotective effect of dapagliflozin by a reduction in urine ACR. Dapagliflozin reduced albuminuria when given in combination with ACE-I or ARBs, and a combination of dapagliflozin and saxagliptin was used to achieve the dual objectives of effective lowering of blood glucose and urinary albumin excretion in people with T2DM and CKD [89]. The renal outcome was not a primary endpoint in the DERIVE study. In this study, decreases from baseline in eGFR were greater with dapagliflozin than with placebo at week 24 (− 2.49 mL/min/1.73 m2, 95% CI − 4.96 to − 0.02); however, eGFR returned to baseline levels at week 27 (3 weeks post-treatment) (0.61 mL/min/1.73 m2, 95% CI − 1.59 to 2.81) [61].
Table 2.
Renal outcome trials with sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus
| Parameters | Canagliflozin [45] | Dapagliflozin [89] | Dapagliflozin [61] |
|---|---|---|---|
| Study acronym (NCT number/ClinicalTrials.gov identifier) | CREDENCE (NCT02065791) | DELIGHT (NCT02547935) | DERIVE(NCT02413398) |
| Number of enrolled patients | 4401 | 461 | 321 |
| Median observation time/ follow-up period | 2.62 years | 24 weeks | 24 weeks |
| Eligibility |
eGFR = 30 to < 90 mL/min/1.73 m2 ACR > 300 to 5000 Treatment with RAAS blockade |
1. HbA1c ≥ 7.0% and ≤ 11.0% 2. Stable antidiabetic treatment during the last 12 weeks up to randomization 3. eGFR 25–75 mL/min/1.73 m2, inclusive 4. Micro- or macroalbuminuria (urine ACR 30—3500 mg/g) 5. Treatment with ACE-I or an ARB for at least 3 months prior to screening |
HbA1c ≥ 7.0% and ≤ 11.0% Renal impairment: CKD 3A |
| Intervention | Cana 100 mg once daily |
Dapa 10 mg or Dapa 10 mg + saxagliptin 2.5 mg |
Dapa 10 mg |
| Control | Placebo | Placebo | Placebo |
| Renal outcomes | |||
| Composite of ESKD, a doubling of the serum creatinine level, or death from renal, or CV causes |
Events per 1000 patient-years: Cana: 43.2 HR 0.70 (95% CI 0.59–0.82, p < 0.00001) |
Not reported | Not reported |
| Change from baseline in urine ACR at Week 24 | Not reported |
Difference versus placebo at Week 24: Dapa − 21.0% (95% CI − 34.1 to − 5.2, p = 0.011) Dapa + saxagliptin − 38.0% (95% CI − 48.2 to − 25.8, p < 0.0001) |
Change from baseline in urine ACR at Week 24 for patients with baseline urine: ACR ≥ 30 mg/g Week 12: difference − 41.7% (95% CI − 57.1 to − 21.0, p < 0.001) Week 24: difference: − 14.0% 95% CI − 42.3, 28.0, p = 0.454 |
| Change from baseline in eGFR at Week 24 | Not reported |
Difference versus placebo at Week 24: Dapa − 2.35 mL/min/1.73 m2 (95% CI − 4.16 to − 0.53, p = 0.011) Dapa + saxagliptin − 2.44 mL/min/1.73 m2 (95% CI − 4.22 to − 0.66, p = 0.0075) |
Difference vs placebo: − 2.49 mL/min/1.73 m2 (95% CI − 4.96 to − 0.02) |
| Doubling of serum creatinine level |
Events per 1000 patient-years: Cana: 20.7 HR 0.60 (95% CI 0.48–0.76, p < 0.001) |
Not reported | Not reported |
| ESKD |
Events per 1000 patient-years: Cana: 20.4 HR 0.68 (95% CI 0.54–0.86, p = 0.002) |
Not reported | Not reported |
| eGFR < 15 mL/min/1.73 m2 |
Events per 1000 patient-years: Cana: 13.6 HR 0.60 (95% CI 0.45–0.80) |
Not reported | Not reported |
| Dialysis initiated or kidney transplantation |
Events per 1000 patient-years: Cana: 13.3 HR 0.74 (95% CI 0.55–1.00) |
Not reported | Not reported |
| Renal death |
Events per 1000 patient-years: Cana: 0.3 |
Not reported | Not reported |
| ESKD, doubling of serum creatinine level, or renal death |
Events per 1000 patient-years: Cana: 27.0 HR 0.66 (95% CI 0.53–0.81) |
Not reported | Not reported |
| Dialysis, kidney transplantation, or renal death |
Events per 1000 patient-years: Cana: 13.6 HR 0.72 (95% CI 0.54–0.97) |
Not reported | Not reported |
ACE-I Angiotensin-converting enzyme inhibitor, ACR albumin:creatinine ratio, ARB angiotensin II receptor blocker, ESKD end-stage kidney disease RAAS renin-angiotensin aldosterone system
Neuen et al. performed a meta-analysis of four CVOTs covering 38,723 participants to assess the composite of dialysis, transplantation, or death due to kidney disease; their results also confirmed the renoprotective effects of SGLT2i. These authors reported that SGLT2i reduced the risk of dialysis, transplantation, or death due to kidney disease (RR 0.67, 95% CI 0.52–0.86, p = 0.0019) in addition to reducing ESRD (RR 0.65, 95% CI 0.53–0.81, p < 0.0001) and AKI (RR 0.75, 95% CI 0.66–0.85, p < 0.0001). The benefit was consistent across all studies and observed across all eGFR subgroups, even in participants with a baseline eGFR of 30–45 mL/min/1.73 m2 (RR 0.70, 95% CI 0.54–0.91, p = 0.0080) and irrespective of baseline albuminuria (Ptrend = 0.66) and use of RAAS blockers (Pheterogeneity = 0.31) [90]. Zelniker et al. showed a similar benefit, with reduction in the terms of composite of worsening of renal function, ESRD, or renal death across all baseline eGFR levels, but greatest in those with preserved renal function at baseline (eGFR of < 60 mL/min/1.73 m2: 33% reduction; eGFR between 60 and 90 mL/min/1.73 m2: 44% reduction; eGFR ≥ 90 mL/min/1.73 m2: 56% reduction; Pinteraction = 0.0258). SGLT2i were also shown to provide 45% risk reduction for composite renal events (HR 0.55, CI 0.48–0.64, p < 0.0001), with a similar benefit in those with and without ASCVD (Pinteraction = 0.71) [91].
Revisions in Treatment Guidelines for DKD Management
Based on the outcomes of the CREDENCE trial in patients with CKD, the ADA 2020 and European Association for the Study of Diabetes (EASD) 2019 consensus statements strongly recommend (strength of recommendation A and 1B, respectively) as a current first-line standard of care SGLT2i in people with T2DM and DKD with eGFR ≥ 30 mL/min/1.73 m2, and particularly in those with > 300 mg/g albuminuria, with or without ASCVD, provided they have an adequate renal function [46, 92, 93].
Safety Concerns and Contraindications With SGLT2i
Sodium-glucose cotransporter 2 inhibitors are increasingly being recognized as antihyperglycemic agents for people with T2DM having established ASCVD or multiple risk factors for CVD, and for those with mild-to-moderate renal impairment. However, healthcare providers need to be cautious and vigilant about the specific safety concerns while prescribing them. Regulatory agencies, including the US FDA, the European Medicines Associations (EMA), and Health Canada have issued safety warnings for several AEs. These include AKI, diabetic ketoacidosis (DKA), genital mycotic infections, urinary tract infections (UTI), bone fractures, lower limb amputations, and acute and chronic pancreatitis. The association of acute and chronic pancreatitis with SGLT2i was reported in the safety review by Health Canada, which concluded that there may be a link between the use of SGLT2i and the risk of acute pancreatitis. However, there was only limited evidence to suggest a link with chronic pancreatitis [94–96]. Table 3 summarizes the most common AEs associated with the use of SGLT2i.
Table 3.
Frequently observed adverse events with sodium-glucose cotransporter 2 inhibitors: evidence from meta-analyses
| Adverse events | Overall SGLT2i [100] | Empagliflozin [58] | Canagliflozin [98] | Dapagliflozin [99] |
|---|---|---|---|---|
| Acute kidney injury | RR 0.59 (95% CI 0.39–0.89; I2 = 0.0%) | Not analyzed | Not analyzed | Not analyzed |
| Diabetic ketoacidosis | RR 0.66 (95% CI 0.30–1.45, I2 = 0.0%) | Not analyzed | Not analyzed | Not analyzed |
| Urinary tract infections | RR 1.02 (95% CI 0.95–1.09, I2 = 0.0%) | OR 1.119 (95% CI 0.886–1.411) |
Cana 100 mg: RR 1.45 (95% CI 0.91–2.29, p = 0.12) Cana 300 mg: RR 1.18 (95% CI 0.73–1.91, p = 0.49) |
RR 1.74 (95% CI 1.21–2.49, p = 0.003) |
| Genital tract infections | Not analyzed | OR 2.814 (95% CI 1.712–4.625) |
Cana 100 mg: RR 3.79 (95% CI 2.05–7.01, p < 0.01) Cana 300 mg: RR 3.99 (95% CI 2.16–7.35, p < 0.01) |
RR: 3.52 (95% CI 2.06–6.03, p < 0.00001) |
| Bone fractures | RR 0.87 (95% CI 0.69–1.09, I2 = 1.3%) | Not analyzed | Not analyzed | Not analyzed |
| Osmotic diuresis-related AE/hypovolemia | Not analyzed | Not analyzed |
Cana 100 mg: RR 5.35 (95% CI 2.43–11.77, p < 0.01) Cana 300 mg: RR 5.35 (95% CI 2.43–11.77, p < 0.01) |
RR: 0.74 (95% CI 0.32–1.71, p = 0.47) |
AE Adverse events, OR odds ratio, RR relative risk, SGLT2i sodium-glucose cotransporter 2 inhibitors
Genital Mycotic Infections and UTIs
Genital mycotic infections and UTIs are the most commonly reported AEs with SGLT2i, and they can be attributed to the specific mechanism of action of urinary glucose excretion of SGLT2i. A three- to sixfold increase in genital infections has been reported in RCTs as well as in observational studies [97]. In the EMPA-REG OUTCOME trial, a significantly higher percentage of men and women had genital mycotic infections (5.0 and 10.0%, respectively) compared with those in the placebo group (1.5 and 2.6%, respectively; p < 0.001); however, the rates of UTI with empagliflozin and placebo were similar [64]. A meta-analysis of 14 RCTs of empagliflozin showed a significantly higher incidence of genital tract infections compared with placebo [58]. A similar trend was observed for canagliflozin compared with placebo in the CANVAS trial for genital mycotic infections (34.9% in men vs. 10.8% in women; 68.8 vs. 17.5%, respectively; both p < 0.001) and UTI (40 vs. 38%, p = 0.38) [81], as well as in the meta-analysis of seven RCTs of canagliflozin [98]. Although rare, genital infections were proportionately higher in dapagliflozin group than in the placebo group (0.9 vs. 0.1%; HR 8.36, 95% CI 4.19–16.68, p < 0.001), both in men and in women in the DECLARE-TIMI 58 study [66]. A meta-analysis of six dapagliflozin RCTs showed a higher incidence of UTI with dapagliflozin compared with placebo [99].
However, a large meta-analysis of 109 studies involving SGLT2i found no difference in the incidence of UTI with SGLT2i compared with placebo (RR 1.02, 95% CI 0.95–1.09) [100].
Diabetic Ketoacidosis
The major concern regarding SGLT2i-associated DKA is that it may present with normal or slightly high blood glucose levels, which can lead to delays in the recognition or diagnosis of DKA and may be potentially fatal. SGLT2i have been associated with increases in ketone-associated AEs, suggesting a causal relationship between SGLT2i and an asymptomatic rise in ketone bodies. This is likely due to downstream insulin deficiency and increased glucagon levels that promote lipolysis and hepatic ketogenesis. The glucosuria induced by SGLT2i lowers plasma glucose, which decreases insulin secretion from the β-cells of the pancreas. This, along with the attenuation of sodium reabsorption in the kidneys, may indirectly increase the ketone reservoir by increasing renal ketone reabsorption [111]. The events of DKA were rarely reported in the major CVOTs conducted to date and were not significantly different for the SGLT2i arms when compared with placebo arms for empagliflozin [66] and canagliflozin [81]. The meta-analysis by Donnan et al. reported similar results [100]. Although rare, DKA was more common in the dapagliflozin group than in the placebo group in the DECLARE TIMI 58 trial (0.3 vs. 0.1%; HR 2.18, 95% CI 1.10–4.30, p = 0.02). However, a pertinent observation was that > 80% of patients with DKA were using insulin at baseline [66]. Zelniker et al. found an increased risk of DKA with SGLT2i versus the placebo arm (HR 2.20, 95% CI 1.25–3.87, p = 0.0060), but again the event rates were low (< 1 per 1000 patient-years) [91].
Acute Kidney Injury
The risk of AKI with SGLT2i is considered to be due to volume depletion resulting from natriuresis, the effect on TGF, and consequent kidney medullary hypoxia. The US FDA issued an initial warning for increased AKI risk in December 2015 and then strengthened the warning in June 2016 for canagliflozin and dapagliflozin [101]. However, the incidence of AKI was significantly lower in patients receiving dapagliflozin compared with placebo (1.5 vs 2.0%; HR 0.69, 95% CI 0.55–0.87) in the DECLARE TIMI-58 study [66]. Similar observations were reported from the EMPA-REG OUTCOME [64] and CANVAS program [81]. A recent meta-analysis reported a consistent and robust reduction in the likelihood of AKI among those participants who had been randomized to receive a SGLT2i (HR 0.66, 95% CI 0.54–0.80). The reports of AKI were similar in frequency to those of kidney disease progression [102]. Furthermore, a Cochrane meta-analysis published in September 2018 concluded that SGLT2i have little or no risk of AKI in people with diabetes and CKD (eGFR < 60 mL/min/1.73 m2) [103]. Thus, the possibility of AKI should not preclude the use of SGLT2i in patients with mild-to-moderate CKD; SGLT2i should be discontinued if GFR falls below 30 mL/min/1.73 m2. The patients can be carefully monitored for any incidence of AKI. Similar monitoring is required for a rare but serious genital infection called Fournier’s gangrene for which the FDA has issued a warning [104].
Lower Extremity Amputations
In the CANVAS program, the rates of lower extremity amputations were higher with canagliflozin (6.30 per 1000 participant-years) than with placebo (3.37 per 1000 participant-years) (HR 1.97, 95% CI 1.41–2.75). Overall amputation risk was strongly associated with a baseline history of prior amputation (major or minor) (HR 21.31, 95% CI 15.40–29.49). The increased risk of amputation was consistent in people with baseline eGFR levels above and below 60 mL/min/1.73 m2 (HR 1.91, 95% CI 1.29–2.83 vs. HR 2.17, 95% CI 1.14–4.10, respectively), as was the risk of fracture (HR 1.29, 95% CI 1.04–1.61 vs. HR 1.18, 95% CI 0.80–1.73, respectively) [81]. In the recent CREDENCE trial, there was no significant difference in the risk of lower limb amputation (HR 1.11, 95% CI 0.79–1.56) and rates of fracture (HR 0.98, 95% CI 0.70–1.37) between the canagliflozin and placebo group [45]. Donnan et al. highlight the lack of data for a causal association of SGLT2i with the risk of amputations and fractures, and they also confirm that the present evidence for this association is available from CANVAS and CANVAS-R trials only [100]. Pharmacovigilance analysis from the US FDA Adverse Event Reporting System (FAERS) also confirms that the use of canagliflozin, but not dapagliflozin or empagliflozin, might be associated with an increased risk of amputations. However, there are limitations to the FAERS data because there is no definite causal link between drug exposure and AE [112]. Conversely, the World Health Organization global database revealed the expected signal for canagliflozin; however, the proportional reporting ratio was also high for empagliflozin and, for toe amputations only, for dapagliflozin [113].
Overall, genital mycotic infections are the most frequent side effect observed with SGLT2i. The incidence of UTI in patients treated with SGLT2i is similar to that seen in all people with T2DM. Hypoglycemia can occur when a SGLT2i is used as an add-on to other agents causing hypoglycemia, such as insulin or sulfonylureas. Volume depletion and hypotension are rare and can be minimized by adjusting diuretic and antihypertensive treatment in patients at risk. The incidences of AKI, amputation (largely feet and toes), and fractures are rare and can be prevented by careful monitoring and avoiding use in high-risk patients [105].
Ongoing SGLT2i Trials Assessing Renal Outcomes
Multiple trials assessing the renal benefits of SGLT2i are ongoing. The RACELINES trial will assess the clinical effects and mechanism of monotherapy and combination therapy of the SGLT2i empagliflozin and dipeptidyl peptidase 4 (DPP-4) inhibitor linagliptin on renal physiology and biomarkers in metformin-treated T2DM patients [106]. The EMPA-KIDNEY trial will compare the renal effect of a SGLT2i and a sulfonylurea. The EMPA-KIDNEY trial will evaluate the effect of empagliflozin on kidney disease progression or CV death in people without T2DM [107]. Despite optimal treatment with RAAS inhibitors, many patients with non-diabetic kidney disease show progressive kidney function loss, which is associated with high residual proteinuria. The recently completed DIAMOND study was conducted to assess the renoprotective effects of dapagliflozin in non-diabetic patients with proteinuria [109]. The DAPA-CKD trial, evaluating the efficacy of dapagliflozin in patients with CKD stages 2–4 and elevated urinary albumin excretion, with and without T2D, will be stopped early due to overwhelming efficacy [110]. The treatment paradigm for CKD, for people with and without T2DM, may change after the release of the ongoing trial results over the next few years. These trials are listed in Table 4 with their primary outcome measures.
Table 4.
Ongoing clinical trials of sodium-glucose cotransporter 2 inhibitors in patients with chronic kidney disease
| NCT number (acronym) | Trial drug | Comparator | Eligibility | Primary outcome | Expected study completion date |
|---|---|---|---|---|---|
| NCT03433248 (RACELINES) | Empagliflozin and linagliptin monotherapy or combination therapy | Gliclazide |
1. Patients with T2DM with HbA1c: 7.0—9.5% 2. On treatment with ACE-I or ARB 3. eGFR ≥ 45 mL/min/1.73 m2 |
Changes in fasting and postprandial GFR at Week 16 | December 2020 |
| NCT03036150 (Dapa-CKD) | Dapagliflozin |
1. Patients with T2DM 2. eGFR ≥ 25 and ≤ 75 mL/min/1.73 m2 3. Evidence of increased albuminuria 3 months or more 4. On treatment with ACE-I or ARB |
Time to the first occurrence of any of the components of the composite: (1) ≥ 50% sustained decline in eGFR or (2) reaching ESRD or CV death or renal death | November 2020 | |
| NCT03594110 (EMPA-KIDNEY) | Empagliflozin |
1. Evidence of CKD at risk of kidney disease progression 2. On treatment with ACE-I or ARB |
Composite primary outcome: Time to first occurrence of (1) kidney disease progression or (2) CV death | June 2022 | |
| NCT03315143 (SCORED) | Sotagliflozin |
1. T2DM with HbA1c ≥ 7% 2. eGFR ≥ 25 and ≤ 60 mL/min/1.73 m2 |
Time to the first MACE Time to CV death or HHF |
Marcj 2022 |
ACE-I angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, CKD chronic kidney disease, CV cardiovascular, eGFR estimated glomerular filtration rate, ESRD end-stage renal disease, GFR glomerular filtration rate, HbA1c glycated hemoglobin, HHF hospitalization for heart failure, T2DM type 2 diabetes mellitus, MACE major adverse cardiac events
Recommendations for Optimal Use of SGLT2 Inhibitors
Sodium-glucose cotransporter 2 inhibitors reduce the risk of progression to DKD and renal death by approximately 40–50% in people with T2DM who are at risk of CV events and are on ACE-Is and ARBs. Thus, SGLT2i have emerged as the new drugs that must be included in the armamentarium for the management for people with T2DM having CVD or mild-to-moderate DKD to curb morbidity and mortality. The recommendations for optimal use of SGLT2i, generated on the basis of evidence from RCTs and meta-analyses, is summarized below.
Consensus Group Recommendations for Optimal Use of SGLT2 Inhibitors in DKD
| Recommendation | Renal/ safety outcomes assessed | Source |
|---|---|---|
| 1. SGLT2i can be used as add-on second-line therapy in patients with T2DM having mild to moderate DKD for delaying the progression of DKDa |
Composite of dialysis, transplantation, or death due to kidney disease Composite of ESRD Incident or worsening nephropathy Rate of decline of eGFR Change from baseline in UACR Change from baseline in eGFR Rate of decline of eGFR Subgroup analysis |
NCT02065791: CREDENCE (Canagliflozin) NCT01131676: EMPA-REG (Empagliflozin) NCT01032629 CANVAS and CANVAS-R (Canagliflozin) NCT00968812: CANTATA-SU (Canagliflozin) NCT01730534: DECLARE-TIMI-58 (Dapagliflozin) NCT02547935: DELIGHT (Dapagliflozin) NCT01986855: VERTIS-RENAL (Ertugliflozin) SRMA: Xu et al. 2017 Zelniker et al. 2019 [91] Neuen et al. 2019 [90] |
| 1.1. Initial reduction in eGFR is expected after initiation of SGLT2i, hence close monitoring of patients is recommended | ||
| 1.2. Patients with eGFR < 60 mL/min/1.73 m2 should be closely monitored as per local practice for renal function parameters | ||
| 1.3. SGLT2i can be used if eGFR > 45 mL/min/1.73 m2 and can be continued if eGFR is between 30 and 45 mL/min/1.73 m2 | ||
| 1.4. SGLT2i should be discontinued if eGFR falls below 30 mL/min/1.73 m2 | ||
| 1.5. SGLT2i should not be used in patients with ESRD or on dialysis | ||
| 2. Dose modulation may be required while prescribing SGLT2i as an add-on to sulfonylureas and insulin | Safety outcomes |
Review articles Gomez-Peralta et al. [108] Fitchett 2019 [105] Monami et al. 2014 [56] |
| 3. Patient selection and education should be an integral part of management while initiating SGLT2i |
Safety data meta-analysis A safety update on SGLT2i |
NCT01032629 and NCT01989754: CANVAS (Canagliflozin) SRMA of RCTs: Liakos et al. 2014 Devi et al. 2017 Xiong et al. 2016 Feng et al. 2019 NCT01986855: VERTIS-RENAL US FDA Drug Safety Communications [95] Fitchett 2019 [56] |
| 3.1. Caution should be taken while prescribing SGLT2i to patients with a history of prior amputation, severe peripheral vascular disease, neuropathy, foot ulcers, hypersensitivity reactions, and those on diuretic therapy | ||
| 3.2. Patients should be educated about perineal and genital hygiene, and signs and symptoms of mycotic infections; early diagnosis and treatment should be encouraged |
CKD Chronic kidney disease, DKD diabetic kidney disease, eGFR estimated glomerular filtration rate, ESRD end-stage renal disease, MA meta-analysis, RCT randomized controlled trial, SGLT2i sodium-glucose cotransporter 2 inhibitor, SRMA systematic review and meta-analysis, T2DM type 2 diabetes mellitus, UACR urine albumin-to-creatinine ratio, US FDA US Food and Drug Administration
aUS FDA has approved canagliflozin for use in DKD and has granted FastTrack designation for development of dapagliflozin to delay the progression of renal failure and prevent cardiovascular and renal deaths in patients with CKD
Acknowledgements
Members of the Working Group of the Endocrine Society of Bengal: Ajitesh Roy, Animesh Maiti, Anirban Sinha, Arjun Baidya, Asish Kumar Basu, Dasarathi Sarkar, Debmalya Sanyal, Dibakar Biswas, Indira Maisnam, Kaushik Pandit, Moutusi Raychaudhuri, Nilanjan Sengupta, Partha Pratim Chakraborty, Pradip Mukhopadhyay, Pradip Raychaudhuri, Pranab Kumar Sahana, Purushottam Chatterjee, Rana Bhattacharjee, Ranen Dsgupta, Ravi Kant Saraogi, Salil Kumar Pal, Sarmishtha Mukhopadhyay, Satinath Mukhopadhyay, Soumik Goswami, Subhankar Chowdhury, and Sujoy Ghosh
Funding
The preparation of this consensus statement and funding of the journal’s Rapid Service Fee were supported by AstraZeneca Pharma India Limited. All authors had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing Assistance
The authors thank Dr. Ranjini Sen of AstraZeneca Pharma India Ltd. for providing medical writing assistance in the development of this manuscript, in collaboration with Ms. Neelam Joglekar, Sciformix, A Covance Company. Medical writing assistance was funded by AstraZeneca Pharma India Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Ajitesh Roy, Animesh Maiti, Anirban Sinha, Arjun Baidya, Asish Kumar Basu, Dasarathi Sarkar, Debmalya Sanyal, Dibakar Biswas, Indira Maisnam, Kaushik Pandit, Moutusi Raychaudhuri, Nilanjan Sengupta, Partha Pratim Chakraborty, Pradip Mukhopadhyay, Pradip Raychaudhuri, Pranab Kumar Sahana, Purushottam Chatterjee, Rana Bhattacharjee, Ranen Dasgupta, Ravi Kant Saraogi, Salil Kumar Pal, Sarmishtha Mukhopadhyay, Satinath Mukhopadhyay, Soumik Goswami, Subhankar Chowdhury and Sujoy Ghosh have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
The US Food and Drug Administration has approved canagliflozin for use in DKD and has granted FastTrack designation for the development of dapagliflozin as therapy to delay the progression of renal failure and prevent cardiovascular and renal deaths in patients with chronic kidney disease.
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12871889.
References
- 1.International Diabetes Federation. IDF diabetes atlas, ninth ed. Brussels: Belgium International Diabetes Federation; 2019. https://diabetesatlas.org/en/resources/. Accessed 23 Dec 2019.
- 2.International Diabetes Federation. Diabetes facts & figureshttps://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html. Accessed 23 Dec 2019.
- 3.Barrett EJ, Liu Z, Khamaisi M, et al. Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab. 2017;102:4343–4410. doi: 10.1210/jc.2017-01922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 5.Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 7.Luk AO, Li X, Zhang Y, et al. Quality of care in patients with diabetic kidney disease in Asia: the Joint Asia Diabetes Evaluation (JADE) Registry. Diabet Med. 2016;33:1230–1239. doi: 10.1111/dme.13014. [DOI] [PubMed] [Google Scholar]
- 8.Prasannakumar M, Rajput R, Seshadri K, et al. An observational, cross-sectional study to assess the prevalence of chronic kidney disease in type 2 diabetes patients in India (START-India) Indian J Endocrinol Metab. 2015;19:520–523. doi: 10.4103/2230-8210.157857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh AK, Farag YM, Mittal BV, et al. Epidemiology and risk factors of chronic kidney disease in India—results from the SEEK (Screening and Early Evaluation of Kidney Disease) study. BMC Nephrol. 2013;14:114. doi: 10.1186/1471-2369-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold SV, Hunt PR, Chen H, et al. Cardiovascular outcomes and mortality in type 2 diabetes with associated cardio-renal-metabolic comorbidities. Diabetes. 2018;67(Suppl 1):1582. doi: 10.1159/000504558. [DOI] [PubMed] [Google Scholar]
- 11.Ang YG, Heng BH, Saxena N, Liew STA, Chong PN. Annual all-cause mortality rate for patients with diabetic kidney disease in Singapore. J Clin Transl Endocrinol. 2016;4:1–6. doi: 10.1016/j.jcte.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis. 2018;25:121–132. doi: 10.1053/j.ackd.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dare AJ, Fu SH, Patra J, et al. Renal failure deaths and their risk factors in India 2001–13: nationally representative estimates from the Million Death Study. Lancet Glob Health. 2017;5:e89–e95. doi: 10.1016/S2214-109X(16)30308-4. [DOI] [PubMed] [Google Scholar]
- 14.Hamada S, Gulliford MC. Multiple risk factor control, mortality and cardiovascular events in type 2 diabetes and chronic kidney disease: a population-based cohort study. BMJ Open. 2018;8:e019950. doi: 10.1136/bmjopen-2017-019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh YT, Kuo JF, Su SL, Chen JF, Chen HC, Hsieh MC. Subnormal estimated glomerular filtration rate strongly predict incident cardiovascular events in type 2 diabetic Chinese population with normoalbuminuria. Medicine (Baltimore) 2016;95:e2200. doi: 10.1097/MD.0000000000002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mechanick JI, Pessah-Pollack R, et al. American Association of Clinical Endocrinologists and American College of Endocrinology protocol for standardized production of clinical practice guidelines, algorithms, and checklists—2017 update. Endocr Pract. 2017;23:1006–1021. doi: 10.4158/EP171866.GL. [DOI] [PubMed] [Google Scholar]
- 17.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 18.KDIGO Working Group Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2011;2013(3):19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association 11. Microvascular complications and foot care: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S135–S151. doi: 10.2337/dc20-S011. [DOI] [PubMed] [Google Scholar]
- 21.Toth-Manikowski S, Atta MG. Diabetic kidney disease: pathophysiology and therapeutic targets. J Diabetes Res. 2015;2015:697010. doi: 10.1155/2015/697010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magee C, Grieve DJ, Watson CJ, Brazil DP. Diabetic nephropathy: a tangled web to unweave. Cardiovasc Drugs Ther. 2017;31:579–592. doi: 10.1007/s10557-017-6755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018;117:662–675. doi: 10.1016/j.jfma.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Forbes JM, Fukami K, Cooper ME. Diabetic nephropathy: where hemodynamics meets metabolism. Exp Clin Endocrinol Diabetes. 2007;115(2):69–84. doi: 10.1055/s-2007-949721. [DOI] [PubMed] [Google Scholar]
- 25.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60:215–225. doi: 10.1007/s00125-016-4157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsimihodimos V, Filippatos TD, Elisaf MS. SGLTi and the kidney: effects and mechanisms. Diabetes Metab Syndr. 2018;12(6):1117–1123. doi: 10.1016/j.dsx.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–2576. doi: 10.1681/ASN.V10122569. [DOI] [PubMed] [Google Scholar]
- 29.Hallow KM, Gebremichael Y, Helmlinger G, Vallon V. Primary proximal tubule hyperreabsorption and impaired tubular transport counterregulation determine glomerular hyperfiltration in diabetes: a modeling analysis. Am J Physiol Renal Physiol. 2017;312:F819–F835. doi: 10.1152/ajprenal.00497.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 31.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S98–S110. doi: 10.2337/dc20-S009. [DOI] [PubMed] [Google Scholar]
- 32.National Kidney Foundation KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Das A, UnnikrishnanAG, Saboo B et al. Indian Guidance on Cardiovascular and Renal Comorbidity Management in Type-2 Diabetes Mellitus. 2018. https://www.japi.org/may_2018_special_issue/Indian_Guidance_on.pdf. Accessed 24 Aug 2019.
- 34.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 35.Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83:517–523. doi: 10.1038/ki.2012.401. [DOI] [PubMed] [Google Scholar]
- 36.Haller H, Ito S, Izzo JL, Jr, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 37.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 38.Bakris GL, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163:1555–1565. doi: 10.1001/archinte.163.13.1555. [DOI] [PubMed] [Google Scholar]
- 39.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 40.Chan GC, Tang SC. Diabetic nephropathy: landmark clinical trials and tribulations. Nephrol Dial Transplant. 2016;31:359–368. doi: 10.1093/ndt/gfu411. [DOI] [PubMed] [Google Scholar]
- 41.Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 42.Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204–2213. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 43.Packham DK, Wolfe R, Reutens AT, et al. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23:123–130. doi: 10.1681/ASN.2011040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Zeeuw D, Akizawa T, Audhya P, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 46.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019;34:ehz486. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 47.Skrtić M, Yang GK, Perkins BA, et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia. 2014;57:2599–2602. doi: 10.1007/s00125-014-3396-4. [DOI] [PubMed] [Google Scholar]
- 48.Verma S, McMurray JJV. SGLT2i and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka H, Takano K, Iijima H, et al. Factors affecting canagliflozin-induced transient urine volume increase in patients with type 2 diabetes mellitus. Adv Ther. 2017;34:436–451. doi: 10.1007/s12325-016-0457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woods TC, Satou R, Miyata K, Katsurada A, Dugas CM, Klingenberg NC. Canagliflozin prevents intrarenal angiotensinogen augmentation and mitigates kidney injury and hypertension in mouse model of type 2 diabetes mellitus. Am J Nephrol. 2019;49:331–341. doi: 10.1159/000499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshimoto T, Furuki T, Kobori H, et al. Effects of sodium-glucose cotransporter 2 inhibitors on urinary excretion of intact and total angiotensinogen in patients with type 2 diabetes. J Investig Med. 2017;65:1057–1061. doi: 10.1136/jim-2017-000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ansary TM, Nakano D, Nishiyama A. Diuretic effects of sodium glucose cotransporter 2 inhibitors and their influence on the renin-angiotensin system. Int J Mol Sci. 2019;20:629. doi: 10.3390/ijms20030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wanner C. EMPA-REG OUTCOME: the nephrologist's point of view. Am J Cardiol. 2017;120:S59–S67. doi: 10.1016/j.amjcard.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 54.Vallon V, Gerasimova M, Rose MA, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol. 2013;306:F194–F204. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ojima A, Matsui T, Nishino Y, Nakamura N, Yamagishi S. Empagliflozin, an inhibitor of sodium-glucose cotransporter 2 exerts anti-inflammatory and antifibrotic effects on experimental diabetic nephropathy partly by suppressing AGEs-receptor axis. Horm Metab Res. 2015;47:686–692. doi: 10.1055/s-0034-1395609. [DOI] [PubMed] [Google Scholar]
- 56.Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:457–466. doi: 10.1111/dom.12244. [DOI] [PubMed] [Google Scholar]
- 57.Liakos A, Karagiannis T, Athanasiadou E, et al. Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:984–993. doi: 10.1111/dom.12307. [DOI] [PubMed] [Google Scholar]
- 58.Devi R, Mali G, Chakraborty I, Unnikrishnan MK, Abdulsalim S. Efficacy and safety of empagliflozin in type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Postgrad Med. 2017;129:382–392. doi: 10.1080/00325481.2017.1259544. [DOI] [PubMed] [Google Scholar]
- 59.Cefalu WT, Stenlöf K, Leiter LA, et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58:1183–1187. doi: 10.1007/s00125-015-3547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilding JP, Blonde L, Leiter LA, et al. Efficacy and safety of canagliflozin by baseline HbA1c and known duration of type 2 diabetes mellitus. J Diabetes Complicat. 2015;29:438–444. doi: 10.1016/j.jdiacomp.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 61.Fioretto P, Del Prato S, Buse JB, et al. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): the DERIVE Study. Diabetes Obes Metab. 2018;20:2532–2540. doi: 10.1111/dom.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:369–384. doi: 10.1016/S2213-8587(13)70208-0. [DOI] [PubMed] [Google Scholar]
- 63.Guidance for Industry: Diabetes Mellitus — Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). 2008. https://www.fda.gov/media/71297/download. Accessed 17 Aug 2019.
- 64.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 65.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 66.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 67.Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study) Circulation. 2018;137:323–334. doi: 10.1161/CIRCULATIONAHA.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.JARDIANCE® (empagliflozin) tablets, for oral use[prescribing information on the Internet]. Initial U.S. Approval: 2014 [revised 2018 Oct]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/204629s018lbl.pdf. Accessed 28 Aug 2019.
- 69.U.S. FDA Approves INVOKANA® (canagliflozin) to Reduce the Risk of Heart Attack, Stroke or Cardiovascular Death in Adults with Type 2 Diabetes and Established Cardiovascular Disease. Available from: https://www.janssen.com/us-fda-approves-invokanar-canagliflozin-reduce-risk-heart-attack-stroke-or-cardiovascular-death. Accessed 28 Aug 2019.
- 70.FARXIGA® (dapagliflozin) tablets, for oral use [prescribing information on the Internet]. Initial U.S. Approval: 2014 [revised 2019 Oct]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202293s018lbl.pdf. Accessed 23 Dec 2019.
- 71.Dapagliflozin Receives Fast Track Designation for Chronic Kidney Disease. Available from: https://www.endocrinologyadvisor.com/home/topics/diabetes/type-2-diabetes/diabetes-tx-gains-fast-track-designation-for-kidney-disease/. Accessed 23 Dec 2019.
- 72.Nistor I, Bolignano D, Haller MC, et al. Why creating standardized core outcome sets for chronic kidney disease will improve clinical practice. Nephrol Dial Transplant. 2017;32:1268–1273. doi: 10.1093/ndt/gfv365. [DOI] [PubMed] [Google Scholar]
- 73.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64:821–835. doi: 10.1053/j.ajkd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 74.Heerspink HJ, Tighiouart H, Sang Y, et al. GFR decline and subsequent risk of established kidney outcomes: a meta-analysis of 37 randomized controlled trials. Am J Kidney Dis. 2014;64:860–866. doi: 10.1053/j.ajkd.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 75.Mol PG, Maciulaitis R, Vetter T. GFR decline as an end point for clinical trials in CKD: a view from Europe. Am J Kidney Dis. 2014;64:838–840. doi: 10.1053/j.ajkd.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Thompson A, Lawrence J, Stockbridge N. GFR decline as an end point in trials of CKD: a viewpoint from the FDA. Am J Kidney Dis. 2014;64:836–837. doi: 10.1053/j.ajkd.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Prischl FC, Wanner C. Renal outcomes of antidiabetic treatment options for type 2 diabetes—a proposed MARE definition. Kidney Int Rep. 2018;22(3):1030–1038. doi: 10.1016/j.ekir.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ridderstråle M, Andersen KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700. doi: 10.1016/S2213-8587(14)70120-2. [DOI] [PubMed] [Google Scholar]
- 79.Heerspink HJ, Johnsson E, Gause-Nilsson I, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18:590–597. doi: 10.1111/dom.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28:368–375. doi: 10.1681/ASN.2016030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 82.Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704. doi: 10.1016/S2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 83.Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018;138:1537–1550. doi: 10.1161/CIRCULATIONAHA.118.035901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu L, Li Y, Lang J, et al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibition on renal function and albuminuria in patients with type 2 diabetes: a systematic review and meta-analysis. PeerJ. 2017;5:e3405. doi: 10.7717/peerj.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grunberger G, Camp S, Johnson J, et al. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the VERTIS-RENAL randomized study. Diabetes Ther. 2018;9:49–66. doi: 10.1007/s13300-017-0337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606–617. doi: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 87.Fioretto P, Stefansson BV, Johnsson E, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia. 2016;59:2036–2039. doi: 10.1007/s00125-016-4017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cherney DZI, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:610–621. doi: 10.1016/S2213-8587(17)30182-1. [DOI] [PubMed] [Google Scholar]
- 89.Pollock C, Stefánsson B, Reyner D, et al. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:429–441. doi: 10.1016/S2213-8587(19)30086-5. [DOI] [PubMed] [Google Scholar]
- 90.Neuen BL, Young T, Heerspink HJL, et al. SGLT2i for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):P845–54. [DOI] [PubMed]
- 91.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 i for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 92.Das SR, Everett BM, Birtcher KK, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72:3200–3223. doi: 10.1016/j.jacc.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.American Diabetes Association (ADA). Living standards of medical care in diabetes. Updates to the Standards of Medical Care in Diabetes. Available from https://care.diabetesjournals.org/living-standards. Accessed 20 Sept 2019.
- 94.European Medical Agency.EMA confirms recommendations to minimise ketoacidosis risk with SGLT2 inhibitors for diabetes. Available from: https://www.ema.europa.eu/en/documents/referral/sglt2-inhibitors-article-20-procedure-ema-confirms-recommendations-minimise-ketoacidosis-risk-sglt2_en.pdf. Accessed 25 Dec 2019.
- 95.United States Food and Drug Administration. Drug safety communication. FDA warns about rare occurrences of a serious infection of the genitalarea with SGLT2 inhibitors for diabetes. Available from: https://www.fda.gov/media/115602/download. Accessed 25 Dec 2019.
- 96.Health Canada. Summary Safety Review - SGLT2 inhibitors (canagliflozin, dapagliflozin and empagliflozin). Available from: https://hpr-rps.hres.ca/reg-content/summary-safety-review-detail.php?lang=en&linkID=SSR00204. Accessed 25 Dec 2019.
- 97.Scheen AJ. An update on the safety of SGLT2 i. Expert Opin Drug Saf. 2019;18:295–311. doi: 10.1080/14740338.2019.1602116. [DOI] [PubMed] [Google Scholar]
- 98.Xiong W, Xiao MY, Zhang M, Chang F. Efficacy and safety of canagliflozin in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e5473. doi: 10.1097/MD.0000000000005473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng M, Lv H, Xu X, Wang J, Lyu W, Fu S. Efficacy and safety of dapagliflozin as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2019;98:e16575. doi: 10.1097/MD.0000000000016575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Donnan JR, Grandy CA, Chibrikov E, et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open. 2019;9:e022577. doi: 10.1136/bmjopen-2018-022577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.FDA Drug Safety Communication. FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-kidney-warnings-diabetes-medicines-canagliflozin. Accessed 22 Aug 2019.
- 102.Gilbert RE, Thorpe KE. Acute kidney injury with sodium-glucose co-transporter-2 inhibitors: a meta-analysis of cardiovascular outcome trials. Diabetes Obes Metab. 2019;21:1996–2000. doi: 10.1111/dom.13754. [DOI] [PubMed] [Google Scholar]
- 103.Lo C, Toyama T, Wang Y, et al. Insulin and glucose-lowering agents for treating people with diabetes and chronic kidney disease. Cochrane Database Syst Rev. 2018;9:CD011798. doi: 10.1002/14651858.CD011798.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.US Food and Drug Administration. FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-occurrences-serious-infection-genital-area-sglt2-inhibitors-diabetes. Accessed 28 Aug 2019.
- 105.Fitchett D. A safety update on sodium glucose co-transporter 2 inhibitors. Diabetes Obes Metab. 2019;21(Suppl 2):34–42. doi: 10.1111/dom.13611. [DOI] [PubMed] [Google Scholar]
- 106.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03433248, Renal Actions of Combined Empagliflozin and LINagliptin in Type 2 diabetES (RACELINES); 2018 Feb 14 [cited 2019 Aug 25]; [about 4 screens]. Available from: https://clinicaltrials.gov/ct2/show/NCT03433248.
- 107.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03594110, EMPA-KIDNEY (The study of heart and kidney protection with empagliflozin); 2018 Feb 20 [cited 2019 Aug 25]; [about 4 screens]. Available from: https://clinicaltrials.gov/ct2/show/NCT03594110.
- 108.Gomez-Peralta F, Abreu C, Lecube A, et al. Practical approach to initiating SGLT2 inhibitors in type 2 diabetes. Diabetes Ther. 2017;8:953–962. doi: 10.1007/s13300-017-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.ClinicalTrials.gov. Effects of Dapagliflozin in Non-diabetic Patients With Proteinuria (DIAMOND). https://clinicaltrials.gov/ct2/show/NCT03190694. Accessed 10 May 2020.
- 110.https://www.astrazeneca.com/media-centre/press-releases/2020/farxiga-phase-iii-dapa-ckd-trial-will-be-stopped-early-after-overwhelming-efficacy-in-patients-with-chronic-kidney-disease.html Accessed 10 May 2020.
- 111.Goldenberg RM, Berard LD, Cheng AYY, et al. SGLT2 inhibitor-associated diabetic ketoacidosis: clinical review and recommendations for prevention and diagnosis. Clin Ther. 2016;38(12):2654–64.e1. doi: 10.1016/j.clinthera.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 112.Fadini G, Avogaro A. SGTL2 inhibitors and amputations in the US FDA adverse event reporting system. Lancet Diabetes Endocrinol. 2017;5:680–681. doi: 10.1016/S2213-8587(17)30257-7. [DOI] [PubMed] [Google Scholar]
- 113.Khouri C, Cracowski JL, Roustit M. SGLT-2 inhibitors and the risk of lower-limb amputation: is this a class effect? Diabetes Obes Metab. 2018;20(6):1531–1534. doi: 10.1111/dom.13255. [DOI] [PubMed] [Google Scholar]
- 114.Fadini GP, Solini A, Manca ML, et al. Effectiveness of dapagliflozin versus comparators on renal endpoints in the real world: a multicentre retrospective study. Diabetes Obes Metab. 2019;21(2):252–260. doi: 10.1111/dom.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8:27–35. doi: 10.1016/S2213-8587(19)30384-5. [DOI] [PubMed] [Google Scholar]