Abstract
Therapeutic efficacy against cancer relies heavily on the ability of the therapeutic agents to reach their final targets. The optimal targets of most cancer therapeutic agents are usually biological macromolecules at the subcellular level, which play a key role in carcinogenesis. Therefore, to improve the therapeutic efficiency of drugs, researchers need to focus on delivering not only the therapeutic agents to the target tissues and cells but also the drugs to the relevant subcellular structures. In this review, we discuss the most recent construction strategies and release patterns of various cancer cell subcellular-targeting nanoformulations, aiming at providing guidance in the overall design of precise nanomedicine. Additionally, future challenges and potential perspectives are illustrated in the hope of enhancing anticancer efficacy and accelerating the translational progress of precise nanomedicine.
Subject terms: Drug delivery, Drug development
Introduction
Nanoparticle-based drug delivery systems (NDDSs) are extensively employed in the therapy, diagnosis, and imaging of cancer due to their characteristics of high cancer-targeting efficacy, low toxicity, and controlled release properties.1 An efficient drug delivery system must avoid the clearance of the reticuloendothelial system, penetrate across blood vessel walls and be enriched at cancer sites to exert their pharmacological effects.2 For this purpose, an ever-increasing number of preclinical studies have reported a large number of engineered nanoformulations with unique physical and chemical properties, with the goal of delivering chemotherapeutic agents, photosensitizers, genes, and other biomolecules to cancer cells in specific and efficient manners.3 However, due to the problems of multidrug resistance (MDR), high variability, and poor patient prognosis, NDDSs have still faced tremendous challenges. It is therefore necessary when designing new treatment strategies to study in-depth the pathogenesis of cancer.
With the development of precision medicine, researchers have realized that variations in key intracellular biomolecules (genes and proteins), which are usually at the subcellular level, play a critical role in carcinogenesis and cancer development.4–6 Designing drug candidates based on molecular-level pathogenesis has become a new pattern and trend of drug discovery. For example, Ying et al. found that the expression level of sterol o-acyltransferase 1, which is responsible for transforming cholesterol into cholesterol ester-storage granules, is closely related to the poor prognosis of patients with liver cancer. Based on this, the research team proved that avasimibe, a small molecular inhibitor of sterol o-acyltransferase 1, had a good antitumor effect on patient-derived tumor tissue xenograft model of hepatocellular carcinoma, and provided new treatment strategies for tumor patients.7 Moreover, high-profile gene therapies also have to deliver the therapeutic genes into the cytoplasm or nucleus, where they can function. As a result, effective NDDSs should not only carry the therapeutic agents to the target tissues and cells but also deliver the drugs to distinct subcellular sites which mean organelles as targets accurately. They are considered to be one of the most promising approaches for cancer treatment. Through their proper design and specific modifications, subcellular-targeting nanoformulations are enriched in tumor cells, are internalized by endocytosis across the subcellular barriers (such as inner body embedding and lysosomal degradation)8 and target-specific subcellular structures (as shown in Fig. 1). This is then followed by the controlled release of therapeutic agents at the target sites, thus improving their antitumor efficacy, reducing their toxic and side effects, and overcoming the most critical limitation of intracellular drug delivery—MDR.9
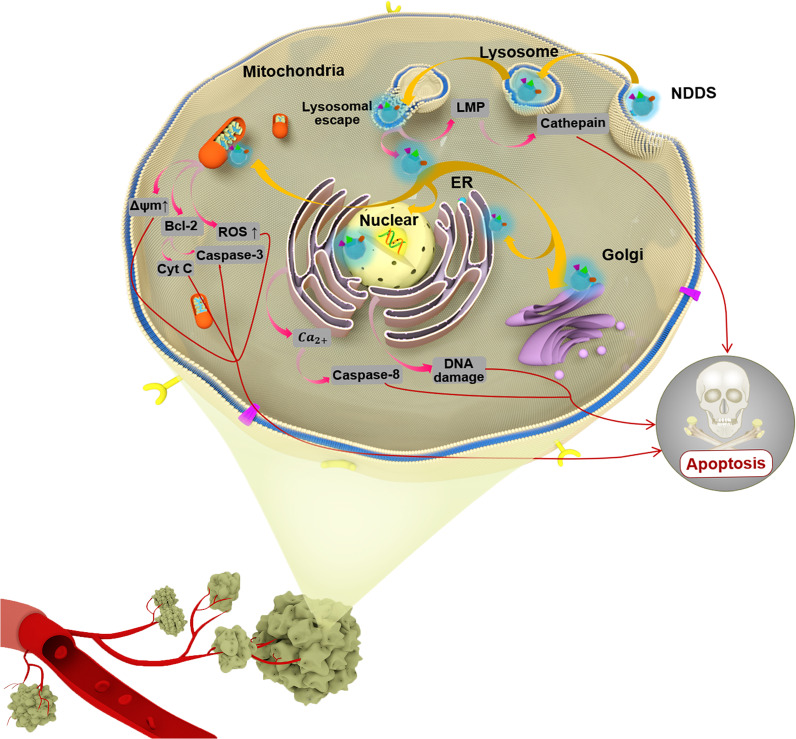
Fig. 1.
Schematic illustration of cancer cell subcellular targeting NDDSs for improving cancer therapeutic efficacy and different poptotic pathways mediated by different organelle-targeted NDDSs. NDDS nanoparticle-based drug delivery system, LMP lysosomal membrane permeabilization, ER endoplasmic reticulum, Bcl-3 B-cell lymphoma 3, ROS reactive oxygen species, Cyt C Cytochrome C
In this review, based on the latest research progress over the past 5 years, we will focus on the important aspects of subcellular-targeting nanoformulations for cancer therapy. First, relevant knowledge including the specific endocytosis pathway of different nanoformulations taken up into cells and the pathological characteristics of tumor cell organelles are the key elements for guiding the construction of NDDSs, especially for the selection of targeting ligands. Next, according to the different subcellular targets of commonly used anticancer therapeutic strategies (chemical therapy, gene therapy, photodynamic therapy (PDT), etc.) applied after surgery, this article will elaborate on how to achieve precise subcellular targeting by functionalizing the surface of nanoparticles (NPs) with ligands and other means in the order of lysosome, nucleus, mitochondria, endoplasmic reticulum (ER), and Golgi apparatus. Furthermore, we will point out that multiple targeting and controlled release are crucial to the design and overall construction of the subcellular-targeting NDDSs. Finally, two challenges and potential directions to pursue in order to boost precise subcellular targeting are illustrated, which will benefit the transformation of NDDSs from laboratory research to clinical practice.
Main
NDDSs can achieve the enrichment of tumor microenvironment, cell internalization, and intracellular delivery through passive or active targeting. In passive targeting, the size, shape, and surface charge of NPs can affect penetration and retention, thus significantly affecting their cell internalization and subcellular localization. For example, positively charged ultrasmall NPs have a higher affinity to the organelles such as mitochondria and nuclei, thereby promoting their intracellular permeability.10 Active targeting usually relies on the modification of localization group such as antibodies, ligands, etc., which have specific interaction with the receptor, thus leading to more significant effect than conventional treatment strategies. In intracellular transport and targeting, we still focus on these two aspects to explore design strategies of subcellular-targeting nanoformulations.
Endocytosis and intracellular trafficking of nanoformulations
There are many targets (such as folate receptors, transferrin (Tf) receptors, antigens) which are usually overexpressed on the surface of cancer cells, and targeting them to maximize the drug accumulation around cancer cells have become a focus research to cancer therapy in recent decades. When NPs reach the cell surface through passive or active targeting, endocytosis is the main mechanism by which they are taken up by cancer cells. Different types of NDDSs rely on different cell endocytosis mechanisms to enter the cell, which ensures they internalize in specific intracellular regions.11 We will briefly review the classic endocytosis pathways for better prediction of the intracellular fate of nanoformulations.
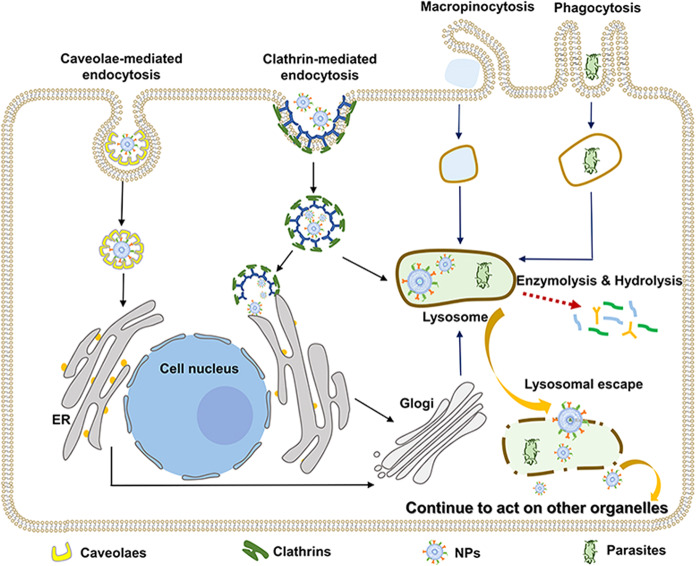
Endocytosis can be divided into clathrin-mediated endocytosis (CME), caveolae-mediated endocytosis (CVME), macropinocytosis, and phagocytosis12 (as shown in Fig. 2). Among these, CME and CVME are the major uptake pathways of various nanoformulations. Generally, large NPs (<120 nm) are internalized mainly through CME, and specific ligand-modified nanoformulations (e.g., epidermal growth factor, folic acid, chemokines, and Tf) can significantly improve the efficiency of this endocytosis pathway. Following CME, the nanoformulations are trafficked through the early endosomes—late endosomes—lysosomes pathway and arrive in the lysosomal lumen, where they may be degraded by lysosomal hydrolases.13 For those nanoformulations whose action sites are other subcellular localizations in the cytoplasm, they are supposed to be designed to avoid endosome/lysosome degradation and retain their biological activity. Using carrier materials that are stable in acidic environments and solution with pH buffering properties can alleviate degradation problem to a certain extent.14 Endosome/lysosome escape capability is a more effective prerequisite.15 The commonly recognized mechanisms of lysosomal escape include proton-sponge effect, membrane fusion, the generation of gas, and the application of CPPs and PCI. Some examples and applications used in nanomedicine are listed in Table 1. On the other hand, nanoformulations with a small particle size (<60 nm) usually rely on CVME to enter cells. These NPs coated by caveolae usually do not enter lysosomes and are directly transferred to the Golgi or ER.16,17 Other endocytosis processes are shown in Fig. 2. As is apparent, the endocytosis process of antitumor NPs is the key step to achieve subcellular enrichment. Deep understanding and exploration of these endocytic pathways are rather significant for developing new delivery strategies for subcellular targeting.
Fig. 2.
Schematic diagram depicting endocytosis and intracellular trafficking pathways of nanoformulations. NPs nanoparticles
Table 1.
The mechanisms and applications of lysosomal escape used in nanomedicine
| Mechanism of lysosomal escape | Device | The key structure for lysosomal escape | Cargoes | Cell line | Reference |
|---|---|---|---|---|---|
| Proton-sponge effect | catHDL/PA | Polyanions bearing both pendant carboxylate groups and alkyl chains | DOX and curcumin | T24 | 117 |
| N-quaternary ammonium-chitosan | Quaternary amine groups | Brucine | HepG2 | 70 | |
| MPN-Coated NPs | The phenolic molecules in the metal–phenolic networks(MPNs) | Calcein | MDA-MB-231 | 118 | |
| FL–C6-NH2-Modified CRP@dOSN | pH-responsive imine bonds | Chitosan | HeLa | 119 | |
| Polymeric-drug conjugate solid NPs containing encapsulated superparamagnetic iron oxide NPs (IO@PNP) | Poly(ethylene glycol)-block-poly(histidine) | DOX | PC3MM2 | 120 | |
| Polyurethane micelles | Hydrazone bonds | DOX | SKOV3 | 121 | |
| mPEG-b-PLA-PHis-ss-OEI | Disulfide bonds | DOX and siRNA | MCF-7/ADR | 122 | |
| Surface-modified single-walled carbon nanotube | Polyethylenimine (PEI)-betaine | DOX and siRNA | A549 | 123 | |
| GA-loaded cp-rHDL NPs (cp-rHDL/GA) | The histidine in CPPs | Gambogic acid (GA) | HepG2 and HT1080 | 124 | |
| Guanidino HPMA copolymer | Guanidine group | KLA peptide | B16F10 | 125 | |
| TPH/PTX nanomicelles | The positively charged nanomicelles and HA | PTX | A549 | 126 | |
| D-alpha-tocopheryl poly (ethylene glycol 1000) succinate and HA dual-functionalized cationic liposomes | The imidazole groups of histidine | PTX | MCF-7/MDR | 127 | |
| m-HA coating PEI-PCL/shRNA complexes | PEI-PCL | PTX and KIAA1199 specific shRNA | MDA-MB-231 | 128 | |
| Dextran nanogels with CAD adjuvant | Cationic amphiphilic drugs adjuvant (nonbiodegradable polymeric dextran NGs, inorganic propylamine-functionalized MSNPs, cationic LNPs, such as (PEGylated) DOTAP-DOPE liposomes, the lipofection reagent Lipofectamine RNAiMAX, and lipid NPs containing the ionizable lipid DLin-MC3-DMA) | siRNA | H1299 | 129 | |
| mPEG-PHis-PSD/PLL/siRNA NP | Poly(l-histidine) | siRNA | NSCLC | 130 | |
| Lipid NPs | The ionizable cationic lipid components | siRNA | HTB-177 | 131 | |
| UCNP (CD/Azo) -siRNA/PEG NPs | GE11 + /TH + NP | siRNA | MDA-MB-468 | 132 | |
| The cationic dextran nanogels | Cationic amphiphilic drugs | siRNA | H1299 | 133 | |
| pH/redox dual-sensitive unimolecular NPs | The imidazole groups | siRNA | MDA-MB-468 | 134 | |
| Poly(2-diethylaminoethyl methacrylate) around the silica nanoparticle core (PDEAEM@SNP) | The tertiary amine group of the PDEAEM shell | siRNA | MDA-MB-231 | 14 | |
| siRNA biomimetic nanocomposites modified by erythrocyte membrane | Citraconic anhydride grafted poly-l-lysine | siRNA | U87MG | 135 | |
| DSPE-PEG-uPA@CaP | The CaP shell | siRNA and Pt | MDA-MB-231 | 136 | |
| UA-GT/PAH-Cit/siRNA NCs | Imidazole-containing moieties | siVEGF | QGY-7703 | 137 | |
| Angiopep LipoPCB(Temozolomide + BAP/siTGF-β) | The zwitterionic lipid (distearoyl phosphoethanol-amine-polycarboxybetaine lipid) | Temozolomide and siTGF-β | GL261 | 138 | |
| PCI | MSNs tethered with lipid bilayers (MSN@tLB) | IR-780 | Zoledronic acid | MCF-7 | 139 |
| Photoactivatable Pt(IV) prodrug-backboned polymeric nanoparticle system (CNPPtCP/si(c-fos)) | Azide complexes | Pt and si (c-fos) | A2780DDP | 140 | |
| Glucose functionalized polydopamine NPs | Polydopamine | Bortezomib borate | MDA-MB-231 and MCF-10A | 141 | |
| C60-DEX-NH2 | Fullerenes | siRNA | MDA-MB-231 and 4T1 | 142 | |
| The lipoic acid and chlorin e6‐conjugated pullulan micelle | Ce6 | Chlorin e6 and DOX | HepG2, HeLa and HCT‐116 | 143 | |
| Membrane fusion | pBA‐CP‐pPEGA | The self‐assembly into tubisomes | \ | HEK293 | 144 |
| Cross-linked N-(2-hydroxypropyl) methacrylamide copolymer micelles | HA2 peptide (GLFEAIEGFIENGWEGMIDGWYG) | H1-S6A and F8A (H1) peptide | MCF-7 | 145 | |
| CPPs | TAT-conjugated iron oxide NPs | TAT peptide | \ | A549 | 146 |
| siRNA @ TMV-TAT | TAT peptide | siRNA | MHCC97-H/GFP | 147 | |
| siRNA /TAT-AP1-ELP complexes | TAT peptide | siRNA | 4T1 | 148 | |
| A hybrid nanoparticulate system based on a cationic helical polypeptide PPABLG (PPABDLG HNPs) | The helical structure of PPABLG features | siRNA | RAW 264.7 | 149 | |
| The generation of gas | siPol2 @ NPs | The chitosan-guanidinate-CO2 NPs | POLR2A siRNA | MDA-MB-453 TNBC | 150 |
| D/N-PDA/Hb@HA | The NO donor | DOX | HeLa | 151 |
Lysosomal accumulation
Many nanoformulations mediated by CME can actively accumulate in lysosomes at the end of the endocytosis pathway. Taking full advantage of this accumulation to delivery antitumor drugs that act on lysosomes can greatly simplify the complexity of the carriers’ design. Second, recent reports have demonstrated chloroquine and its derivatives,18 rapamycin,19 HSP70 antagonist,20 and cathepsin B21 can act on the lysosomes and their components to trigger lysosomal membrane permeabilization (LMP), which can bypass the classical caspase apoptosis pathway and thus produce antitumor effects on drug-resistant cells.22 Third, the lysosomal pathological features lay a foundation for precise drug release.23 Given the evidence discussed above, lysosomal targeting and destruction could represent potential pharmacological delivery strategies.
Lysosomal characteristics
Lysosomes are single-membrane acidic vesicles (pH 4.5–5.0) that contain more than 60 hydrolytic enzymes that can break down biomolecules (such as proteins, lipids, carbohydrates, and nucleic acids).24 They play important roles in maintaining cellular homeostasis, inducing cell apoptosis, nutrient sensing, and immune responses.22 However, malignant transformation usually leads to changes in lysosomal volume, composition, and subcellular localization. In cancer cells, increased lysosomal fragility caused by increases in sphingomyelin makes lysosomes more vulnerable to LMP in response to stimuli, such as surfactants, heat, and reactive oxygen species (ROS), thus causing cell death.25
Delivery strategies of lysosomal precise therapy
Receptor-mediated endocytosis can usually increase the possibility of the NPs’ final arrival in lysosomes,13 so ligand modifications play important roles in lysosomal targeting. When NDDSs are modified by the specific aptamer of receptors on the surface of tumor cells, such as Tf26 and the anti-human epidermal growth factor receptor-2 monoclonal antibody,27 the receptor–ligand complex is mediated by receptor–ligand interactions, collected into transport vesicles and delivered into the early endosome-late endosome-lysosome pathway, resulting in its accumulation in lysosomes. Owen et al. reported NPs modified by different anti-HER2 mAbs (trastuzumab and 73JIgG) that bind to different epitopes on HER2 have variable amounts reaching the lysosome.28 Lysosome-targeting fragments can also be used to promote lysosomal accumulation. For example, alkylated piperidine fragments could target lysosomes and then self-assemble to construct anticancer prodrug molecules.29 In addition to surface modification, other physicochemical properties of NPs affect the efficiency of lysosomal accumulation. Lysosomal accumulation of internalized NPs is related to NP rigidity, size,30 and surface charge,31 and smaller and softer NPs with certain positive and negative charges have much greater uptake rates into lysosomes in cancer cells. Therefore, the main means of delivering drugs to lysosomes is to design and develop the appropriate targeting sequences, assisted by optimizing the physical and chemical properties of nanoformulations.
After reaching lysosomes, NDDSs need to respond to the lysosomal microenvironment effectively to release their cancer therapeutic agents, which need to act rapidly on the lysosome and trigger LMP. This response mainly relies on some pH-sensitive liposomes and stimulus-responsive polymers containing specific pH-triggered switches (such as disulfide bonds,32 hydrazone bonds, acrylic acid, and diethylaminophenyl units33) and enzyme response switches (such as cathepsin B-sensitive dipeptide linker,34 glycosidic bond hydrolyzed by glycosidase,35 vSIRPα-probe activated by lysosomal endopeptidases36). Additional important triggering methods are the delivery of photosensitizers37 and magnetic agents24 to lysosomes by NDDSs. When the tumor is exposed to external near-infrared light or a magnetic field, the sensitive agents will produce a considerable amount of ROS and heat, stimulating the destruction of the fragile lysosomal membrane, and induce tumor cell death. As shown by Zhang et al., their novel photosensitizer supramolecular nanogel is sensitive to lysosomal pH and aggregates in the lysosomes for enhanced PDT of multidrug-resistant cancer.37
Nucleus targeting
Chemotherapy is still the cornerstone of cancer treatment and the vast majority of conventional chemotherapeutic drugs need to work in the nucleus of cancer cells to induce apoptosis.38 Alternatively, cancer gene therapy, which transfers genes (such as the CRISPR/Cas9 nuclease system, nucleic acid aptamers, DNA, and siRNA) to the chromosomes of tumor cells to regulate or replace abnormal genes, is gradually emerging.39 Their efficacy depends on the efficient transfer of the drugs or complete therapeutic exogenous gene into the nucleus.40 In recent studies, the nucleus has been commonly used as the site of action for free radicals and heat to cooperate with chemotherapy or gene therapy to improve the antitumor effect,41 which means transporting photosensitizers or theranostics to the nucleus to produce ROS with potentially damaging effects.42,43 However, the NDDSs targeting the cancer cell membrane generally only release foreign genes or anticancer agents into the cytoplasm, and then they can only enter the nucleus through free diffusion. The efficiency of diffusion is limited, and <1% of the therapeutic agents in the cytoplasm enter the nucleus and reach the final target.38 Therefore, enhanced therapeutic agent efficiency by nuclear targeted delivery is anticipated to be necessary for efficient cancer treatments and overcoming MDR.
Nuclear characteristics
The nucleus is the site of storage, replication, and transcription of genetic material and it plays important roles in cell proliferation, metabolism, growth, and differentiation. Due to the strong shielding effect of the bilayer nuclear membrane, nuclear pore complexes (NPCs) with lengths of ~90 nm and transverse diameters of 70 nm are the only channels for bidirectional exchange between the cytoplasm and nucleoplasm. The inner walls of NPCs are tethered with phenylalanine-glycine nucleoporins (FG Nups), thus limiting the inner diameter to only ~40 nm.44 As a result, the low efficiency of nuclear membrane penetration has greatly hindered applications of nuclear targeting NDDSs.
Construction strategies of cancer cell nucleus-targeting NDDSs
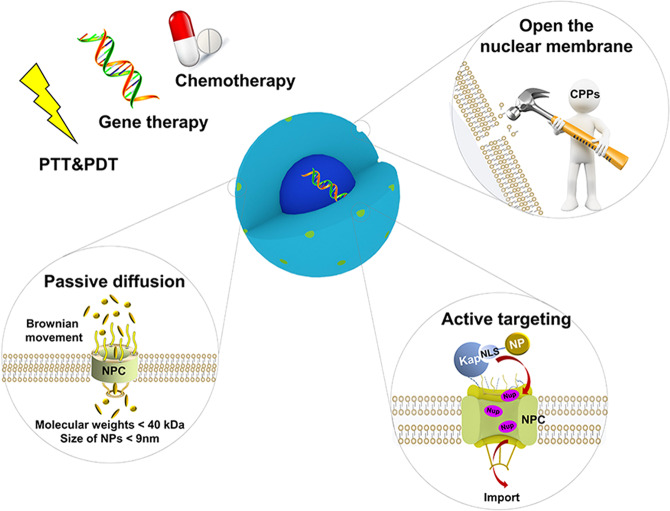
In general, the NDDSs’ ability to efficiently access the cancer cell nucleus from the cytoplasm arises from three aspects: passive diffusion, active targeting, and pore formation in the nuclear envelope membrane (as shown in Fig. 3).
Fig. 3.
Three construction ways for nucleus-targeting NDDSs to access the cancer cell nucleus from the cytoplasm: passive diffusion, active targeting, and pore formation in the nuclear envelope membrane. PTT photothermal therapy, PDT photodynamic therapy, NPC nuclear pore complex, CPPs cell membrane penetrating peptides, Kap karyopherin, NLS nuclear localization signal sequence, NP nanoparticle
Passive diffusion
The structure of the NPCs limits the translocation of nanoformulations into the nucleus by passive diffusion. Based on principles of Brownian motion, the key influencing factors of passive cancer cell nucleus-targeting NPs such as size, shape, and charge have been extensively studied as follows.
Size is the critical factor affecting the passive diffusion of NPs into the nucleus. Lim’s group has demonstrated that ions and small molecules with molecular weights <40 kDa can diffuse freely through the NPCs.44 For NDDSs, NPs capable of passive nuclear diffusion are generally smaller than 9 nm.45 Therefore, it is necessary for nucleus-targeting NPs to regulate their size by rational preparation or to achieve size reduction of large NPs activated by special pH conditions or enzymes.46 In particular, how to compress and fold gene macromolecules to minimize the size of the gene nanocarrier system should be considered. The existing research has mainly focused on how to condense DNA/RNA into stable complexes through the electrostatic interactions between cation nanocarriers and anion nucleic acids.39
Although small NPs are able to diffuse into the nucleus, the charge and shape of the NPs also play important roles in nuclear uptake. Positively charged NPs are more favorable for passage into the nucleus, but intravenous injection of positively charged NPs may induce hemolysis. To address this problem, a charge reversal strategy from negative to positive in endosomes and lysosomes has been applied.47 NDDSs that recover a positive charge in lysosomes can not only promote lysosomal escape but also enhance nuclear targeting, thus enhancing the cytotoxicity of the anticancer drug compared with free drugs.48 Other studies have shown that NPs with a higher aspect ratio (shaped like rods or worms) achieve higher nuclear concentrations compared with the lower aspect ratio NPs,49which can be ascribed to the structure of the NPCs.
Active targeting
Although the ultrasmall NPs can carry therapeutic agents into the cancer cells’ nucleus, most of the marketed NDDSs, whose sizes are usually between 100 and 200 nm, are excluded from the nucleus.38 Fortunately, NPs larger than NPC can realize nuclear active targeting by surface ligand modification after lysosomal escape.
Nuclear localization signal sequences (NLSs),50 including from the SV40 T antigen, adenovirus, transactivator of transcription (TAT) peptide, NF-κB, KRRRR et al.51,52 are the most classical ligands used for nuclear targeting. NLSs can be recognized by karyopherins (Kaps) and rapid binding between Kaps and FG Nups cause FG Nups to shrink back into more malleable forms.53,54 Therefore, NLSs modified NPs with a large particle size could enter the nucleus via active translocation. Thus far, most reported sizes of active nuclear targeting NDDSs were extended to 50 nm, which means gold NPs55 and mesoporous silica NPs (MSNs)56 have been extensively used in nucleus active targeting because of their advantages of easy control of particle size and surface modification. For example, Tang et al.57 synthesized copper sulfide NPs encapsulated by a silica shell layer, which were modified by RGD and TAT peptides at the same time. Mediated by RGD to enter cancer cells, these NPs can effectively target the nucleus with the help of TAT. When illuminated by a 980 nm laser, copper sulfide NPs release heat to rapidly increase the temperature and damage the DNA. Li et al.58 developed a kind of gold NPs with simultaneous surface modification of siRNA and NLSs. The NLS-mediated NPs translocated to the nucleus and the siRNA acted on gene promoter DNA methylation, thus inducing long-term gene silencing in the nucleus of cancer cells. Meanwhile, a promising strategy to transfer larger NPs to the nucleus involves optimizing the NLS density.59 For instance, compared to the high density of 2 NLS2/nm, NPs modified with the intermediate density of 0.9 NLS2/nm can achieve a 3.7-fold increased nuclear accumulation.60 In addition to NLSs ligands, boronic acid groups can also translocate anticancer NPs with a large size from the cell surface to the nucleus through the importin α/β-mediated pathway. In the future, the development and discovery of new NLSs will provide a wider range of options for targeted ligands of nuclear targeting NDDSs.
Opening the nuclear membrane
In addition to improving the physicochemical properties of the nanoformulations to pass through NPCs as readily as possible, another effective method is to open the nuclear membrane with the help of cell membrane penetrating peptides (CPPs)61 to enhance the nuclear translocation of antitumor NDDSs. Researchers have gradually mastered some common properties of CPPs and have synthesized a series of CPPs with stronger penetration and higher efficiency, such as CB5005,62 which consists of a membrane permeation sequence cascaded with the NF-κB NLS. Further study found this kind of CPP had a unique affinity to brain glioma and its application in adriamycin delivery could effectively penetrate the membranes of cancer cells and the nucleus, allowing the chemotherapy drugs to directly damage the DNA.
In short, nuclear delivery efficiency may depend on the physicochemical properties of the NPs including size, shape, charge, and surface modifications. Intensive study of these factors may allow for the development of efficient cell nucleus-targeting NDDSs. Meanwhile, besides light responses,41 further research is required to explore the means of controlling drug release from carriers in the nucleus.
Mitochondria targeting
As indispensable energy reservoirs, mitochondria are also important as targets of anticancer drugs. Lonidamine, amlodipine, ceramide, and some natural substances (resveratrol, berberine, betulinic acid) are the main antitumor therapeutic agents acting on mitochondria. They usually activate the apoptotic effector proteins Bax and Bak, release cytochrome c, and form apoptotic bodies, inducing cancer cells’ death.63,64 Paclitaxel (PTX), doxorubicin (DOX), and camptothecin, in addition to acting on recognized targets, also act on mitochondria to varying degrees to induce apoptosis.65
Mitochondria in cancer cells show greater susceptibility than those in normal tissues. Thus, there is the potential to deliver radiosensitizers,66 photosensitizers,67 and theranostics68 to the mitochondria of cancer cells, aiming at ROS production and oxidative stress, which induce mitochondrial permeability transitions and fundamentally affect the energy supply of cancer cells. All of the above have demonstrated that mitochondria targeting is of great significance for improving antitumor therapy.
Mitochondrial characteristics
Mitochondria are double-membrane-bound organelles with independent DNA69 and they participate in multiple cellular functions, including energy production, calcium buffering, lipid synthesis, signaling, cell proliferation, and apoptosis.70 In the process of Adenosine triphosphate synthesis, protons are pumped from the mitochondrial matrix to the intermembrane space, which generates a proton gradient and establishes the mitochondrial membrane potential (MMP, about −160 mv).Cancer cells tend to experience mitochondrial dysfunction, such as an increased MMP (−220 mv), accumulation of hydrogen peroxide, reduced oxidative phosphorylation, increased ROS production, Ca2+ overload, and the Warburg effect,71,72 which mean that mitochondria in cancer cells are more susceptible to external disturbances than normal cells.
Construction strategies of cancer cell mitochondria-targeting NDDSs
In view of the large negative MMP and the precise membrane structure of mitochondria in cancer cells, cancer cell mitochondria-targeting NDDSs usually achieve active subcellular targeting with the aid of two different targeting ligands: delocalized lipophilic cations (DLCs) and specific mitochondrial-targeting sequences (MTSs). Similarly, disturbing the mitochondrial membrane integrity by CPPs also helps NPs penetrate into mitochondria (as shown in Fig. 4).
Fig. 4.
Schematic illustration of mitochondria-targeted drug delivery strategies mediated by active targeting and CPPs. PDT photodynamic therapy, DLCs delocalized lipophilic cations, MMP mitochondrial membrane potential, TPP triphenylphosphonium, DQA di-quaternary ammonium, NHCs nitrogen-containing heterocycles, CPPs cell membrane penetrating peptides, MTSs mitochondrial-targeting sequences
Active targeting
DLCs, including 4-carboxybutyl triphenylphosphonium bromide (TPP), quaternary ammonium salts, nitrogen-containing heterocycles, et al.,73 can easily pass through lipid bilayers and accumulate in the mitochondrial matrix due to their high lipophilicity and stable cationic charge, so they have become the most popular constituent molecules in mitochondrial-targeting NDDSs.74 Among them, TPP has been the most extensively studied and it can both induce lysosomal escape and localize from the cytoplasm to the mitochondria. TPP-anchored poly(amidoamine) dendrimer,75 TPP-Lonidamine-DOX self-assembled NPs,76 PLGA-b-PEG NPs with surface modification of TPP,77 and silica NPs with surface modification of TPP78 can carry different therapeutic molecules to mitochondria in cancer cells. However, cationic materials represented by TPP induce inevitable systemic toxicity. Chemical modification of TPP or application of a core-shell structure, which shields the positive charges during circulation but is then removed in the lysosomal environment to expose the TPP, can maximize the safety of the drug. For instance, compared with liposomes where STPP is embedded in the lipid bilayer, liposomal loading with PTX and modification with a novel triphenyphosphonium-PEG-PE conjugate can more easily interact with the mitochondria and avoid the nonspecific cytotoxicity of STPP, to enhance their antitumor effects.79
In consideration of the safety concerns of DLCs, researchers have preferred to develop new MTSs to achieve precise intracellular localization. The precise membrane structure and internal structure of mitochondria provide a basis for determining the specific loci of MTSs. MTSs such as the KLA peptide80 and the amphipathic tail-anchoring peptide81 commonly contain 20–30 amino acids and α-helix structures that are rich in base, hydroxyl, and hydrophobic residues. Anticancer drugs, DNA, and nanocarriers conjugated to MTSs or DNA sequences encoding MTSs integrated into therapeutic DNA, are supposed to target mitochondria.82 For example, Kazuaki et al. designed a dual-function lipid-based drug delivery system that is capable of intracellular trafficking, such as endosomal escape mediated by octaarginine (a kind of CPP), and then delivery to mitochondria mediated by MTSs.83 However, it should be realized that extensive applications of MTSs are limited by their poor stability and their inability to target tumor locations. It is essential to improve the physicochemical and biopharmaceutical properties of these peptides and conjugate them with cancer cell-targeting fragments before clinical applications.
It is worth mentioning that the special mitochondrial microenvironment of cancer cells may be a trigger for drug release from vehicles. For example, Yue et al.84 reported the use of TL-CPT-PEG1K-TPP copolymers. After uptake by the cancer cells, the NPs were guided to the mitochondria by TPP, with hundreds of fold increased accumulation. The thioketal linker (TL) in the copolymers was sensitive to a high concentration of ROS in the cancer cells’ mitochondria to release CPT. Hu et al.85 constructed a kind of MSNs loaded with Fe2+. After mediated by the MTSs to enter the cancer cells’ mitochondria, the Fe2+ reacted with the increased amount of H2O2 and generated cell-damaging hydroxyl radicals. NIR exposure could promote chemical reactions and this delivery system has overcome the limitations that conventional PDT needs to rely on O2 and controllably exert their anticancer effects.
Opening the mitochondria envelope
Similar to the nuclear targeting delivery strategies, CPPs can also be used to enhance NDDSs penetration in accurate delivery of mitochondria targeting. Compared with the cell membrane, the mitochondrial membrane is more hydrophobic and has more negative potential, thus increasing the positive polarity and hydrophobicity of CPPs is conducive to helping nanoformulations cross the mitochondrial membrane. Commonly used mitochondrial CPPs generally have highly hydrophobic residues, such as cyclohexyl and SS peptides. In addition, some cationic small molecules, including rhodamine, pyridinium, and cyanine, which have inherent capabilities of mitochondrial penetration, can be modified on the surface of liposomes86 or self-assembled into NPs with imaging functions87 to selectively target the mitochondria.
In conclusion, an in-depth study of the cancer cells’ special mitochondrial microenvironment and development of novel targeting sequences may benefit the further design of efficient and safe mitochondrial-targeting NDDSs.
ER and Golgi targeting nanoformulations
With the development of modern oncology, the discovery of new and valuable anticancer targets and cellular pathways has fostered the study of cancer therapeutic agents acting on organelles other than the nucleus and mitochondria. The ER and Golgi have gradually attracted attention due to their large intracellular surface area and important roles in endocytosis.
Characteristics of the ER and Golgi
As the largest subcellular structure in the cell, the ER is a series of lamellar and tubular cavities composed of membranes that are weakly alkaline and it stores large amounts of calcium. ER controls the biosynthesis, folding, and assembly of proteins and other biological macromolecules, as well as playing an important role in cell survival and homeostasis.88 When stimulated, the ER will release calcium ions and active caspase-8 to initiate the apoptotic program.89 Eeyarestatin, bortezomib, natural polyphenols, terpenes, and other ER stress inducers90 have been identified.91 Delivering anticancer drugs to cause sustained and excessive ER stress, thus inducing cell death, has become a new anticancer strategy.
The Golgi apparatus is closely linked to the ER. It is usually comprised of three different compartments, including the cis-Golgi network, medial-Golgi, and trans-Golgi network, which have a pH gradient from cis-Golgi network (pH 6.7) to trans-Golgi network (pH 6.0).92 It is an important organelle of cell secretory pathways that can modify, label, store and transport proteins, lipids, and polysaccharides. Recent studies have shown that the Golgi’s function is significantly improved in cancer cells, and its structural integrity affects certain signaling pathways, particularly those related to migration, invasion, and angiogenesis.93,94 Therefore, delivering intra-Golgi protein inhibitors to cancer cells’ Golgi has the potential to block multiple molecular pathways associated with the development of cancer.
Design of ER or Golgi targeting nanoformulations
In the delivery process of therapeutic agents acting on the ER or Golgi apparatus, it is necessary to consider the different endocytosis pathways of NPs entering cancer cells. That is, mainly because the CVME pathway can actively transport NPs into the ER and Golgi. Obviously, it is very beneficial to deliver antitumor agents to achieve CVME by specific design of their nanoformulations. The other key to designing NDDSs is to enhance their retention time in the target substructure and to avoid their being discharged by exocytosis. For example, Xue et al. reported a pH-responsive photothermal ablation agent that was assembled with bovine serum albumin to form NPs. Due to their hypertrophic morphology, they could accumulate in the Golgi apparatus of cancer cells during endocytosis. Meanwhile, NPs can be activated for effective photothermal therapy in response to the acidic environment of the Golgi.95
In addition, we must take into consideration that a significant proportion of NDDSs enter the lysosomes of cancer cells through the CME. For these NPs, only by modifying the appropriate ER and Golgi target sequences can they selectively target to these subcellular organelles after endolysosomal escape to the cytoplasm. Studies have shown that several biocompatible metal complexes could be used to target the ER. Kwon et al. reported an effective strategy for IR(III) delivery targeting the ER. The IR(III) complex can not only target the ER actively but also produce ROS in response to the PDT reagent, which results in oxidative damage to proteins.96 E3/19K of adenovirus,97 phosphotetrapeptide (4P),98 KKXX peptide,99 propylene oxide,100 the sulfonyl group,101 and ER-targeting photosensitizer TCPP-TER102 have also been applied to construct NPs targeting the ER.
In terms of Golgi targeting, Huang et al. demonstrated that L-cysteine is a kind of effective ligand for the Golgi. Carbon quantum dots and silica NPs could target the Golgi to monitor its changes when they are modified with l-cysteine.103 Gong’s team repeatedly proved that chondroitin sulfate (CS) nanomicelles targeted the Golgi since the glycosyltransferases in the Golgi could specifically bind to CS.104,105
However, it should be pointed out that compared with the targeting of the mitochondria and nuclei, the subcellular targeting of the ER and Golgi is still in its infancy, with not enough information available to apply comprehensive design strategies.
Other subcellular-targeting nanoformulations
Apart from the above, there are several other important subcellular structures that are also susceptible to therapeutic agents.
The mutation of and abnormal expression of cytoskeleton-associated proteins play important roles in cancer cell migration, so targeting the cytoskeleton may be a potential anticancer therapy.106 For drugs (such as PTX and vincristine) acting on the cytoskeleton, the current delivery strategy is mainly to design NDDSs that are degraded in the lysosome, releasing the therapeutic agents into the cytoplasm through lysosome escape, and then achieve the drug targeting by the interaction between the drug molecules and the protein targets.107
As a complex of RNA and protein, many key molecules and proteins in ribosomes are secondary regulators of epigenetic regulation and cancer progression.108,109 In recent years, ribosomes have been gradually regarded as a potential target in the development of anticancer drugs.110,111 Discovery and delivery of drug molecules acting on ribosomes remains in a preliminary stage. Delivery of antitumor drugs to ribosomes will also be an important branch of subcellular targeting in the near future.
Key factors in the rational design of subcellular-targeting anticancer nanomedicine
The above has described different strategies of precise delivery of antitumor agents to subcellular organelles in cancer cells. To guide the rational design and clinical transformation of subcellular-targeting anticancer nanomedicine comprehensively, we will emphasize below two key factors and principles that need to be considered when constructing efficient nanomedicine.
Dual targeting and multiple targeting
The initial premise of the subcellular-targeting NDDSs discussed above is that they have overcome the first step of initial delivery and tend to accumulate in the region of the tumor. Therefore, in the rational design of subcellular targeting anticancer nanomedicine, we need to use dual-targeting strategies, taking into account both cancer cell targeting and subcellular targeting. For example, Qu’s team112 designed folate and TAT-modified Fe3O4 core/mesoporous silica shell NPs to deliver camptothecin, López et al.113 developed mesoporous silica particles with asymmetric modification of folate and TPP, and Xie et al.114 constructed hollow carbonitride nanospheres modified by hyaluronic acid (HA) and mitochondrial localization peptide D. These NDDSs achieved the organic combination of cancer enrichment and subcellular level targeting, which greatly improving the efficiency of the antitumor agents. Furthermore, it should be noted that the overlapping interactions between two target ligands and their relative densities may have influences on their targeting ability. Meanwhile, scientists are also making efforts to synthesize multifunctional targeting sequences, such as one sequence having both cancer-targeting and subcellular targeting functions or having both navigation and imaging functions.
In many cases, targeting only one organelle may not be able to reach the expected therapeutic effect. One solution chosen by scientists is to simultaneously target multiple subcellular organelles or structures. For example, Yao et al.115 have developed HA-modified hydroxyapatite (HAP) NPs (HAP-HA). HA acts as a tumor-targeting active ligand and can bind to the CD44 receptor overexpressed on the surface of cancer cells. HAP can load and deliver DOX to the nucleus and mitochondria of tumor cells to maximize the expected therapeutic effect. Multiple targeting is based on the principle of organelle interaction network and functional synergy. Achieve simultaneous targeting of mitochondria and nucleus, ER and nucleus, as well as ER and mitochondria is of great significance for enhancing therapeutic efficacy.
Accurate response and controlled release
The differences between nanoformulations and free drugs lie not only in the protection and transport by the carriers but also in the controllable release of the cargoes in specific locations.116 Thus, subcellular-targeting nanoformulations are supposed to release their payload in a controlled manner to ensure that the goods cannot be released before reaching the specific target, but only be released on demand when they reach the target successfully. This response relies on the characteristics of the microenvironment in different organelles, such as the acidity of lysosomes,32 the weak basicity of the ER, the weak acidity of the Golgi,95 and the high expression of ROS84 and H2O285 in the mitochondria. In-depth explorations of intracellular environments, components, and functionality will drive innovation in the development of promising subcellular-targeting NDDSs in the field of anticancer nanomedicine. It needs to be emphasized that in some programmed stimulus-response drug delivery systems, the use of two or more stimuli in sequential or coordinated action also requires comprehensive tests in vivo to achieve accurate spatiotemporal control of each trigger factor.
Conclusions and perspectives
With the development of medical biology and nanotechnology, research into and applications of subcellular-targeting NDDSs have become hot topics and trends over the past 5 years. Great advances in nanotechnology have stimulated the quick development of various subcellular-targeting nanoformulations as listed in Table 2. They are generally modified with subcellular-targeting function groups to efficiently cross through the intracellular obstacles and reach the molecular target, where they control their payloads release in response to the specific subcellular microenvironment (e.g., the acidic environment of the lysosome and Golgi). This direct delivery of therapeutic agents to their final destination maximizes the therapeutic efficacy of various cancer therapies. Although progress in preclinical studies has been made, we have to point out that some limitations still remain. Here, we list the current challenges and potential future directions of this topic.
Table 2.
Summary of the application of various subcellular-targeting nanoformulations in the past 5 years
| Subcellular structures | Targeting molecules | Cargoes | Vehicles | Size and zeta | Cell lines | Others | Reference |
|---|---|---|---|---|---|---|---|
| ER | TCPP-TER | Porphyrin | Ds-sP/TCPP-TER NPs | 100 nm | 4T1 cells | PDT | 102 |
| Fluorescent dansyl group | Tri-substituted triazine and 5-fluorouracil | A supramolecular self-assembled hexameric rosette structure | \ | HeLa cells | \ | 101 | |
| \ | DOX | Ag NPs | 75 nm | MCF-7/KCR cells | \ | 152 | |
| Phosphoric acid tetrapeptide (1P) | Phosphoric acid tetrapeptide (1P) | The crescent-shaped supramolecular assemblies | \ | HeLa cells | \ | 98 | |
| \ | Ir(III) | The Ir(III) complexes | \ | HEK293T, U-2 OS or HeLa cells | PDT | 96 | |
| Adenovirus E3/19 K protein | The tumor-associated antigen L6 | Cancer vaccine | \ | EL4-L6 cells and B16F10 cells | \ | 97 | |
| Golgi | CS | DOX and retinoic acid | CS nanomicelles | 40.2 ± 1.42 nm | Hepatic stellate cells | \ | 104 |
| CS | PTX and retinoic acid | CS nanomicelles | 192.7 ± 1.8 nm | 4T1-Luc cells | \ | 105 | |
| Cyanine dyes | BSA-pH-PTT | \ | HepG2 cells | PTT | 95 | ||
| L-cysteine | Carbon quantum dots | Silica NPs | 8.5 ± 3.5 nm | HEp-2 cells | \ | 103 | |
| Lysosome | Anti-HER2 mAb | \ | Antibody drug conjugate polymeric NPs | \ | BT-474 cells(HER2+ cell line) | \ | 28 |
| Anti-HER2 aptamer (human epidermal growth factor receptor-2, HApt) | \ | Gold nanostars | 90 nm, −8.05 mv | SK-BR-3 cells | \ | 27 | |
| Tf | Dihydroartemisinin | Nanoscale Graphene oxide | 100–200 nm | EMT6 cells | \ | 26 | |
| EGF | \ | Iron oxide magnetic NPs | 14 ± 4 nm | MDA-MB-231 cells | Magnetic fluid hyperthermia | 24 | |
| \ | Photosensitizer | Supramolecular nanogels and organosilica nanodots | 75 nm | A549/DDP cells | \ | 37 | |
| Alkylated piperidine fragment | Ferrocene analogs | N‐alkylamino ferrocene‐based prodrugs | \ | BL-2 cells | The prodrug reacts with ROS. | 29 | |
| \ | 5,6-dimethylxanthenone-4-acetic acid | Direct-acting antiviral NPs | 55 ± 2 nm | HeLa cells | PTT/PDT | 33 | |
| Mitochondria | HA | Coumarin-6 | HA/PEG/BD Nanodrugs | 150 nm | A549 cells | \ | 74 |
| TPP | BSA, MAO-A, Cetuximab, IgG, or anti-MTCO2 | CPD–TPP–protein@BS–NPs | \ | HeLa, HepG2, and SH-SY5Y cells | \ | 78 | |
| TPP | Lonidamine and DOX | TPP-LND-DOX NPs | 110 nm | 4T1, MCF-7, and MCF-7/ADR cells | \ | 76 | |
| TPP | Lonidamine and α-tocopheryl succinate | poly(D,L-lactic-co-glycolic acid)-block (PLGA-b)-poly(ethylene glycol)-TPP polymer | \ | HeLa, IMR-32, and 3T3-L1 cells | \ | 77 | |
| TPP | α-tocopheryl succinate and obatoclax moieties | TOS-TPP-Obt-NPs | 131.6 nm, 42.9 ± 1.20 mV | MDA-MB-231 cells | \ | 153 | |
| TPP | Gd | TiO2(Gd) NPs | 17.6 ± 0.1 mV | MCF-7 cells | Radiation therapy | 66 | |
| TPP | \ | Poly(amidoamine) dendrimer | \ | HeLa cells | \ | 75 | |
| DSPE-PEG2K-TPP | Lonidamine and IR-780 | Thermosensitive liposomes | 125.0 ± 63.36 nm, 23.5 ± 3.12 mV | Lewis Lung Carcinoma cells | PTT/PDT | 68 | |
| TPP-PEG-PE | PTX | Liposome | 145–175 nm,1.66 ± 5.49 mV | HeLa cells | \ | 79 | |
| \ | Fenton reagent | Upconversion NPs | \ | HepG2 cells | \ | 85 | |
| Nuclear | Triplex-forming oligonucleotides | \ | Tiopronin-covered gold NPs (Au-TIOP NPs) | <10 nm | MCF-7 cells | \ | 45 |
| Acridine based compounds | Chlorambucil | Acridin-9-methanol NPs | 60 nm | HeLa cells | \ | 154 | |
| AS1411 aptamers | Ce6 | Ca-AS1411/Ce6/hemin@pHis-PEG (CACH-PEG) NCP | \ | 4T1 cells | PDT | 42 | |
| DGR or RGD, and KRRRR | Antisense single‐stranded DNA oligonucleotide | TD NCP/ASO-NPs | 76–198 nm | MDA-MB-231 cells | Gene interference therapies | 52 | |
| FA and TAT | Camptothecin | MSNs | \ | HeLa and A549 cells | Magnetic guidance | 112 | |
| H1 peptide | HA2 | Cross-linked N-(2-hydroxypropyl) methacrylamide copolymer micelles | \ | MCF-7 cells | \ | 145 | |
| Membrane-permeable sequence (CB5005M) | DOX | \ | \ | Human glioma cells (U87) | Coordinately administered | 62 | |
| NLS | siRNA | Au NPs | \ | MCF-7, HeLa, and HepG2 cells | \ | 58 | |
| NLS | Iridium (III) | LNPdePEG-FA | 150 nm | HeLa cells | \ | 46 | |
| NLS | \ | PPAP-DMA | 150.6 ± 15.6 nm | HeLa cells | PDT | 43 | |
| NLS | Photosensitizer | Exosomes | 132.6 nm | 4T1 cells | PDT | 41 | |
| NLS | Albumin-Rhodamine | Chitosan NPs | 150 nm | L929 cells | \ | 59 | |
| RGD and NLS | \ | Au NPs | \ | HSC-3 cells | \ | 55 | |
| TAT | DOX | MSNs | 43 nm | MCF-7/ADR cells | \ | 155 | |
| RGD and TAT | CuS | CuS@MSN-TAT-RGD NPs | 40 nm, −23.9 ± 0.7 mV | HeLa cells | PTT | 57 | |
| TAT | DOX | MSNs | 25/50/67/105 nm | HeLa cells | \ | 56 | |
| TAT | DOX | NaYF4:Er/Yb@NaGdF4–PEG | 58.8 nm | HeLa cells | \ | 54 | |
| TAT | anti-p65 antibody and TAT peptide | MSNs | 40 nm | 4T1 cells | \ | 156 | |
| HA and TAT | 9-Nitro-20(S)-camptothecin | CHR–PCL–TAT–ALAL–HA (HATPC) micelles | 121.6 ± 5.79 nm | SKOV3 tumor cells | \ | 51 | |
| Nuclear and mitochondria | HA and HAP | DOX | Hydroxyapatite NPs | 179.50 ± 24.50 nm | HepG2 cells | \ | 115 |
| HA and KLA | DOX | Carbon nitride nanosphere | 236.53 nm | A549 cells | \ | 114 |
In terms of cell biology research, the current progress related to the fate of subcellular-targeting nanomedicine may involve some uncertainties. (1) There is controversy since different researchers have come to different conclusions about the endocytosis pathway and mechanism of the same type of nanoformulation. (2) There is a lack of support from raw data and targeted research related to the stability of most currently existing nanoformulations in lysosomes, especially regarding how to ensure subcellular-targeting groups are able to function after escaping from the lysosome. (3) The intertumor heterogeneity is currently less considered in the design of subcellular-targeting NDDSs, which are mainly based on the common pathological features of organelles. Therefore, there is an urgent need for more comprehensive studies on different types of cancer cells (such as MDR cells) at the organelle/molecular level. In addition, precision medicine is based on gene mutation information, and individualized treatment, especially in subcellular delivery of gene therapeutic agents, should pay more attention to understanding the internal regulation of living systems by combining them with gene sequencing technology.
In terms of clinical transformation, the translation efficiency of complex nanoformulations is quite low. A high targeting ability of multiple modified structures is closely related to their instability, and a high sensitivity to intracellular environmental changes is often accompanied by systemic toxicity. This imbalance between efficacy and side effects makes demands on the exploration of multifunctional targeting groups (e.g., have both cancer-targeting and subcellular-targeting functions, have both navigation and imaging functions) on the one hand, and drives the development of diversification triggering and release strategies at the subcellular level (especially the nucleus) on the other hand. Furthermore, exploiting controllable preparation of nanoformulations in combination with other novel techniques such as microfluidic technology will control or optimize their properties more accurately.
In terms of monitoring methods, observing dynamic nanoformulations’ behavior in vivo and in tumor cells is indispensable to the biological and medical research of nanomedicine. However, the various visualization imaging techniques in the field of nanomedicine have their own advantages and disadvantages. For instance, the analysis conducted by transmission electron microscopy is static while having high resolution. Two-photon microscopy can observe tumor tissues directly in real time and in vivo, but it is limited by the imaging depth and the resolution at the subcellular level. Most of the organelle fluorescent dyes need to be used after cell membrane rupture and inactivation. To solve these problems will require complementation with numerous technologies on the basis of the existing tools, especially imaging methods for visualizing the actual process of nanoformulations entering single cancer cells. In addition, subcellular pharmacokinetics also affect the final efficacy of nanomedicine and should be paid more attention to, since it can be used for screening and transformation.
In general, subcellular-targeting NDDS are expected to play a greater role in cancer treatment and, where appropriate, of other diseases. It is also an inevitable trend in the field of personalized cancer medicine and precision nanomedicine. This review emphasizes the importance of subcellular targeting in the precise treatment of tumors, and encourages the development of novel subcellular-targeting strategies. The application of multidisciplinary and more concentrated efforts in the research into subcellular-targeting NDDSs and clinical transformation can further enhance our understanding of personalized cancer medicine for precise treatment and effectively guide the future design of nanoformulations.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21873057), Shandong Provincial Natural Science Foundation of China (Grant No. ZR2019MB041), the Major Basic Research Project of Shandong Natural Science Foundation, P.R. China (Grant No. ZR2018ZC0232) and the Fundamental Research Funds of Shandong University (Grant No. 2018JC006).
Competing interests
The authors declare no competing interests.
References
- 1.van der Meel R, et al. Smart cancer nanomedicine. Nat. Nanotechnol. 2019;14:1007–1017. doi: 10.1038/s41565-019-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai Y, Xu C, Sun X, Chen X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem. Soc. Rev. 2017;46:3830–3852. doi: 10.1039/C6CS00592F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stratton MR. Exploring the genomes of cancer cells: progress and promise. Science. 2011;331:1553–1558. doi: 10.1126/science.1204040. [DOI] [PubMed] [Google Scholar]
- 5.Rozenblatt-Rosen O, et al. The Human Tumor Atlas Network: charting tumor transitions across space and time at single-cell resolution. Cell. 2020;181:236–249. doi: 10.1016/j.cell.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murciano-Goroff YR, Taylor BS, Hyman DM, Schram AM. Toward a more precise future for oncology. Cancer Cell. 2020;37:431–442. doi: 10.1016/j.ccell.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Y, et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature. 2019;567:257–261. doi: 10.1038/s41586-019-0987-8. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, et al. Design of nanocarriers based on complex biological barriers in vivo for tumor therapy. Nano Today. 2017;15:56–90. doi: 10.1016/j.nantod.2017.06.010. [DOI] [Google Scholar]
- 9.Rajendran L, Knölker HJ, Simons K. Subcellular targeting strategies for drug design and delivery. Nat. Rev. Drug Discov. 2010;9:29–42. doi: 10.1038/nrd2897. [DOI] [PubMed] [Google Scholar]
- 10.Kang JW, So PTC, Dasari RR, Lim DK. High resolution live cell Raman imaging using subcellular organelle-targeting SERS-sensitive gold nanoparticles with highly narrow intra-nanogap. Nano Lett. 2015;15:1766–1772. doi: 10.1021/nl504444w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu CG, Han YH, Kankala RK, Wang SB, Chen AZ. Subcellular performance of nanoparticles in cancer therapy. Int. J. Nanomedicine. 2020;15:675–704. doi: 10.2147/IJN.S226186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundquist P, Artursson P. Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev. 2016;106:256–276. doi: 10.1016/j.addr.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Xu S, Olenyuk BZ, Okamoto CT, Hamm-Alvarez SF. Targeting receptor-mediated endocytotic pathways with nanoparticles: rationale and advances. Adv. Drug Deliv. Rev. 2013;65:121–138. doi: 10.1016/j.addr.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khaled SZ, et al. One-pot synthesis of pH-responsive hybrid nanogel particles for the intracellular delivery of small interfering RNA. Biomaterials. 2016;87:57–68. doi: 10.1016/j.biomaterials.2016.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Z, Ukidve A, Kim J, Mitragotri S. Targeting strategies for tissue-specific drug delivery. Cell. 2020;181:151–167. doi: 10.1016/j.cell.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Q, et al. Enzyme-activatable polymer–drug conjugate augments tumour penetration and treatment efficacy. Nanotechnol. 2019;14:799–809. doi: 10.1038/s41565-019-0485-z. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Yu W, Chen Z, Zhang J, Zhang N. Enhanced gene transfection efficiency in CD13-positive vascular endothelial cells with targeted poly(lactic acid)-poly(ethylene glycol) nanoparticles through caveolae-mediated endocytosis. J. Control. Release. 2011;151:162–175. doi: 10.1016/j.jconrel.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, et al. Targeting autophagy enhances the anticancer effect of artemisinin and its derivatives. Med. Res. Rev. 2019;39:2172–2193. doi: 10.1002/med.21580. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence RE, et al. Structural mechanism of a Rag GTPase activation checkpoint by the lysosomal folliculin complex. Science. 2019;366:971–977. doi: 10.1126/science.aax0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen W, Liu W, Shao Y, Chen L. VER-155008, a small molecule inhibitor of HSP70 with potent anti-cancer activity on lung cancer cell lines. Exp. Biol. Med. 2014;239:638–645. doi: 10.1177/1535370214527899. [DOI] [PubMed] [Google Scholar]
- 21.Matarrese P, et al. Cathepsin B inhibition interferes with metastatic potential of human melanoma: an in vitro and in vivo study. Mol. Cancer. 2010;9:207. doi: 10.1186/1476-4598-9-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galluzzi L, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domagala A, et al. Typical and atypical inducers of lysosomal cell death: a promising anticancer strategy. Int. J. Mol. Sci. 2018;19:2256. doi: 10.3390/ijms19082256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domenech M, Marrero-Berrios I, Torres-Lugo M, Rinaldi C. Lysosomal membrane permeabilization by targeted magnetic nanoparticles in alternating magnetic fields. ACS Nano. 2013;7:5091–5101. doi: 10.1021/nn4007048. [DOI] [PubMed] [Google Scholar]
- 25.Erdal H, et al. Induction of lysosomal membrane permeabilization by compounds that activate p53-independent apoptosis. Proc. Natl Acad. Sci. USA. 2005;102:192–197. doi: 10.1073/pnas.0408592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Wei Y, Zhai S, Chen Q, Xing D. Dihydroartemisinin and transferrin dual-dressed nano-graphene oxide for a pH-triggered chemotherapy. Biomaterials. 2015;62:35–46. doi: 10.1016/j.biomaterials.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 27.Lee H, Dam DHM, Ha JW, Yue J, Odom TW. Enhanced human epidermal growth factor receptor 2 degradation in breast cancer cells by lysosome-targeting gold nanoconstructs. ACS Nano. 2015;9:9859–9867. doi: 10.1021/acsnano.5b05138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen SC, et al. Targeting HER2 + breast cancer cells: lysosomal accumulation of anti-HER2 antibodies is influenced by antibody binding site and conjugation to polymeric nanoparticles. J. Control. Release. 2013;172:395–404. doi: 10.1016/j.jconrel.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Daum S, et al. Lysosome-targeting amplifiers of reactive oxygen species as anticancer prodrugs. Angew. Chemie Int. Ed. 2017;56:15545–15549. doi: 10.1002/anie.201706585. [DOI] [PubMed] [Google Scholar]
- 30.Huang WC, et al. Engineering chimeric receptors to investigate the size- and rigidity-dependent interaction of pegylated nanoparticles with cells. ACS Nano. 2016;10:648–662. doi: 10.1021/acsnano.5b05661. [DOI] [PubMed] [Google Scholar]
- 31.Borkowska M, et al. Targeted crystallization of mixed-charge nanoparticles in lysosomes induces selective death of cancer cells. Nat. Nanotechnol. 2020;15:331–341. doi: 10.1038/s41565-020-0643-3. [DOI] [PubMed] [Google Scholar]
- 32.Sun B, et al. Disulfide bond-driven oxidation- and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano Lett. 2018;18:3643–3650. doi: 10.1021/acs.nanolett.8b00737. [DOI] [PubMed] [Google Scholar]
- 33.Liang P, et al. Tumor-microenvironment-responsive nanoconjugate for synergistic antivascular activity and phototherapy. ACS Nano. 2018;12:11446–11457. doi: 10.1021/acsnano.8b06478. [DOI] [PubMed] [Google Scholar]
- 34.Yu N, et al. Dually enzyme- and acid-triggered self-immolative ketal glycoside nanoparticles for effective cancer prodrug monotherapy. Nano Lett. 2020;20:5465–5472. doi: 10.1021/acs.nanolett.0c01973. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, et al. Controlled loading of albumin-drug conjugates ex vivo for enhanced drug delivery and antitumor efficacy. J. Control. Release. 2020;13:S0168–S3659. doi: 10.1016/j.jconrel.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Ko YJ, et al. Versatile activatable vSIRPα-probe for cancer-targeted imaging and macrophage-mediated phagocytosis of cancer cells. J. Control. Release. 2020;323:376–386. doi: 10.1016/j.jconrel.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, et al. Endosome/lysosome-detained supramolecular nanogels as an efflux retarder and autophagy inhibitor for repeated photodynamic therapy of multidrug-resistant cancer. Nanoscale Horiz. 2020;5:481–487. doi: 10.1039/C9NH00643E. [DOI] [PubMed] [Google Scholar]
- 38.Pan L, Liu J, Shi J. Cancer cell nucleus-targeting nanocomposites for advanced tumor therapeutics. Chem. Soc. Rev. 2018;47:6930–6946. doi: 10.1039/C8CS00081F. [DOI] [PubMed] [Google Scholar]
- 39.Hu Q, Li H, Wang L, Gu H, Fan C. DNA nanotechnology-enabled drug delivery systems. Chem. Rev. 2019;119:6459–6506. doi: 10.1021/acs.chemrev.7b00663. [DOI] [PubMed] [Google Scholar]
- 40.Van Der Aa MAEM, et al. The nuclear pore complex: the gateway to successful nonviral gene delivery. Pharm. Res. 2006;23:447–459. doi: 10.1007/s11095-005-9445-4. [DOI] [PubMed] [Google Scholar]
- 41.Cheng H, et al. Chimeric peptide engineered exosomes for dual-stage light guided plasma membrane and nucleus targeted photodynamic therapy. Biomaterials. 2019;211:14–24. doi: 10.1016/j.biomaterials.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, et al. G-quadruplex-based nanoscale coordination polymers to modulate tumor hypoxia and achieve nuclear-targeted drug delivery for enhanced photodynamic therapy. Nano Lett. 2018;18:6867–6875. doi: 10.1021/acs.nanolett.8b02732. [DOI] [PubMed] [Google Scholar]
- 43.Han K, et al. Acidity-triggered tumor-targeted chimeric peptide for enhanced intra-nuclear photodynamic therapy. Adv. Funct. Mater. 2016;26:4351–4361. doi: 10.1002/adfm.201600170. [DOI] [Google Scholar]
- 44.Sakiyama Y, Mazur A, Kapinos LE, Lim RYH. Spatiotemporal dynamics of the nuclear pore complex transport barrier resolved by high-speed atomic force microscopy. Nat. Nanotechnol. 2016;11:719–723. doi: 10.1038/nnano.2016.62. [DOI] [PubMed] [Google Scholar]
- 45.Huo S, et al. Ultrasmall gold nanoparticles as carriers for nucleus-based gene therapy due to size-dependent nuclear entry. ACS Nano. 2014;8:5852–5862. doi: 10.1021/nn5008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan Y, Li C, Li F, Chen D. PH-activated size reduction of large compound nanoparticles for in vivo nucleus-targeted drug delivery. Biomaterials. 2016;85:30–39. doi: 10.1016/j.biomaterials.2016.01.057. [DOI] [PubMed] [Google Scholar]
- 47.Xu P, et al. Targeted charge-reversal nanoparticles for nuclear drug delivery. Angew. Chemie Int. Ed. 2007;46:4999–5002. doi: 10.1002/anie.200605254. [DOI] [PubMed] [Google Scholar]
- 48.Zhou, Z. et al. Charge-reversal drug conjugate for targeted cancer cell nuclear drug delivery. Adv. Funct. Mater. 10.1002/adfm.200900825 (2009).
- 49.Hinde E, et al. Pair correlation microscopy reveals the role of nanoparticle shape in intracellular transport and site of drug release. Nat. Nanotechnol. 2017;12:81–89. doi: 10.1038/nnano.2016.160. [DOI] [PubMed] [Google Scholar]
- 50.Cheng Y, et al. Dual-targeted peptide-conjugated multifunctional fluorescent probe with AIEgen for efficient nucleus-specific imaging and long-term tracing of cancer cells. Chem. Sci. 2017;8:4571–4578. doi: 10.1039/C7SC00402H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Y, et al. Novel polymeric micelles as enzyme-sensitive nuclear-targeted dual-functional drug delivery vehicles for enhanced 9-nitro-20(: S)-camptothecin delivery and antitumor efficacy. Nanoscale. 2020;12:5380–5396. doi: 10.1039/C9NR10574C. [DOI] [PubMed] [Google Scholar]
- 52.Cheng Y, et al. A multifunctional peptide-conjugated AIEgen for efficient and sequential targeted gene delivery into the nucleus. Angew. Chemie Int. Ed. 2019;58:5049–5053. doi: 10.1002/anie.201901527. [DOI] [PubMed] [Google Scholar]
- 53.Kapinos LE, Huang B, Rencurel C, Lim RYH. Karyopherins regulate nuclear pore complex barrier and transport function. J. Cell Biol. 2017;216:3609–3624. doi: 10.1083/jcb.201702092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu JN, et al. Simultaneous nuclear imaging and intranuclear drug delivery by nuclear-targeted multifunctional upconversion nanoprobes. Biomaterials. 2012;33:7282–7290. doi: 10.1016/j.biomaterials.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 55.Kang B, Mackey MA, El-Sayed MA. Nuclear targeting of gold nanoparticles in cancer cells induces DNA damage, causing cytokinesis arrest and apoptosis. J. Am. Chem. Soc. 2010;132:1517–1519. doi: 10.1021/ja9102698. [DOI] [PubMed] [Google Scholar]
- 56.Pan L, et al. Nuclear-targeted drug delivery of tat peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 2012;134:5722–5725. doi: 10.1021/ja211035w. [DOI] [PubMed] [Google Scholar]
- 57.Li N, et al. Nuclear-targeted photothermal therapy prevents cancer recurrence with near-infrared triggered copper sulfide nanoparticles. ACS Nano. 2018;12:5197–5206. doi: 10.1021/acsnano.7b06870. [DOI] [PubMed] [Google Scholar]
- 58.Li N, et al. Nuclear-targeted siRNA delivery for long-term gene silencing. Chem. Sci. 2017;8:2816–2822. doi: 10.1039/C6SC04293G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tammam SN, Azzazy HME, Breitinger HG, Lamprecht A. Chitosan nanoparticles for nuclear targeting: the effect of nanoparticle size and nuclear localization sequence density. Mol. Pharm. 2015;253:30–36. doi: 10.1021/acs.molpharmaceut.5b00478. [DOI] [PubMed] [Google Scholar]
- 60.Tammam SN, Azzazy HME, Lamprecht A. The effect of nanoparticle size and NLS density on nuclear targeting in cancer and normal cells; impaired nuclear import and aberrant nanoparticle intracellular trafficking in glioma. J. Control. Release. 2017;253:30–36. doi: 10.1016/j.jconrel.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 61.Xu J, et al. Cell-penetrating peptide: a means of breaking through the physiological barriers of different tissues and organs. J. Control. Release. 2019;309:106–124. doi: 10.1016/j.jconrel.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, et al. Functionalized cell nucleus-penetrating peptide combined with doxorubicin for synergistic treatment of glioma. Acta Biomater. 2016;42:90–101. doi: 10.1016/j.actbio.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 63.Jeena MT, Kim S, Jin S, Ryu JH. Recent progress in mitochondria-targeted drug and drug-free agents for cancer therapy. Cancers (Basel) 2020;12:4. doi: 10.3390/cancers12010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou J, et al. The anticancer efficacy of paclitaxel liposomes modified with mitochondrial targeting conjugate in resistant lung cancer. Biomaterials. 2013;34:3626–3638. doi: 10.1016/j.biomaterials.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 65.Malhi SS, et al. Intracellular delivery of redox cycler-doxorubicin to the mitochondria of cancer cell by folate receptor targeted mitocancerotropic liposomes. Int. J. Pharm. 2012;432:63–74. doi: 10.1016/j.ijpharm.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 66.Chen Y, et al. Enhancement of mitochondrial ROS accumulation and radiotherapeutic efficacy using a Gd-doped titania nanosensitizer. Theranostics. 2019;9:167–178. doi: 10.7150/thno.28033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, et al. Intrinsically cancer-mitochondria-targeted thermally activated delayed fluorescence nanoparticles for two-photon-activated fluorescence imaging and photodynamic therapy. ACS Appl. Mater. Interfaces. 2019;11:41051–41061. doi: 10.1021/acsami.9b14552. [DOI] [PubMed] [Google Scholar]
- 68.Yue C, et al. Mitochondria-targeting near-infrared light-triggered thermosensitive liposomes for localized photothermal and photodynamic ablation of tumors combined with chemotherapy. Nanoscale. 2017;9:11103–11118. doi: 10.1039/C7NR02193C. [DOI] [PubMed] [Google Scholar]
- 69.Douiev L, Soiferman D, Alban C, Saada A. The effects of ascorbate, N-acetylcysteine, and resveratrol on fibroblasts from patients with mitochondrial disorders. J. Clin. Med. 2016;6:1. doi: 10.3390/jcm6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Z, et al. Hierarchical targeted hepatocyte mitochondrial multifunctional chitosan nanoparticles for anticancer drug delivery. Biomaterials. 2015;52:240–250. doi: 10.1016/j.biomaterials.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Milane L, Trivedi M, Singh A, Talekar M, Amiji M. Mitochondrial biology, targets, and drug delivery. J. Control. Release. 2015;207:40–58. doi: 10.1016/j.jconrel.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 72.Carroll B, Korolchuk VI, Sarkar S. Amino acids and autophagy: Cross-talk and co-operation to control cellular homeostasis. Amino Acids. 2015;47:2065–2088. doi: 10.1007/s00726-014-1775-2. [DOI] [PubMed] [Google Scholar]
- 73.Ma X, Gong N, Zhong L, Sun J, Liang XJ. Future of nanotherapeutics: Targeting the cellular sub-organelles. Biomaterials. 2016;97:10–21. doi: 10.1016/j.biomaterials.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 74.Song J, et al. Mitochondrial targeting nanodrugs self-assembled from 9-O-octadecyl substituted berberine derivative for cancer treatment by inducing mitochondrial apoptosis pathways. J. Control. Release. 2019;294:27–42. doi: 10.1016/j.jconrel.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 75.Biswas S, Dodwadkar NS, Piroyan A, Torchilin VP. Surface conjugation of triphenylphosphonium to target poly(amidoamine) dendrimers to mitochondria. Biomaterials. 2012;33:4773–4782. doi: 10.1016/j.biomaterials.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, et al. Mitochondrial-targeting lonidamine-doxorubicin nanoparticles for synergistic chemotherapy to conquer drug resistance. ACS Appl. Mater. Interfaces. 2017;9:43498–43507. doi: 10.1021/acsami.7b14577. [DOI] [PubMed] [Google Scholar]
- 77.Marrache S, Dhar S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc. Natl Acad. Sci. USA. 2012;109:16288–16293. doi: 10.1073/pnas.1210096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan P, et al. Mitochondria-targeting, intracellular delivery of native proteins using biodegradable silica nanoparticles. Angew. Chemie Int. Ed. 2019;58:7657–7661. doi: 10.1002/anie.201901699. [DOI] [PubMed] [Google Scholar]
- 79.Biswas S, Dodwadkar NS, Deshpande PP, Torchilin VP. Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium-PEG-PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo. J. Control. Release. 2012;159:393–402. doi: 10.1016/j.jconrel.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan J, et al. Mitochondria-targeted tetrahedral DNA nanostructures for doxorubicin delivery and enhancement of apoptosis. J. Mater. Chem. B. 2020;8:492–503. doi: 10.1039/C9TB02266J. [DOI] [PubMed] [Google Scholar]
- 81.Shah BP, et al. Core-shell nanoparticle-based peptide therapeutics and combined hyperthermia for enhanced cancer cell apoptosis. ACS Nano. 2014;8:9379–9387. doi: 10.1021/nn503431x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamada Y, Harashima H. Mitochondrial drug delivery systems for macromolecule and their therapeutic application to mitochondrial diseases. Adv. Drug Deliv. Rev. 2008;60:1439–1462. doi: 10.1016/j.addr.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 83.Kajimoto K, Sato Y, Nakamura T, Yamada Y, Harashima H. Multifunctional envelope-type nano device for controlled intracellular trafficking and selective targeting in vivo. J. Control. Release. 2014;190:593–606. doi: 10.1016/j.jconrel.2014.03.058. [DOI] [PubMed] [Google Scholar]
- 84.Yue C, et al. ROS-responsive mitochondria-targeting blended nanoparticles: chemo- and photodynamic synergistic therapy for lung cancer with on-demand drug release upon irradiation with a single light source. Theranostics. 2016;6:2352–2366. doi: 10.7150/thno.15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu P, et al. Near infrared-assisted Fenton reaction for tumor-specific and mitochondrial DNA-targeted photochemotherapy. Biomaterials. 2017;141:86–95. doi: 10.1016/j.biomaterials.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 86.Biswas S, Dodwadkar NS, Sawant RR, Koshkaryev A, Torchilin VP. Surface modification of liposomes with rhodamine-123-conjugated polymer results in enhanced mitochondrial targeting. J. Drug Target. 2011;19:552–561. doi: 10.3109/1061186X.2010.536983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang Y, et al. Pyridinium-substituted tetraphenylethylenes functionalized with alkyl chains as autophagy modulators for cancer therapy. Angew. Chemie Int. Ed. 2020;59:10042–10051. doi: 10.1002/anie.202001906. [DOI] [PubMed] [Google Scholar]
- 88.Xiao H, et al. A new endoplasmic reticulum-targeted two-photon fluorescent probe for imaging of superoxide anion in diabetic mice. Biosens. Bioelectron. 2017;91:449–455. doi: 10.1016/j.bios.2016.12.068. [DOI] [PubMed] [Google Scholar]
- 89.Wang C, et al. Investigation of endosome and lysosome biology by ultra pH-sensitive nanoprobes. Adv. Drug Deliv. Rev. 2017;113:87–96. doi: 10.1016/j.addr.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang, Y. et al. An NIR-fluorophore-based therapeutic endoplasmic reticulum stress inducer. Adv. Mater. 10.1002/adma.201800475 (2018). [DOI] [PubMed]
- 91.Zhang H, et al. Targeting VCP enhances anticancer activity of oncolytic virus M1 in hepatocellular carcinoma. Sci. Transl. Med. 2017;9:7996. doi: 10.1126/scitranslmed.aam7996. [DOI] [PubMed] [Google Scholar]
- 92.Zhang H, et al. An off-on COX-2-specific fluorescent probe: targeting the golgi apparatus of cancer cells. J. Am. Chem. Soc. 2013;135:11663–11669. doi: 10.1021/ja4056905. [DOI] [PubMed] [Google Scholar]
- 93.Nishita M, et al. Ror2 signaling regulates Golgi structure and transport through IFT20 for tumor invasiveness. Sci. Rep. 2017;26:7. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee JS, et al. TMEM165, a Golgi transmembrane protein, is a novel marker for hepatocellular carcinoma and its depletion impairs invasion activity. Oncol. Rep. 2018;135:11663–11669. doi: 10.3892/or.2018.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xue F, et al. A smart drug: a pH-responsive photothermal ablation agent for Golgi apparatus activated cancer therapy. Chem. Commun. 2017;53:6424–6427. doi: 10.1039/C7CC03168H. [DOI] [PubMed] [Google Scholar]
- 96.Nam JS, et al. Endoplasmic reticulum-localized Iridium(III) complexes as efficient photodynamic therapy agents via protein modifications. J. Am. Chem. Soc. 2016;138:10968–10977. doi: 10.1021/jacs.6b05302. [DOI] [PubMed] [Google Scholar]
- 97.Sher Y-P, Lin S-I, Chai KM, Chen I-H, Liu S-J. Endoplasmic reticulum-targeting sequence enhanced the cellular immunity of a tumor-associated antigen L6-based DNA vaccine. Am. J. Cancer Res. 2019;9:2028–2036. [PMC free article] [PubMed] [Google Scholar]
- 98.Feng Z, et al. Enzymatic assemblies disrupt the membrane and target endoplasmic reticulum for selective cancer cell death. J. Am. Chem. Soc. 2018;140:9566–9573. doi: 10.1021/jacs.8b04641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sneh-Edri H, Likhtenshtein D, Stepensky D. Intracellular targeting of PLGA nanoparticles encapsulating antigenic peptide to the endoplasmic reticulum of dendritic cells and its effect on antigen cross-presentation in vitro. Mol. Pharm. 2011;8:1266–1275. doi: 10.1021/mp200198c. [DOI] [PubMed] [Google Scholar]
- 100.Sahay G, Gautam V, Luxenhofer R, Kabanov AV. The utilization of pathogen-like cellular trafficking by single chain block copolymer. Biomaterials. 2010;31:1757–1764. doi: 10.1016/j.biomaterials.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghosh C, Nandi A, Basu S. Supramolecular self-assembly of triazine-based small molecules: targeting the endoplasmic reticulum in cancer cells. Nanoscale. 2019;11:3326–3335. doi: 10.1039/C8NR08682F. [DOI] [PubMed] [Google Scholar]
- 102.Deng H, et al. Endoplasmic reticulum targeting to amplify immunogenic cell death for cancer immunotherapy. Nano Lett. 2020;20:1928–1933. doi: 10.1021/acs.nanolett.9b05210. [DOI] [PubMed] [Google Scholar]
- 103.Li RS, et al. Chiral nanoprobes for targeting and long-term imaging of the Golgi apparatus. Chem. Sci. 2017;8:6829–6835. doi: 10.1039/C7SC01316G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luo J, et al. Golgi apparatus-targeted chondroitin-modified nanomicelles suppress hepatic stellate cell activation for the management of liver fibrosis. ACS Nano. 2019;13:3910–3923. doi: 10.1021/acsnano.8b06924. [DOI] [PubMed] [Google Scholar]
- 105.Li H, et al. Chondroitin sulfate-linked prodrug nanoparticles target the Golgi apparatus for cancer metastasis treatment. ACS Nano. 2019;13:9386–9396. doi: 10.1021/acsnano.9b04166. [DOI] [PubMed] [Google Scholar]
- 106.Bao YW, Hua XW, Chen X, Wu FG. Platinum-doped carbon nanoparticles inhibit cancer cell migration under mild laser irradiation: multi-organelle-targeted photothermal therapy. Biomaterials. 2018;183:30–42. doi: 10.1016/j.biomaterials.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 107.Huang Y, et al. Enzyme responsiveness enhances the specificity and effectiveness of nanoparticles for the treatment of B16F10 melanoma. J. Control. Release. 2019;316:208–222. doi: 10.1016/j.jconrel.2019.10.052. [DOI] [PubMed] [Google Scholar]
- 108.Sulima, S. O., Hofman, I. J. F., De Keersmaecker, K. & Dinman, J. D. How ribosomes translate cancer. Cancer Discov.10.1158/2159-8290.CD-17-0550 (2017). [DOI] [PMC free article] [PubMed]
- 109.Ebright RY, et al. Deregulation of ribosomal protein expression and translation promotes breast cancer metastasis. Science. 2020;367:1468–1473. doi: 10.1126/science.aay0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mullard A. Small molecules against RNA targets attract big backers. Nat. Rev. Drug Discov. 2017;16:813–815. doi: 10.1038/nrd.2017.239. [DOI] [PubMed] [Google Scholar]
- 111.Frankowski KJ, et al. Metarrestin, a perinucleolar compartment inhibitor, effectively suppresses metastasis. Sci. Transl. Med. 2018;10:eaap8307. doi: 10.1126/scitranslmed.aap8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Z, et al. A smart nanoassembly for multistage targeted drug delivery and magnetic resonance imaging. Adv. Funct. Mater. 2014;24:3612–3620. doi: 10.1002/adfm.201303662. [DOI] [Google Scholar]
- 113.López V, et al. Janus mesoporous silica nanoparticles for dual targeting of tumor cells and mitochondria. ACS Appl. Mater. Interfaces. 2017;9:26697–26706. doi: 10.1021/acsami.7b06906. [DOI] [PubMed] [Google Scholar]
- 114.Xie R, et al. Mitochondria and nuclei dual-targeted hollow carbon nanospheres for cancer chemophotodynamic synergistic therapy. Mol. Pharm. 2019;16:2235–2248. doi: 10.1021/acs.molpharmaceut.9b00259. [DOI] [PubMed] [Google Scholar]
- 115.Xiong H, Du S, Ni J, Zhou J, Yao J. Mitochondria and nuclei dual-targeted heterogeneous hydroxyapatite nanoparticles for enhancing therapeutic efficacy of doxorubicin. Biomaterials. 2016;94:70–83. doi: 10.1016/j.biomaterials.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 116.Sun Q, Zhou Z, Qiu N, Shen Y. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv. Mater. 2017;29:14–32. doi: 10.1002/adma.201606628. [DOI] [PubMed] [Google Scholar]
- 117.Kim H, et al. Polymer-coated pH-responsive high-density lipoproteins. J. Control. Release. 2016;228:132–140. doi: 10.1016/j.jconrel.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 118.Chen J, et al. Metal-Phenolic coatings as a platform to trigger endosomal escape of nanoparticles. ACS Nano. 2019;13:11653–11664. doi: 10.1021/acsnano.9b05521. [DOI] [PubMed] [Google Scholar]
- 119.Li JL, et al. Dual drug delivery system based on biodegradable organosilica core-shell architectures. ACS Appl. Mater. Interfaces. 2018;10:5287–5295. doi: 10.1021/acsami.7b17949. [DOI] [PubMed] [Google Scholar]
- 120.Herranz-Blanco B, et al. pH-Switch nanoprecipitation of polymeric nanoparticles for multimodal cancer targeting and intracellular triggered delivery of doxorubicin. Adv. Healthc. Mater. 2016;5:1904–1916. doi: 10.1002/adhm.201600160. [DOI] [PubMed] [Google Scholar]
- 121.Song N, et al. Inspired by nonenveloped viruses escaping from endo-lysosomes: a pH-sensitive polyurethane micelle for effective intracellular trafficking. Nanoscale. 2016;8:7711–7722. doi: 10.1039/C6NR00859C. [DOI] [PubMed] [Google Scholar]
- 122.Gao Y, et al. PH/Redox dual-responsive polyplex with effective endosomal escape for codelivery of siRNA and doxorubicin against drug-resistant cancer cells. ACS Appl. Mater. Interfaces. 2019;11:16296–16310. doi: 10.1021/acsami.9b02016. [DOI] [PubMed] [Google Scholar]
- 123.Cao Y, et al. Enhanced lysosomal escape of pH-responsive polyethylenimine-betaine functionalized carbon nanotube for the codelivery of survivin small interfering RNA and doxorubicin. ACS Appl. Mater. Interfaces. 2019;11:16296–16310. doi: 10.1021/acsami.9b02016. [DOI] [PubMed] [Google Scholar]
- 124.Ding Y, et al. Dual-functional bio-derived nanoparticulates for apoptotic antitumor therapy. Biomaterials. 2015;11:9763–9776. doi: 10.1016/j.biomaterials.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 125.Wang F, et al. Charge-reversible multifunctional HPMA copolymers for mitochondrial targeting. ACS Appl. Mater. Interfaces. 2017;9:27563–27574. doi: 10.1021/acsami.7b09693. [DOI] [PubMed] [Google Scholar]
- 126.Wang H, et al. Tumor- and mitochondria-targeted nanoparticles eradicate drug resistant lung cancer through mitochondrial pathway of apoptosis. J. Nanobiotechnology. 2020;18:8. doi: 10.1186/s12951-019-0562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Assanhou AG, et al. Reversal of multidrug resistance by co-delivery of paclitaxel and lonidamine using a TPGS and hyaluronic acid dual-functionalized liposome for cancer treatment. Biomaterials. 2015;73:284–295. doi: 10.1016/j.biomaterials.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 128.Dong Q, et al. Tumor environment differentiated “nanodepot” programmed for site-specific drug shuttling and combinative therapy on metastatic cancer. J. Control. Release. 2018;283:59–75. doi: 10.1016/j.jconrel.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 129.Pharmacy P, et al. Cationic amphiphilic drugs boost the lysosomal escape of small nucleic acid therapeutics in a nanocarrier-dependent manner. ACS Nano. 2020;14:4774–4791. doi: 10.1021/acsnano.0c00666. [DOI] [PubMed] [Google Scholar]
- 130.Shi M, et al. Stimuli-responsive release and efficient siRNA delivery in non-small cell lung cancer by a poly(l-histidine)-based multifunctional nanoplatform. J. Mater. Chem. B. 2020;8:1616–1628. doi: 10.1039/C9TB02764E. [DOI] [PubMed] [Google Scholar]
- 131.Maugeri M, et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 2019;10:4333. doi: 10.1038/s41467-019-12275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen G, et al. NIR-induced spatiotemporally controlled gene silencing by upconversion nanoparticle-based siRNA nanocarrier. J. Control. Release. 2018;282:148–155. doi: 10.1016/j.jconrel.2017.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Joris F, et al. Repurposing cationic amphiphilic drugs as adjuvants to induce lysosomal siRNA escape in nanogel transfected cells. J. Control. Release. 2018;269:266–276. doi: 10.1016/j.jconrel.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 134.Chen G, Wang Y, Xie R, Gong S. Tumor-targeted pH/redox dual-sensitive unimolecular nanoparticles for efficient siRNA delivery. J. Control. Release. 2017;259:105–114. doi: 10.1016/j.jconrel.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu Y, et al. Charge conversional biomimetic nanocomplexes as a multifunctional platform for boosting orthotopic glioblastoma RNAi therapy. Nano Lett. 2020;20:1637–1646. doi: 10.1021/acs.nanolett.9b04683. [DOI] [PubMed] [Google Scholar]
- 136.Dong Y, et al. PH-sensitive shell-core platform block DNA repair pathway to amplify irreversible DNA damage of triple negative breast cancer. ACS Appl. Mater. Interfaces. 2019;11:38417–38428. doi: 10.1021/acsami.9b12140. [DOI] [PubMed] [Google Scholar]
- 137.Han L, Tang C, Yin C. Enhanced antitumor efficacies of multifunctional nanocomplexes through knocking down the barriers for siRNA delivery. Biomaterials. 2015;44:111–121. doi: 10.1016/j.biomaterials.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 138.Qiao C, et al. Traceable nanoparticles with dual targeting and ROS response for RNAi-based immunochemotherapy of intracranial glioblastoma treatment. Adv. Mater. 2018;30:e1705054. doi: 10.1002/adma.201705054. [DOI] [PubMed] [Google Scholar]
- 139.Liu J, et al. NIR light-activated dual-modality cancer therapy mediated by photochemical internalization of porous nanocarriers with tethered lipid bilayers. J. Mater. Chem. B. 2017;5:8289–8298. doi: 10.1039/C7TB02095C. [DOI] [PubMed] [Google Scholar]
- 140.Zhang Q, et al. Photoactivatable prodrug-backboned polymeric nanoparticles for efficient light-controlled gene delivery and synergistic treatment of platinum-resistant ovarian cancer. Nano Lett. 2020;20:3039–3049. doi: 10.1021/acs.nanolett.9b04981. [DOI] [PubMed] [Google Scholar]
- 141.Li Y, et al. Photothermally triggered cytosolic drug delivery of glucose functionalized polydopamine nanoparticles in response to tumor microenvironment for the GLUT1-targeting chemo-phototherapy. J. Control. Release. 2020;317:232–245. doi: 10.1016/j.jconrel.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 142.Wang J, et al. Visible light-switched cytosol release of siRNA by amphiphilic fullerene derivative to enhance RNAi efficacy in vitro and in vivo. Acta Biomater. 2017;59:158–169. doi: 10.1016/j.actbio.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 143.Kim K, Lee CS, Na K. Light-controlled reactive oxygen species (ROS)-producible polymeric micelles with simultaneous drug-release triggering and endo/lysosomal escape. Chem. Commun. 2016;52:2839–2842. doi: 10.1039/C5CC09239F. [DOI] [PubMed] [Google Scholar]
- 144.Brendel JC, et al. Secondary self-assembly of supramolecular nanotubes into tubisomes and their activity on cells. Angew. Chemie Int. Ed. 2018;57:16678–16682. doi: 10.1002/anie.201808543. [DOI] [PubMed] [Google Scholar]
- 145.Zhou Z, Liu Y, Wu L, Li L, Huang Y. Enhanced nuclear delivery of anti-cancer drugs using micelles containing releasable membrane fusion peptide and nuclear-targeting retinoic acid. J. Mater. Chem. B. 2017;5:7175–7185. doi: 10.1039/C7TB01177F. [DOI] [PubMed] [Google Scholar]
- 146.Hauser AK, et al. Targeted iron oxide nanoparticles for the enhancement of radiation therapy. Biomaterials. 2016;105:127–135. doi: 10.1016/j.biomaterials.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tian Y, et al. Integration of cell-penetrating peptides with rod-like bionanoparticles: virus-inspired gene-silencing technology. Nano Lett. 2018;18:5453–5460. doi: 10.1021/acs.nanolett.8b01805. [DOI] [PubMed] [Google Scholar]
- 148.Yi A, Sim D, Lee YJ, Sarangthem V, Park RW. Development of elastin-like polypeptide for targeted specific gene delivery in vivo. J. Nanobiotechnology. 2020;18:15. doi: 10.1186/s12951-020-0574-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.He H, et al. Suppression of hepatic inflammation via systemic siRNA delivery by membrane-disruptive and endosomolytic helical polypeptide hybrid nanoparticles. ACS Nano. 2016;10:1859–1870. doi: 10.1021/acsnano.5b05470. [DOI] [PubMed] [Google Scholar]
- 150.Xu J, et al. Precise targeting of POLR2A as a therapeutic strategy for human triple negative breast cancer. Nat. Nanotechnol. 2019;14:388–397. doi: 10.1038/s41565-019-0381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.He Y, et al. Sequential intra-intercellular delivery of nanomedicine for deep drug-resistant solid tumor penetration. ACS Appl. Mater. Interfaces. 2020;12:8978–8988. doi: 10.1021/acsami.9b20062. [DOI] [PubMed] [Google Scholar]
- 152.Gopisetty, M. K. et al. Endoplasmic reticulum stress: major player in size-dependent inhibition of P-glycoprotein by silver nanoparticles in multidrug-resistant breast cancer cells. J. Nanobiotechnology.10.1186/s12951-019-0448-4 (2019). [DOI] [PMC free article] [PubMed]
- 153.Mallick A, More P, Syed MMK, Basu S. Nanoparticle-mediated mitochondrial damage induces apoptosis in cancer. ACS Appl. Mater. Interfaces. 2016;8:13218–13231. doi: 10.1021/acsami.6b00263. [DOI] [PubMed] [Google Scholar]
- 154.Jana A, et al. Photocontrolled nuclear-targeted drug delivery by single component photoresponsive fluorescent organic nanoparticles of acridin-9-methanol. Bioconjug. Chem. 2013;24:1828–1839. doi: 10.1021/bc400170r. [DOI] [PubMed] [Google Scholar]
- 155.Li X, Pan L, Shi J. Nuclear-targeting MSNs-based drug delivery system: global gene expression analysis on the MDR-overcoming mechanisms. Adv. Healthc. Mater. 2015;4:2641–2648. doi: 10.1002/adhm.201500548. [DOI] [PubMed] [Google Scholar]
- 156.Chen YP, et al. Catcher in the rel: nanoparticles-antibody conjugate as NF-κB nuclear translocation blocker. Biomaterials. 2020;246:119997. doi: 10.1016/j.biomaterials.2020.119997. [DOI] [PubMed] [Google Scholar]