Abstract
Two models were developed to estimate Lyme borreliosis (LB) cases. One was based on the seroprevalence of Borrelia infections in human samples. This model used corrections for false negative and false positive results from published test sensitivity and specificity measures. A second model based on Borrelia infections in sentinel dogs was used to quantify the prevalence of Lyme disease Borrelia infections in humans; the reference baseline for this model was human and canine infections in Germany. A comparison of the two models is shown and differences discussed. The relationships between incidence, prevalence and total infection burden for LB were derived from published data and these were used in both models to calculate annual incidence, prevalence and total LB infections. The modelling was conservative and based on medical insurance records coded for erythema migrans. Linear model growth rates were used in place of the commonly adopted exponential growth. The mean of the two models was used to create estimates for various countries and continents. Examples from the analyses for LB estimated for 2018 include: incidence – USA 473,000/year, Germany 471,000/year, France 434,000/year and UK 132,000/year; prevalence – USA 2.4 million, Germany 2.4 million, France 2.2 million and UK 667,000; total infections – USA 10.1 million, Germany 10.0 million, France 9.3 million and UK 2.8 million. Estimates for the world for 2018 are: incidence 12.3 million/year; prevalence 62.1 million; and total infection burden 262.0 million. These figures are far higher than officially published data and reflect not only the underestimation of diagnosed cases, which is acknowledged by health agencies, but also undiagnosed and misdiagnosed cases.
Keywords: Lyme disease, Borreliosis, Linear regression models, Seropositive, Companion animals, Sentinel animals
1. Introduction
There are few published data on the incidence and prevalence of Lyme disease, and although there are official reports from some countries the responsible medical authorities recognise that the true number of cases are higher. For example at a press conference the Centers for Disease Control and Prevention (CDC) in America said that two studies indicated the incidence of Lyme disease was 10 times higher than official reports of about 30,000 cases (Hinckley et al., 2014)(Nelson et al., 2015). Lindgren et al. stated that about 85,000 cases were reported annually in Europe though the number largely underestimated cases as reporting was highly inconsistent with many Lyme disease cases undiagnosed (Lindgren & Jaenson, 2006)(Centers for Disease Control and Prevention, 2013). And Vanderkerckhove et al. commented that the LD incidence in Western Europe was assumed to be increasing, yet remained to be confirmed (Vandekerckhove, De Buck, & Van Wijngaerden, 2019).
In order to quantify the true burden of Lyme borreliosis (LB) infections two models have been developed to estimate the incidence and prevalence of LB. Model (A) is based on the seroprevalence in general human populations such as blood donors, and Model (B) on published data for the infection rates in companion animals. The data sources were identified by searches of PubMed. For the model using seroprevalence in companion animals, only data for dogs were included to limit the influence of confounding variables.
Studies of seroprevalence of Borrelia infections in humans were normally carried out to determine infection rates for those at risk owing to employment, and frequently included forestry workers, farmers, military personnel, etc. Typically, these studies included a control group selected as a reference, generally from blood donors or member of general populations, or thought to be at low, or no risk of LB. Only data from the control groups have been included in this study, and used as indicative of infection rates in the general public. A total of 38 studies from 19 countries were identified where there were clearly defined controls with seropositivity data. These are shown in Table A1 and listed in the bibliography. Additionally 8 studies were identified in which the relationship between total infection rates (seropositivity) and LB prevalence and LB incidence were defined, These are shown in Table 1 and included in the bibliography. The resulting ratios relating these three parameters have been used with both models to derive LB incidence and LB prevalence from total infection rates. Both models use annual growth rates determined from linear regression of data from all studies. The models were then used to calculate LB infection estimates for the year 2018. The models are considered to be conservative in using linear growth rather than exponential growth. The model using sentinel animal data is normalised to the incidence of LB in Germany where there are published data based on insurance company records for human infection rates and also data for seroprevalence in dogs. The human data were from insurance company records recorded using the World Health Organisation (WHO) code ICD10: A69.2 Erythema chronicum migrans (EM rash). This again gives a conservative estimate since an EM rash is frequently not present or not recognised in cases of LB.Table 1.
Table 1.
LB incidence and prevalence ratios compared with total seropositive cases.
| Study | Seropositive prevalence (SP) | LB prevalence (P) | Ratio LB Prev/SP | LB incidence (I) | Ratio LB Ins/SP | |
|---|---|---|---|---|---|---|
| Arteaga (2007) | 14.6% | 3.6% | 24.7% | nd | nd | |
| Fahrer (1998) | 26.0% | nd | nd | 0.80% | 3.1% | |
| Faulde (2014) | 9.1% | nd | nd | 0.70% | 7.7% | |
| Guy (1989) | 25.0% | 5.0% | 20.0% | nd | nd | |
| Kuiper (1991) | 19.7% | 6.0% | 30.5% | nd | nd | |
| Kuiper (1993) | 28.0% | 5.5% | 19.7% | nd | nd | |
| Müller (2012) | 9.1% | nd | nd | 0.26% | 2.9% | |
| Willhelmsson (2016) | 39.0% | nd | nd | 2.00% | 5.1% | |
| Mean | 23.7% | 4.69% | ||||
A comparison between the two models is made and the means of the studies used to calculate disease parameters for other countries, continents and a world total. In the case of the USA, for which there exist extensive databases for seroprevalence of Borrelia infections in companion animals, Model (B) was used to estimate LB for all of the contiguous states. Various tables and charts are included with these estimates and additional tables and charts are included in supplementary information files.
2. Methods
2.1. Definition of Lyme borreliosis
Many health authorities limit the definition of LB. For example, until 2018 the CDC limited cases to those caused by Borrelia burgdorferi carried by Ixodes scapularis ticks. This was widened to include Borrelia mayonii. There are more than 50 named species of Borrelia with 21 included in the Lyme disease group. Furthermore, in Europe, Borrelia burgdorferi is rare and the infecting species include: B.afzelii, B. bavariensis, B. bissettiae, B.garinii, B.spielmanii and others. Some test kits are manufactured from whole cell antigens which will represent the local Borrelia species. Others use a synthetic peptide. In all cases these will detect non-target species to some degree. There is little published on this; for the purpose of this study LB is defined as those cases that test positive with commercial Lyme disease test kits used in each study. In the case of non-human animal data, it is assumed that the test kits are detecting Borrelia infection in companion animals and that these will be the same species as present in humans in that region.
2.2. Regression models
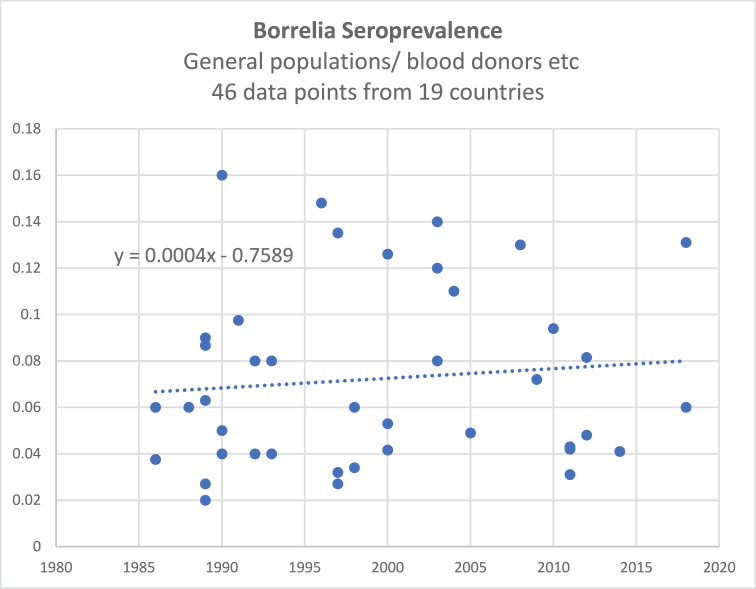
Two models were developed to estimate the prevalence of LB. One based on the seroprevalence of borrelial infections in general populations and the second on seroprevalence in companion animals. Regression analysis was used to compute the outcome variables. We used linear regression methodology for both models based on observation of the graphical data and also to generate conservative estimates, rather than using exponential time sequence regression. The seropositive rate versus year of study is shown in Appendix Fig. A1. A trend line using linear regression has an R2 Pearson coefficient of 0.0096 indicating a good fit to the data.
2.3. Model (A) seroprevalence of Borrelia infections in general populations
This model takes published data for the seropositive prevalence of Borrelia infections in groups of people selected to be controls in studies of Lyme disease associated with ‘at risk’ occupations. For example, blood donors or indoor workers are frequently selected and seroprevalence in these groups reported. These are then used to calculated LB infection rates and produce forecasts for 2018.
2.3.1. False negatives and false positives
The seropositive data published in the source data do not accurately reflect the true seroprevalence. Not only are the serology tests not 100% sensitive producing false negative results, the tests also are not 100% specific and give false positive results owing to cross reactivity with other antigens. Corrections are made in the model for false negatives from the sensitivity of testing derived from a meta-analysis of 19 independent studies of commercial test kits used for LB diagnosis (Cook & Puri, 2016).
A correction is also applied for false positives based on the test specificity. Specificity of commercial test kits was identified in the cited Cook & Puri, 2016 study (unpublished data). These are used with an equation relating false positive rates to the specificity of the test and seroprevalence of infection which was developed and shown in a prior paper by the authors and shown in Table A5 (Cook & Puri, 2017).
2.3.2. Input parameters
Study seropositive = SP.
Test sensitivity = Se.
Test specificity = Sp.
Seropositive corrected for sensitivity = SPc = SP/Se.
False positives = (1 - Sp)(1 - SPc); equation derived and used in (Cook & Puri, 2017).
True seropositive TP = SPc – false positives = SP/Se – (1 - Sp)(1 - SPc).
The results are then extended using linear growth to generate estimates for 2018.
True seropositive in 2018 = TP2018 = TP + g(2018 - year of study), where g is the annual growth rate (linear trend).
The published studies and input data are shown in Appendix A Table A1. The growth rate of infections over time are shown in Appendix A Fig. A1.
Analysis of published data quantifies the frequency of ‘asymptomatic’ LB infections in comparison to the prevalence and incidence of LB. These statistics have been identified in 8 studies and are shown in Table 1. (Arteaga, Perez, Barral, Anda, PedroGarcia-Monco, & Golightly, 2007; Fahrer, Sauvain, Zhioua, Van Hoecke, & Gern, 1998; Faulde & Freise, 2014; Guy, Bateman, Martyn, Heckels, & Lawton, 1989; Kuiper et al., 1993, 1991; Wilhelmsson et al., 2016). The ratio of LB prevalence to seropositive prevalence gives the percentage of people testing positive compared with those subjects who have a diagnosis of Lyme disease i.e. who are symptomatic. The average of the four studies shown in Table 1 where these data are available indicates that 23.7% of people who are seropositive are symptomatic i.e. have clinical LB. This indicates that for every person with clinical LB approximately 3 people are infected but asymptomatic. The ratio of LB incidence to seroprevalence gives the percentage of people diagnosed annually compared with the seropositive prevalence in the population. The average of three studies in Table 1 indicates that 4.7% of seropositive patients are diagnosed each year with LB. These two ratios are used in both models to calculate incidence and prevalence from seropositive data for 2018.
Total infections 2018 = SP2018 × population2018 × Ratio LB incidence/seroprevalence.
LB incidence 2018 = SP2018 × population2018 × Ratio LB incidence/seroprevalence.
LB prevalence2018 = SP2018 × population2018 × Ratio LB prevalence/seroprevalence.
2.4. Model (B) based on Borrelia seroprevalence in companion animals
The observation of sentinel animals is not new and the use of canaries to detect poisonous carbon monoxide in mines was an old technique to identify risk to human life (Burrell & Seibert, 1914). The use of animals to detect the risk of arthropod-borne infectious viral disease was discussed by Shope et al., in 1961 (Shope, Causey, & Causey, 1961). Zeman and Januska studied the human incidence of LB and tick-borne encephalitis (TBE) and various small mammals and game animals, finding that the data correlated with the low human risk of TBE compared the ubiquitous presence of LB (Zeman & Januška, 1999). The use of dogs as sentinels to help identify the risk of Borrelia infections in humans has been demonstrated by a number of workers (Beugnet & Marié, 2009; Duncan, Correa, Levine, & Breitschwerdt, 2005; Falco et al., 1993; Miró, Montoya, Roura, Gálvez, & Sainz, 2013; Stone, Lacombe, & Rand, 2005). Lindenmayer et al. reported a highly significant correlation (P < 0.001) between Borrelia infection prevalence in dogs and the incidence in humans, with a log-linear relationship (Lindenmayer, Marshall, & Onderdonk, 1991). Mead et al. analysed data for 46 US states and for over 900,000 dogs. Comparing the data collected between 2001 and 2006 to human infections also demonstrated a strong correlation (P < 0.03) (Mead, Goel, & Kugeler, 2011).
There have been studies claiming no correlation between animal ownership and human infections and these are described by Smith et al. (Smith, Ballantyne, Morgan, & Wall, 2012). The evidence included a study in which the risk of infection was higher in dogs than in humans (Eng, Wilson, Spielman, & Lastavica, 1988). However, this did not prove lack of correlation, only that the infection rate in dogs was higher than in humans. The Goossens reference (Goossens, 2001) stated that there was no positive correlation observed between the seropositivity of hunters and hunters’ dogs. However, the study results compared seroprevalence in hunters with dogs, and hunters without dogs. This does not address the issue of correlation, only that hunters with or without dogs had similar and high risk of Borrelia infections. In this case the risk factor was the sport of hunting compared with not hunting, rather than dog ownership.
For the present study, PubMed searches were used to identify papers published between 1983 and 2017 (inclusive) in which Borrelia infection rates in dogs were quantified. A total of forty four studies carried out in twenty one countries were identified and are listed with references in Appendix A Table A2.
The benchmark for computing human infections was the ratio of seropositive dogs to the human prevalence of LB. These were taken from studies carried out in Germany, and selected because of the existence of comprehensive LB disease data. Infection rates were derived from published data of medical insurance companies where cases were recorded using the WHO code ICD10: A69.2 Lyme disease erythema chronicum migrans through Borrelia burgdorferi (Müller et al., 2012) (Seifert, 2010). The actual data are shown in Table 2. The ratio of these parameters was then used to calculate LB incidence for each country for which animal data were available. The data were not corrected for test sensitivity and specificity. This was considered valid for two reasons: animal testing was frequently carried out using the same test kit for which sensitivity and specificity were the same for many of the studies; and the model used ratios of the parameters and not the absolute value of seropositivity.
Table 2.
Borrelia seroprevalence in dogs and Lyme disease incidence in Germany.
| Data for Germany (estimates for 2018) | ||
|---|---|---|
| Seroprevalence in dogs | 10.9% | SPG |
| Population of Germany (millions) | 82.29 | PG |
| Human infections using animal seroprevalence | 8,949,123 | SPG∗PG = TG |
| LB incidence/seroprevalence | 4.7% | R1 |
| Initial estimated incidence | 419,723 | TGR1 |
| Seifert/Műller incidence (mean of studies) | 485,751 | IG |
| Seifert/Műller incidence for 2018 | 485,751 | IG + g(2018–2009) |
| Ratio to adjust human incidence (β) | 1.16 | (IG/TG)R1 |
2.4.1. Methodology for model (B) seroprevalence in dogs
The method used the seroprevalence in dogs as the input parameter for seroprevalence in humans which is used to calculate the LB incidence. The result was then compared with the actual LB incidence in the benchmark country (Germany). The correction factor needed to adjust the canine seropositive rate to generate the actual LB infection was then used to adjust all other canine seropositive data.
Seroprevalence in country (C) dogs = SPC
Seroprevalence in dogs in Germany = SPG
Population of Germany = PG
LB infection rate in Germany = IG
Initial estimate for LB seroprevalence = SPGPG = TG
Ratio of LB incidence to seroprevalence = R1.
Initial estimate for LB incidence = SPGPGR1
Actual LB incidence = Seifert/Muller (mean of studies) = IG See Table 2.
Initial LB incidence 2018 = IG + g(2018–2009), where g = annual growth rate of positive serology in dogs.
Ratio to adjust to initial LB incidence estimate to actual LB incidence = β = (IG/TG)R1.
Model (B) is also used to calculate LB data for the United States. The infection rate in dogs is based on data for the contiguous states published for the years 2012–2018. (Companion Animal Parasite Council, 2019). The mean seroprevalence in dogs for the period is 6.3% with an annual growth rate of −0.13%, which is a small decline rather than growth. These are shown in Appendix A Table A3. The cause of decline is not known and may not be statistically significant.
Analysis of data from all countries shows a large variation in sample sizes used in the various studies. For example, the data for Canada where the seroprevalence is 0.2% with a sample of 86,251 dogs. This causes extreme bias if combined with studies where sample sizes were typically tens to hundreds and the weighted mean seroprevalence is 0.6% compared to 8.1% for an average. See Appendix Table A4. Hence simple averages are used and considered valid since intra-analytical test sensitivity, specificity and other experimental variables will be consistent.
The input data for seropositive dogs in Germany is shown in Table 3 and the incidence of LB in the German population is shown in Table 4. Examples for 6 countries is shown in Appendix Table A6.
Table 3.
Canine seroprevalence data for Germany.
| Animal data | Year | Ref: | Sample size | Positive | Seropositive animals (SP) | Seropositive 2018 | CI 95% |
|---|---|---|---|---|---|---|---|
| Töpfer | 2015 | Töpfer (2005) | 207 | 11 | 5.3% | 5.3% | (2%–8%) |
| “ | 2015 | 207 | 15 | 7.2% | 7.2% | (4%–11%) | |
| “ | 2015 | 207 | 46 | 22.2% | 22.2% | (16%–28%) | |
| Barth | 2007 | Barth et al. (2014) | 200 | 21 | 10.5% | 10.3% | (6%–15%) |
| Käsbohrer | 1990 | Käsbohrer and Schönberg (1990) | 189 | 19 | 10.1% | 9.5% | (8%–11%) |
| Mean | 1010 | 112 | 11.1% | 10.9% | (7%–15%) |
Table 4.
Lyme disease incidence from two German studies.
| Human Data | Year (Note.1) | Ref: | LB incidence | Population study date | LB incidence growth to 2018 | LB incidence with population growth |
|---|---|---|---|---|---|---|
| Seifert | 2009 | Seifert (2010) | 743,000 | 82,000,000 | 743,001 | 745,629 |
| Müller | 2008 | Müller et al. (2012) | 228,501 | 82,000,000 | 228,502 | 229,310 |
| Mean | 485,751 | 485,752 | 487,470 |
Note 1: Müller date is that of the investigation not date of publication.
Results
3.1. Model A output
The output for Model A seroprevalence in humans with the 2018 estimates for the incidence, prevalence and total infections are shown in Table 5. Where there were multiple studies for a given country the weighted mean data is computed. The data do not encompass uncertainties related to laboratory variables which have not been estimated. The data for LB incidence, prevalence and total infections are shown in Fig. 1, Fig. 2, Fig. 3 with mean and estimated error bars.
Table 5.
Model A seroprevalence in humans: Estimates of LB incidence, prevalence and total infections for 2018. Mean seroprevalence for multiple studies in one country.
| Country | Number of Studies | Region/Area | Sero-positives (SP) | True positives (Linear growth to 2018) | LB Incidence | LB Prevalence | Borrelia Infections |
|---|---|---|---|---|---|---|---|
| Austria | 1 | Tyrol/Rural | 7.2% | 9.1% | 37,430 | 189,185 | 798,059 |
| Belgium | 2 | National/Urban + Rural | 3.7% | 2.9% | 15,691 | 79,311 | 334,566 |
| England | 1 | Northwest/Rural | 2.7% | 2.1% | 54,005 | 272,964 | 1,151,474 |
| Finland | 1 | Southwest/Urban | 4.0% | 4.1% | 10,648 | 53,821 | 227,038 |
| France | 2 | See Table A1 | 9.6% | 13.9% | 438,962 | 2,218,688 | 9,359,322 |
| Germany | 5 | See Table A1 | 6.4% | 11.8% | 456,187 | 2,305,752 | 9,726,595 |
| Ireland | 2 | See Table A1 | 6.6% | 8.6% | 19,384 | 97,975 | 413,296 |
| Italy | 1 | Tuscany/Urban + Rural | 4.9% | 5.3% | 146,497 | 740,454 | 3,123,537 |
| Lithuania | 1 | National/Urban | 4.0% | 4.3% | 5806 | 29,344 | 123,787 |
| Netherlands | 3 | See Table A1 | 6.8% | 9.0% | 72,454 | 366,213 | 1,544,836 |
| Poland | 2 | See Table A1 | 9.6% | 13.3% | 106,225 | 536,901 | 2,264,865 |
| Scotland | 1 | National/Urban + Rural | 4.2% | 3.8% | 9463 | 47,832 | 201,774 |
| Sweden | 9 | See Table A1 | 8.7% | 12.3% | 57,556 | 290,911 | 1,227,178 |
| Switzerland | 1 | Alpine/Urban + Rural | 6.0% | 7.9% | 31,310 | 158,251 | 667,567 |
| Turkey | 2 | See Table A1 | 5.1% | 5.1% | 197,050 | 995,969 | 4,201,401 |
| Mean | 5.5% | 6.7% |
Fig. 1.
Estimated ‘incidence’ of Lyme borreliosis for 2018
Fig. 2.
Estimated ‘prevalence’ of Lyme borreliosis for 2018
Fig. 3.
Estimated ‘total infections’ in 2018
The true seropositive rate corrected for false negative and false positives and with linear regression to 2018 varies from 2.7% for England to 9.3% for Poland. The LB incidence varies from 5806 cases for Lithuania to 456,187 for Germany. The highest rate for prevalence was for Germany with 2,306,000 cases.
3.2. Model B output
Table 6. Human Borrelia computed from canine infection rates shows the results of the model calculations based on data from Table 3, Table 4. The mean seroprevalence from the dog studies was 8.1% and the model calculations give the seroprevalence in humans of 8.7%. The human infection rates vary considerably from 0.1% in Portugal and 1.0% in Italy, to 19.3% in the Netherlands and 29.3% in Serbia. The estimated seroprevalence rate for England is 6.4% and 7.3% for the United States. The mean seroprevalence for all countries is 8.7%.
Table 6.
Human Borrelia infections computed from canine infection rates.
| Animal Borrelia infection data (dogs) |
Human Lyme Borrelia infections |
|||||||
|---|---|---|---|---|---|---|---|---|
| Country | Sero-prevalence of Borrelia infections in animals AP | Year of study (Note 1) | Sero-prevalence 2018 | Adjusted for human seropositive rate (β) | Pop. 2018 (Mils) | Incidence 2018 | LB prevalence (Total LB cases) | Total infections |
| Brazil | 4.9% | 2001 | 4.5% | 5.2% | 210.9 | 518,570 | 2,621,060 | 11,056,692 |
| Bulgaria | 10.6% | 2015 | 10.5% | 12.2% | 7.0 | 39,971 | 202,030 | 852,245 |
| Czech republic | 6.5% | 2006 | 6.3% | 7.2% | 10.6 | 36,119 | 182,562 | 770,119 |
| England | 6.2% | 1988 | 5.6% | 6.4% | 55.6 | 169,064 | 854,518 | 3,604,703 |
| Finland | 6.3% | 2014 | 6.2% | 7.2% | 5.5 | 18,629 | 94,157 | 397,193 |
| France | 12.2% | 1999 | 11.8% | 13.6% | 67.2 | 429,591 | 2,171,325 | 9,159,526 |
| Germany | 11.1% | 2008 | 10.9% | 12.6% | 82.3 | 485,814 | 2,455,496 | 10,358,277 |
| Hungary | 0.4% | 2006 | 0.2% | 0.2% | 9.7 | 842 | 4253 | 17,943 |
| Italy | 1.0% | 2010 | 0.8% | 1.0% | 59.3 | 27,275 | 137,859 | 581,545 |
| Japan | 10.2% | 2016 | 10.2% | 11.7% | 127.2 | 700,862 | 3,542,433 | 14,943,417 |
| Korea | 1.1% | 2017 | 1.1% | 1.2% | 51.2 | 30,014 | 151,704 | 639,947 |
| Mexico | 7.7% | 2008 | 7.5% | 8.7% | 130.8 | 530,927 | 2,683,517 | 11,320,163 |
| Netherlands | 17.0% | 2000 | 16.6% | 19.3% | 17.1 | 154,267 | 779,728 | 3,289,210 |
| Poland | 12.5% | 2016 | 12.5% | 14.4% | 38.1 | 257,677 | 1,302,402 | 5,494,061 |
| Portugal | 0.2% | 2012 | 0.1% | 0.1% | 10.3 | 447 | 2258 | 9527 |
| Romania | 6.5% | 2011 | 6.4% | 7.4% | 19.6 | 67,875 | 343,067 | 1,447,195 |
| Serbia | 25.5% | 2010 | 25.3% | 29.3% | 8.7 | 119,663 | 604,823 | 2,551,387 |
| Spain | 8.1% | 2008 | 7.9% | 9.2% | 46.4 | 199,292 | 1,007,301 | 4,249,203 |
| Sweden | 7.5% | 2009 | 7.3% | 8.5% | 10.0 | 39,732 | 200,823 | 847,152 |
| USA | 6.3% | 2017 | 6.3% | 7.3% | 327.2 | 1,114,439 | 5,632,816 | 23,761,497 |
| Mean | 8.1% | 7.5% | 8.7% | |||||
3.3. Comparison of the two models
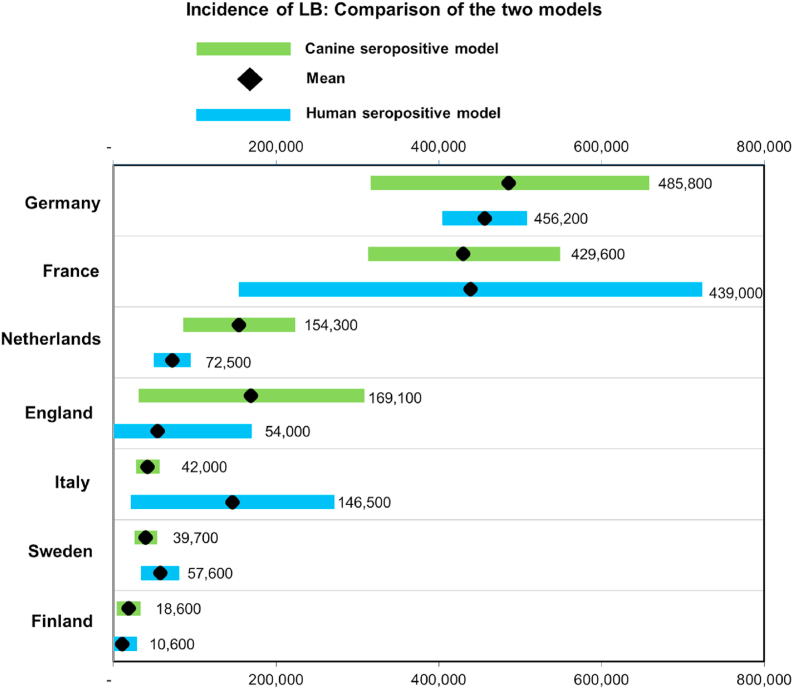
Data were extracted from the two models where both sets of estimates were available for specific countries. A chart of these is shown in Fig. 4.
Fig. 4.
Estimated incidence of Lyme borreliosis. A comparison of the two models
There is a difference in means between the two models of 8%. The largest differences between the two models are for Italy, England and the Netherlands. Analysis of the studies indicates the following:
-
a)
In the case of England there is a difference of 68% between models. The study providing input data for Model (A) (seroprevalence in humans) was carried out using a test based on the B31 strain of Borrelia with an ELISA test cut-off threshold selected of an ‘arbitrary 30 units’. Sera over that threshold were considered by the authors to be ‘strongly positive’ (Morgan-Capner et al., 1989). This indicates that the test did not specifically target the Borrelia species in England, and by using a cut-off to detect ‘strongly positive’ results had low intrinsic sensitivity. This will underestimate the true seroprevalence. The actual sensitivity was not given by the authors; however, a sensitivity of 70% compared to model sensitivity for ELISA tests, a not unrealistic estimate, would match the model to the sentinel animal model.
-
b)
The Netherlands data gave a mismatch between models of 53%. Analysis of the sentinel animal study indicated that all dogs were from rural areas. The probability of tick bites and tick-borne disease will be higher in rural animals compared with dogs living in urban environments. This results in a higher calculated estimate for human infections than if urban dogs were also included.
-
c)
The largest difference between the models is for Italy. The estimate based on Model (B) seroprevalence in dogs is very low at only 10% of the estimate from human seroprevalence. The data are from three studies. The Mannelli study found no dogs seropositive on an estate near Pisa (Mannelli et al., 1999). Not only did the study find all dogs seronegative, but also all deer and human estate workers. This is somewhat anomalous since of the 15 studies carried out in Italy to identify Borrelial infections in ‘at risk’ workers and the general public, this study was the only one that found all test subjects negative. The other 14 human infections studies found Borrelia infection rates between 0.6% and 27% for the general public and ‘at risk’ workers. The Piantadosi study of hunting dogs in southern Italy also found a low seroprevalence of 0.3% (Piantedosi et al., 2017). This study was carried out in the Ambito Territoriale Caccia hunting district of Avellino, and a hunting district of Salerno. It is possible that the results for hunting dogs are not representative of Borrelia infections in dogs for the whole country.
Based on the mean data shown in Fig. 4 the prevalence and total infection rates were computed for the countries where input data was available for both models. The results are shown in Table 7. Two separate means are calculated for estimation of infection levels for other countries, one for England and the second for all other countries. These are shown in Table 8. The data for England are used for estimates of infections in the UK and Ireland, as shown in Table 9. The mean seroprevalence for all countries is used to estimate infections for continents and the world with the results shown in Table 10. Estimates for a group of countries are shown in Table 11.
Table 7.
Mean infections in countries with data for both models.
| Country | Incidence | Prevalence | Total infections |
|---|---|---|---|
| Finland | 14,600 | 73,700 | 311,000 |
| Sweden | 48,700 | 246,100 | 1,038,000 |
| Italy | 94,300 | 476,700 | 2,011,000 |
| England | 112,000 | 566,100 | 2,388,000 |
| Netherlands | 113,000 | 571,100 | 2,409,000 |
| France | 434,000 | 2,194,000 | 9,254,000 |
| Germany | 471,000 | 2,380,000 | 10,040,000 |
Table 8.
Mean seroprevalence of the two models.
| Seropositive rate | Model A | Model B | Mean of Models |
|---|---|---|---|
| England | 2.07% | 6.48% | 4.28% |
| Mean all countries | 6.7% | 8.7% | 7.7% |
Table 9.
Borrelia infection rates for the UK and Ireland.
| Country | Population (millions) | LB Incidence | LB Prevalence | Total Infections |
|---|---|---|---|---|
| England | 55.6 | 112,000 | 564,000 | 2,378,000 |
| Ireland | 4.8 | 10,000 | 49,000 | 205,000 |
| Northern Ireland | 1.9 | 4000 | 19,000 | 80,000 |
| Scotland | 5.3 | 11,000 | 54,000 | 227,000 |
| Wales | 3.0 | 6000 | 30,000 | 128,000 |
| UK | 65.8 | 132,000 | 667,000 | 2,813,000 |
Table 10.
Borrelia infection rates for the continents and world total.
| Continent | At risk population (millions) | LB Incidence | LB Prevalence | Total Infections |
|---|---|---|---|---|
| Africa | 488.5 | 1,770,000 | 8,930,000 | 37,650,000 |
| Asia | 1781.9 | 6,440,000 | 32,560,000 | 137,360,000 |
| Europe | 739.0 | 2,670,000 | 13,510,000 | 56,970,000 |
| North America | 204.8 | 740,000 | 3,740,000 | 15,790,000 |
| Oceania | 15.2 | 50,000 | 280,000 | 1,170,000 |
| South America | 169.9 | 610,000 | 3,110,000 | 13,100,000 |
| World | 3399.3 | 12,290,000 | 62,110,000 | 262,000,000 |
Table 11.
Additional examples of LB rates for various countries.
| Estimates for some Countries | Population (millions) 2018 | At risk population | LB Incidence | LB Prevalence | Total Infections |
|---|---|---|---|---|---|
| Australia | 25.1 | 10.0 | 36,000 | 183,000 | 770,000 |
| Brazil | 210.9 | 84.4 | 305,000 | 1,541,000 | 6,500,000 |
| Bulgaria | 7.0 | 7.0 | 25,000 | 128,000 | 540,000 |
| Canada | 37.0 | 14.8 | 53,000 | 270,000 | 1,140,000 |
| Cuba | 11.1 | 4.4 | 16,000 | 81,000 | 340,000 |
| Czech rep | 10.6 | 10.6 | 38,000 | 194,000 | 820,000 |
| Denmark | 5.8 | 5.8 | 21,000 | 107,000 | 450,000 |
| Finland | 5.5 | 2.2 | 8000 | 40,000 | 170,000 |
| Hungary | 9.7 | 3.9 | 14,000 | 71,000 | 300,000 |
| Japan | 127.2 | 50.9 | 184,000 | 929,000 | 3,920,000 |
| Korea | 51.2 | 20.5 | 74,000 | 375,000 | 1,580,000 |
| Mexico | 130.8 | 52.3 | 189,000 | 955,000 | 4,030,000 |
| New Zealand | 4.9 | 2.0 | 7000 | 36,000 | 150,000 |
| Portugal | 10.2 | 4.1 | 15,000 | 73,000 | 310,000 |
| Romania | 19.6 | 19.6 | 71,000 | 358,000 | 1,510,000 |
| Serbia | 8.7 | 8.7 | 31,000 | 159,000 | 670,000 |
| South Africa | 57.7 | 23.1 | 83,000 | 422,000 | 1,780,000 |
| Spain | 46.4 | 18.6 | 67,000 | 339,000 | 1,430,000 |
| Switzerland | 8.5 | 8.5 | 31,000 | 156,000 | 660,000 |
| USA | 327.2 | 130.9 | 473,000 | 2,392,000 | 10,090,000 |
The estimates are based on the mean of the two models where data are available as input. At risk population based on US model (see text).
As mentioned above, there is a large difference between the models for England owing to the use of a ‘low’ sensitivity test. With the Model (A) seropositive rate of 2.07% compared to 6.48% for Model (B) (sentinel animals). This suggests that the estimates will be significantly under-estimated, again giving a conservative estimate for infection and disease rates. A closer estimate for UK and Ireland to compensate for this bias would be approximately 50% higher than the table data.
The majority of data is for European countries which predominantly fall into the category of temperate and with ecologies dominated by forests and significant rainfall. These would not be representative of the other continents. In order to avoid bias the estimates for continental and worldwide infections do not use the total populations which are replaced by an estimate of ‘at risk’ populations. The United States was selected as more representative of other continental masses with a mixture of climates and ecologies. With the north-east characterised by large forested areas and significant rainfall, in contrast south-central areas such as Arizona and New Mexico classed as ‘desert’ at low elevations with low levels of rainfall. Using the database for seroprevalence in dogs, each of the contiguous states was assign to low, medium or high risk of infection, risk factor were based on the seroprevalence and ‘at risk’ human population calculated This generates an estimate of the US ‘at risk’ population of 38% of the total population (unpublished data). With the large variety of geography, climate and ecology present in the United States an assumption is made that the model can be used as a proxy for risks in other continents and the world at large. Although the methodology is not rigorous by reducing total populations regional and continental it contributes to making the model outputs conservative.
4. Discussion
4.1. General comments regarding the two models
-
1)
Data from the two studies in Germany for the incidence of Lyme disease were based on reports from medical insurance companies and coded in their records as ICD10: A69.2 Lyme disease erythema chronicum migrans through Borrelia burgdorferi. In studies in which an EM rash was not an inclusion condition for entry into a study between 25% and 60% of patients had such a rash (Steere et al., 1977)(Muhlemann & Wright, 1987). This will result in the models significantly underestimating LB infections. The occurrence of EM rash in cases of LB as derived from analysis of 12 studies carried out by MJC was 40.4% (unpublished data), which suggests the models underestimate LB infections by a factor of 2.5. This suggests that the models are conservative.
-
2)
Some of the animal and human studies were based on national data collection and others in specific locations within each country, hence in some cases the infection rates may not be representative of the entire country. In the case of Model (A) (seroprevalence of Borrelia infections in humans) Table A1 shows details of each study including the region and whether rural or urban or mixed area. The De Keukeleire study demonstrated a rural seropositivity of 4.3% and an urban level of 3.1%. The use of common test methods for both sample each of near 200 individuals suggests that the difference is valid. However, differences in test kits, methodologies and cut-off values make other comparisons less relevant. Some studies included multiple locations including urban areas and the mean of multiple sampling sites has been used. Sample sizes ranged between 50 for Finland and Switzerland to 23,628 for Germany. These have been taken into account when calculating the upper and lower bounds shown in Fig. 1, Fig. 2, Fig. 3, Fig. 4.
-
3)
The mean incidence for the two models differs by 8%. Considering that the two models use independent inputs, and some studies have small sample sizes, the correlation is good. Also, in general the results are within the error boundaries apart from the cases were there were reasons associated with study methodologies.
-
4)The change of human infection rates over time demonstrated in the graph of historical trends (Fig. A1) was very low at 0.04% per year. Also there is wide dispersion of the data with infection rates as high as 16% in 1990. Additionally, the possibility of changes of test sensitivity has not influenced the data since a meta-analysis of commercial test kit accuracy shows that there has been no significant change in test sensitivity over the last 20 years (Cook & Puri, 2016). This suggests that the underlying infections rates have been relatively stable over time and that LB has been established for a long period throughout the world. This does not correlate with official published data for LB diagnosed cases. Data from the CDC show significant growth over the period from 1993 to the present, although the counting methods have changed at different times. The UK data from Public Health England also show significant growth, again despite counting methodology changes. The growth of official data can be explained by:
-
a.Increased recognition by the general public.
-
b.Increase recognition and diagnosis by clinicians.
-
c.Diagnosis of cases from the large body of infected and symptomatic patients that either have been misdiagnosed or undiagnosed in the past.
-
a.
-
5)
In the studies of the general public and/or blood donors it is implied by the study authors or stated explicitly that all subjects are asymptomatic i.e. ‘healthy’. However, the symptoms of LB vary from mild to life threatening. In fact people with perfect health are in the minority with one study of the global burden of disease indicating that less than 5% of the world population had no health problems. (Vos, Bell, & Bertozzi-Villa, 2015) The study reported that there were over two billion people with more than five health problems. The leading causes of disability included lower back pain, neck pain, major depression, headaches, hearing loss and Alzheimer’s disease. With 95% of the world population suffering from at least one health issue and with approximately 30% with more than five problems, the assumption that all blood donors and other groups selected are healthy controls must be false. It also suggests that many of the groups of people defined in these models as infected but asymptomatic will in fact be people with low level health issues and clinical LB. No attempt has been made to quantify these cases; however, they would increase the extrapolated values for the incidence and prevalence of LB.
-
6)
The output of Model A (seroprevalence in the general population) shown in Table 5 is based on specific regional data. In countries with a single study the data are extrapolated to the total national population. This may over- or underestimated the actual seroprevalence where the study data were from rural or urban areas. In counties where the studies are carried out in rural areas the model will over-estimate the results for the nation at large. Where the study was based on urban samples the model will underestimate the results. No attempt has been made to compensate for the bias.
-
7)
In Model (B) seroprevalence in dogs, consideration was given to the issue of companion animal behaviour, climatic differences and local ecology. Searches for information on this issue did not identify useful quantitative data for the different countries. It is probable that dogs in rural areas and hence the owners would be more likely to be at risk than in urban areas. In this model no parameter representing these variables was used.
4.2. Are there supporting data to validate the results of these models?
The CDC at a press conference associated with the 2013 National Conference on Lyme Borreliosis and Other Tick-Borne Diseases, provided useful data (Centers for Disease Control and Prevention, 2013). The release referred to two studies which indicated that the data published by the CDC for prior years of approximately 30,000 cases per year was underestimating the number. The Hinckley et al. study using laboratory test data indicated between 240,000 and 440,000 cases in 2008 (Hinckley et al., 2014). The second study, by Nelson et al. of medical insurance claims from 2005 to 2010 gave an estimate of 329,000 cases per year on average for that time period (Nelson et al., 2015). These studies indicated that diagnosed cases were over 10 times higher than the cases reported to the CDC and comparable to the estimated incidence in this study or 430,000 cases.
There is additional support provided from a 2019 paper in which Delong et al. used three different models to estimate post-treatment Lyme disease (PTLD) in the US (Delong, Hsu, & Kotsoris, 2019). In one scenario they used a treatment failure rate of 20% and linear growth of disease from 329,000 cases in 2005. This generated an estimated prevalence of PTLD of just over 1,500,000 cases. This is similar the model presented here with a prevalence of 2,171,000 cases.
In the case of LB in the UK, the official figures for 2012 as an example were 1040 cases for England and Wales and 207 cases for Scotland, making a total of 1247 cases. Cairns et al. published a study based on analysis of cases recorded in the Clinical Practice Research Datalink, a primary care database for the years 2001 until 2012 (Cairns & Godwin, 2005). They found an incidence for 2012 of 12.1 cases per 100,000 population for the UK, for a total of 7738 cases, which is six times greater than the official reported data. Taking a linear growth based on the Cairns data to the year 2018 gives an incidence of 19.8 cases per 100,000 per year, equivalent to 12,900 cases. This is still significantly lower than the model, however the authors state that cases diagnosed by specialists may not be included and the study did not include misdiagnosed and undiagnosed cases.
4.3. Is there evidence of misdiagnosis?
Literature searches for evidence of Borrelia infections related to Alzheimer’s disease identified 11 studies. One study (Galbussera 2008) was carried out in Italy, which has a very low incidence of Lyme disease (0.001 cases per 100,000) and so a low probability of detecting Borrelia in the sample of 50 Alzheimer’s cases (Sykes & Makiello, 2016). Of the remaining 10 studies (Bu et al., 2015; Gutacker et al., 1998; MacDonald, 1986, 2006; Marquard, Kurz, Bremer, & Dose, 2011; Marques, Weir, Fahle, & Fisher, 2000; McLaughlin, Ng Ying Kin, Chen, Nair, & Chan, 1999; Miklossy, 1994; Pappolla et al., 1989; Riviere, Riviere, & Smith, 2002) four found no association between Alzheimer’s disease and Borrelia infections. All of these have been included in the weighted average of 19% of Alzheimer’s disease patients with Borrelia infections.
Also estimates have been made for multiple sclerosis (MS) and myalgic encephalitis/chronic fatigue syndrome (ME/CFS). In the cases of MS a misdiagnosis rate of 19%, the same as Alzheimer’s, is used and for ME/CFS a rate of 30% is used based on data from a survey carried out in the UK (Bloor, 2014), and undiagnosed cases by national medical authorities where 30% of patients reported that independent/private clinicians had diagnosed the cases. These data were used to estimate the misdiagnosed and undiagnosed cases for the US of 1.7 million for 2018. These are shown in Table 12.
Table 12.
Misdiagnosed cases. Estimates for the USA.
| Disease | % LD | Year | Prevalence | Comment | Reference |
|---|---|---|---|---|---|
| MS | 2010 | 727,344 | Wallin et al. (2019) | ||
| Lyme cases | 19% | Note 1 | 140,377 | Low CI 95% used | Chmielewska-Badora, Cisak, and Dutkiewicz (2000) |
| Alzheimer’s disease | 2010 | 4,700,000 | Hebert, Weuve, Scherr, and Evans (2013) | ||
| Lyme cases | 19% | 906,824 | Mean of 10 studies | See textSection 6 | |
| ME/CFS | 2,736,000 | Valdez et al. (2019) | |||
| Lyme cases | 10% | Note 2 | 273,600 | Bloor survey 48% CFS | Bloor (2014) |
| Total Misdiagnosed | 1,320,801 | ||||
| Undiagnosed | 30% | 396,240 | Bloor survey | Bloor (2014) | |
| Total un/misdiagnosed | 1,717,041 | ||||
Note 1. For MS related Lyme cases rather than using the Chmielewska-Badora mean data a highly conservative lower 95% confidence value is used.
Note 2. For ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) cases a conservative estimate of 10% is used rather than the 48% of Lyme patients given a CFS diagnosis.
The estimated total of undiagnosed and misdiagnosed cases of LB in the US is 1.7 million. This is 35% higher than the model estimate. It also only includes three diseases. Sometimes LB is called the ‘great imitator’ owing to the wide variety of symptoms that can extend to every organ of the body. A recently published book identifies over 300 medical conditions that present with the same symptoms as LB (Çetin, 2018) and with LB mimicking a large number of diseases not included in the estimate it suggests that the model is conservative and underestimates the actual incidence and prevalence of the disease.
5. Conclusions
The two independent methodologies agree on average to within 10%. Where there are large differences between the two models for specific countries (England, The Netherlands and Italy), analysis of the original studies identified reasons to account for the differences in most cases. The estimated incidence for LB in Europe for 2018 is 2,520,000 cases, for North America 790,000 cases and the estimate for the world of over 11 million cases per year. The prevalence of LB for Europe is 12.7 million cases and for North America 4.0 million and for the world 59.0 million cases. The world-wide infection burden is estimated to be almost 250 million. The majority of these are either asymptomatic or sufficiently minor so that people do not consider it necessary to visit a doctor. These figures are much higher than corresponding officially published ones. They are considered to be conservative and under-estimate actual infection rates since the models are based on recorded cases that are diagnosed based on an EM rash.
Author Contributions
MJC conceived and developed the models and wrote the draft manuscript. BKP validated the models and methodology and wrote and edited the manuscript. Both authors read and approved the final version of the manuscript
Funding
The authors received no funding for this research.
Declaration of competing interest
The authors have no financial competing interests. The first author (MJC) was diagnosed with Lyme disease in 2009.
Acknowledgements
The authors would like to thank the editors and reviewers. Their inputs and suggestions were very helpful and allowed us to improve the manuscript.
Handling editorDr. J Wu
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Appendix. Input data tables and charts
Table A1.
Input data: Seroprevalence in general human populations 34 studies. The references for Tables A1 and A2 are available from the corresponding author
| Country | Year | Year of study | Sero-prevalence | Sample size | Sample type | Region/Area | Climate |
|---|---|---|---|---|---|---|---|
| Austria | 2015 | 2009 | 7.2% | 1010 | Blood donors (BD) | Tyrol/Rural | Alpine |
| Belgium | 2016 | 2011 | 4.3% | 209 | BD | South/Rural | Temperate |
| Belgium | 2016 | 2011 | 3.1% | 193 | BDs | South/Urban | Temperate |
| England | 1989 | 1989 | 2.7% | 75 | Controls | Northwest/Rural | Temperate |
| Finland | 1995 | 1993 | 4.0% | 50 | Healthy people | Southwest/Urban | Humid Continental |
| France | 1997 | 1997 | 3.2% | 31 | BD | Ile de Paris/Mixed | Temperate Continental |
| France | 1993 | 1990 | 16.0% | 100 | General population | Urban Central | Temperate Continental |
| Germany | 1996 | 1992 | 4.0% | 200 | BD | Brandenburg/∖mixed | Temperate Continental |
| Germany | 2012 | 2010 | 9.4% | 6954 | Adults | National/Mixed | Temperate Continental |
| Germany | 1998 | 1992 | 8.0% | 133 | BD | Bavaria/Mixed | Temperate Continental |
| Germany | 1993 | 1993 | 8.0% | 3736 | BD | Berlin/Mixed | Temperate Continental |
| Germany | 1989 | 1988 | 6.0% | 334 | Healthy people | North Bavaria/Mixed | Temperate Continental |
| Germany | 2012 | 2012 | 4.8% | 12614 | Children and adolescents | National/Mixed | Temperate Continental |
| Germany | 1996 | 1996 | 14.8% | 4896 | General population | South Bavaria/Mixed | Temperate Continental |
| Ireland | 1998 | 1998 | 3.4% | 1224 | Gen pop/hi med low prev | National/Mixed | Temperate Island |
| Ireland | 1991 | 1991 | 9.8% | 400 | Gen pop/hi med low prev | National/Mixed | Temperate Island |
| Italy | 2005 | 2005 | 4.9% | 365 | BD | Tuscany/Mixed | Temperate Continental |
| Lithuania | 1994 | 1990 | 4.0% | 163 | Urban | National/Urban | Temperate Continental |
| Netherlands | 1993 | 1990 | 5.0% | 151 | Office workers | National/Urban | Temperate |
| Netherlands | 1991 | 1989 | 9.0% | 1052 | BD | National/Rural + Urban | Temperate |
| Netherlands | 1991 | 1989 | 6.3% | 127 | controls matched age/residence | National/Rural? | Temperate |
| Poland | 1998 | 1998 | 6.0% | 100 | BD | Lublin/Urban | Temperate Continental |
| Poland | 2019 | 2018 | 13.1% | 199 | BD | National/urban | Temperate Continental |
| Scotland | 2015 | 2011 | 4.2% | 1440 | BD | National/Rural + Urban | Temperate Island |
| Slovenia | 2000 | 2000 | 12.6% | 143 | Children young adults | National/Rural + Urban | Temperate Continental |
| Sweden | 2001 | 2000 | 4.2% | 408 | BD | Southeast/Rural + Urban | Temperate continental |
| Sweden | 1997 | 1997 | 13.5% | 185 | Island residents | Koster Islands | Temperate continental |
| Sweden | 1993 | 1989 | 2.0% | 50 | BD’s non endemic/City/hospital | North/Urban | Temperate continental |
| Sweden | 1993 | 1989 | 8.7% | 150 | BD | Stockholm/Urban | Temperate continental |
| Sweden | 2014 | 2004 | 11.0% | 90 | BD | Not stated | Temperate continental |
| Sweden | 2001 | 2000 | 5.3% | 249 | Clerks | Southwest/Urban | Temperate continental |
| Sweden | 2007 | 2003 | 8.0% | 200 | ND | Regional/Rural + Urban] | Temperate continental |
| Sweden | 2007 | 2003 | 14.0% | 200 | BD | Regional/Rural + Urban] | Temperate continental |
| Sweden | 2007 | 2003 | 12.0% | 200 | BD | Regional/Rural + Urban] | Temperate continental |
| Switzerland | 1991 | 1986 | 6.0% | 50 | General population | Alpine Rural + Urban | Alpine temperate |
| Turkey | 2018 | 2014 | 4.1% | 368 | General population | Erzincan/Mixed | Dry |
| Turkey | 2019 | 2018 | 6.0% | 193 | BD | Düzce/Rural | Dry |
Table A2.
Input data: Full list of studies and data for seroprevalence in dogs.
|
Country/State |
Sero-prevalence animals AP |
Sample n |
Positive n |
Publication Year |
Year of study or publication |
|
|---|---|---|---|---|---|---|
| Brazil |
9.7% |
237 |
23 |
2001 |
2001 |
|
| Brazil | 0.04% | 2553 | 1 | 2003 | 2003 | |
| Brazil | 4.9% | 1395 | 9 | 2008 | 2008 | |
| Bulgaria | 22.6% | 106 | 24 | 2003 | 2003 | |
| Bulgaria | 6.7% | 46 | 3 | 2006 | 2006 | |
| Bulgaria | 2.4% | 25 | 1 | 2015 | 2015 | |
| Bulgaria | 10.6% | 59 | 9 | 2008 | 2008 | |
| Germany/Munich | 10.5% | 200 | 21 | 2014 | 2007 | |
| Germany | 5.3% | 207 | 11 | 2005 | 2001 | |
| Germany | 7.2% | 207 | 15 | 2005 | 2001 | |
| Germany | 22.2% | 207 | 46 | 2005 | 2001 | |
| Berlin | 10.1% | 189 | 19 | 1990 | 1990 | |
| Germany | 11.1% | 202 | 22 | 2004 | 2000 | |
| Czech Republic | 7% | 399 | 2006 | 2006 | ||
| England/Hampshire | 2.6% | 115 | 3 | 1988 | 1988 | |
| England/Hereford | 9.8% | 41 | 4 | 1988 | 1988 | |
| England | 6.2% | 78 | 4 | 1988 | 1988 | |
| Finland east | 0.0% | 24 | 0 | 2014 | 2011 | |
| Finland west | 3.3% | 92 | 3 | 2014 | 2011 | |
| Finland south | 1.8% | 163 | 3 | 2014 | 2011 | |
| Aland | 20.0% | 20 | 4 | 2014 | 2011 | |
| Finland | 6.3% | 75 | 3 | 2014 | 2011 | |
| France | 25.0% | 88 | 22 | 1988 | 1986 | |
| France | 10.4% | 183 | 19 | 1998 | 1992 | |
| France | 1.1% | 919 | 10 | 2009 | 2009 | |
| France | 12.2% | 304 | 15 | 2000 | 1998 | |
| Hungary | 0.4% | 1305 | 5 | 2014 | 2014 | |
| Italy | 0.0% | 23 | 0 | 1999 | 1999 | |
| Italy | 0.3% | 1335 | 4 | 2017 | 2017 | |
| Italy Central | 1.5% | 1965 | 29 | 2014 | 2014 | |
| Italy | 1.0% | 1108 | 11 | |||
| Japan | 10.2% | 314 | 32 | 2016 | 2016 | |
| Korea | 1.1% | 532 | 6 | 201 | 2017 | |
| Mexico | 6.8% | 384 | 26 | 2008 | 2008 | |
| Mexico | 0.2% | 1706 | 4 | 2016 | ||
| Mexico | 16.0% | 850 | 136 | 1999 | 1999 | |
| Mexico | 7.7% | 980 | 55 | 2008 | 2004 | |
| Netherlands | 17.0% | 75 | 2000 | 2000 | ||
| Poland | 19.7% | 243 | 48 | 2016 | 2016 | |
| Poland | 5.3% | 157 | 8 | 2016 | 2013 | |
| Poland | 12.5% | 200 | 28 | 2016 | 2015 | |
| Portugal | 0.2% | 557 | 1 | 2012 | 2012 | |
| Romania | 6.5% | 276 | 18 | 2011 | 2011 | |
| Serbia | 25.5% | 486 | 124 | 2010 | 2010 | |
| Spain tarragona malloca | 0.7% | 460 | 3 | 2006 | 2006 | |
| Spain | 21.0% | 308 | 37 | 1995 | 1995 | |
| Spain | 6.3% | 649 | 41 | 2008 | 2008 | |
| Spain | 11.6% | 146 | 17 | 2000 | 2000 | |
| Spain | 1.1% | 95 | 1 | 1997 | 1997 | |
| Spain | 8.1% | 297 | 20 | 2002 | 2002 | |
| Sweden | 10.3% | 54 | 6 | 2009 | 2009 | |
| Sweden | 4.7% | 611 | 29 | 2000 | 1993 | |
| Sweden | 7.5% | 333 | 17 | 2005 | 2001 | |
| Turkey | 23.2% | 400 | 93 | 2008 | 2008 | |
| USA | 0.00% | 0 | 0 | 2019 | 2018 | |
| 7.8% |
Table A3.
Input data: Seroprevalence in US dogs mean for years 2012–18
| Year | Seroprevalence | Sample size |
|---|---|---|
| 2012 | 6.85% | 2,367,261 |
| 2013 | 6.26% | 2,756,275 |
| 2014 | 6.43% | 3,323,397 |
| 2015 | 6.19% | 3,975,831 |
| 2016 | 6.43% | 4,172,861 |
| 2017 | 6.27% | 4,833,554 |
| 2018 | 5.64% | 5,646,390 |
| Mean | 6.30% | 3,867,938 |
| Annual Growth (decline) | −0.13% |
Source: Companion Animal Parasite Council. Parasite Prevalence Maps: Tick Borne disease agents: Lyme disease: Dogs. https://www.capcvet.org/maps/#2014/all/lyme-disease/dog/united-states/. Published 2019. Accessed April 23, 2019.
Table A4.
Effect of Canadian sample size on the mean of dog seroprevalence
| Sample size Seroprevalence |
||||||
|---|---|---|---|---|---|---|
| With Canada |
Without Canada and US |
|||||
| Seroprevalence | Positive | Sample size | Seroprevalence | Sample size | ||
| 0.02% | 17 | 86251 | ||||
| 4.90% | 68 | 1395 | 4.90% | 1395 | ||
| 10.60% | 6 | 59 | 10.60% | 59 | ||
| 6.50% | 26 | 399 | 6.50% | 399 | ||
| 6.20% | 5 | 78 | 6.20% | 78 | ||
| 6.30% | 5 | 75 | 6.30% | 75 | ||
| 12.20% | 37 | 304 | 12.20% | 304 | ||
| 11.10% | 22 | 202 | 11.10% | 202 | ||
| 0.40% | 5 | 1305 | 0.40% | 1305 | ||
| 1.00% | 11 | 1108 | 1.00% | 1108 | ||
| 10.20% | 32 | 314 | 10.20% | 314 | ||
| 1.10% | 6 | 532 | 1.10% | 532 | ||
| 7.70% | 75 | 980 | 7.70% | 980 | ||
| 17.00% | 13 | 75 | 17.00% | 75 | ||
| 12.50% | 30 | 243 | 12.50% | 243 | ||
| 0.20% | 0 | 157 | 0.20% | 157 | ||
| 6.50% | 36 | 557 | 6.50% | 557 | ||
| 25.50% | 124 | 486 | 25.50% | 486 | ||
| 8.10% | 24 | 297 | 8.10% | 297 | ||
| 7.50% | 25 | 333 | 7.50% | 333 | ||
| 95150 | 8899 | |||||
| 0.60% | Mean | 8.18% | ||||
Table A5.
Serology test sensitivities and specificities
| Test method | Sensitivity | Specificity |
|---|---|---|
| ELISA | 62.30% | 95.0% |
| C6 | 53.90% | 97.9% |
| Western Blot | 62.40% | 95.7% |
| Overall | 59.5% | 96.2% |
Source: Cook MJ, Puri BK. Commercial test kits for the detection of Lyme borreliosis: a meta-analysis of test accuracy. Int J Gen Med. 2016; 9:427–440. doi:https://doi.org/10.2147/IJGM.S122313
Table A6.
| Country | Canineseropositive | Human seropositive |
|---|---|---|
| England | 6.5% | 2.1% |
| Finland | 6.3% | 4.1% |
| France | 12.2% | 13.9% |
| Germany | 11.1% | 11.8% |
| Italy | 1.0% | 5.3% |
| Netherlands | 17.0% | 9.0% |
| Sweden | 7.5% | 12.3% |
Fig. A1.
Chart of seroprevalence in humans versus year of study (xlsx).
Fig. A2.
Chart of seroprevalence in dogs versus year of study (xlsx).
References
- Arteaga F., Perez A.G., Barral M., Pedro Anda, Garcia-Monco J.C., Golightly M.G. Disparity between serological reactivity to Borrelia burgdorferi and evidence of past disease in a high-risk group. Clinical Infectious Diseases. 2007;27(5):1210–1213. doi: 10.1086/514970. [DOI] [PubMed] [Google Scholar]
- Barth C., Straubinger R.K., Krupka I., Müller E., Sauter-Louis C., Hartmann K. Comparison of different diagnostic assays for the detection of Borrelia burgdorferi-specific antibodies in dogs. Veterinary Clinical Pathology/American Society for Veterinary Clinical Pathology. 2014;43(4):496–504. doi: 10.1111/vcp.12213. [DOI] [PubMed] [Google Scholar]
- Beugnet F., Marié J.L. Emerging arthropod-borne diseases of companion animals in Europe. Veterinary Parasitology. 2009;163(4):298–305. doi: 10.1016/j.vetpar.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Bloor K. 2014. Lyme and tick-borne infections patient survey.http://lymeresearchuk.org/wp-content/uploads/2012/04/prof-diag-final-WPress.pdf Retrieved. [Google Scholar]
- Burrell G.A., Seibert F.M. Experiments with small animals and carbon monoxide. Journal of Industrial and Engineering Chemistry. 1914;6(3):241–244. doi: 10.1021/ie50063a027. [DOI] [Google Scholar]
- Bu X.L., Yao X.Q., Jiao S.S., Zeng F., Liu Y.H., Xiang Y. A study on the association between infectious burden and Alzheimer’s disease. European Journal of Neurology. 2015;22(12):1519–1525. doi: 10.1111/ene.12477. [DOI] [PubMed] [Google Scholar]
- Cairns V., Godwin J. Post-lyme borreliosis syndrome: A meta-analysis of reported symptoms. International Journal of Epidemiology. 2005;34(6):1340–1345. doi: 10.1093/ije/dyi129. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2013. CDC provides estimate of Americans diagnosed with Lyme disease each year.http://www.cdc.gov/media/releases/2013/p0819-lyme-disease.html Retrieved from. [Google Scholar]
- Çetin B. Lyme hastaligi “Iklim degisiminin ilk pandemisi”. 1st. Birlesik Matbaaclilic Buca OSB Mah Begos 2 Bolge; Izmir: 2018. [Google Scholar]
- Chmielewska-Badora J., Cisak E., Dutkiewicz J. Lyme borreliosis and multiple sclerosis: Any connection? A seroepidemic study. Annals of Agricultural and Environmental Medicine. 2000;7(1):141–143. doi: 10.1002/(SICI)1097-0177(199909)216:1<1::AID-DVDY1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Companion Animal Parasite Council . 2019. Parasite prevalence Maps: Tick borne disease agents: Lyme disease: Dogs.https://www.capcvet.org/maps/#2014/all/lyme-disease/dog/united-states/ Retrieved. [Google Scholar]
- Cook M.J., Puri B.K. Commercial test kits for the detection of Lyme borreliosis: A meta-analysis of test accuracy. International Journal of General Medicine. 2016;9:427–440. doi: 10.2147/IJGM.S122313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M.J., Puri B.K. Application of Bayesian decision-making to laboratory testing for Lyme disease and comparison with testing for HIV. International Journal of General Medicine. 2017;10:113–123. doi: 10.2147/IJGM.S131909. https://www.dovepress.com/articles.php?article_id=32303 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delong A., Hsu M., Kotsoris H. Estimation of cumulative number of post- treatment Lyme disease cases in the US , 2016 and 2020. BMC Public Health. 2019;19(352):1–8. doi: 10.1186/s12889-019-6681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan A.W., Correa M.T., Levine J.F., Breitschwerdt E.B. The dog as a sentinel for human infection: Prevalence of Borrelia burgdorferi C6 antibodies in dogs from southeastern and mid-atlantic states. Vector Borne and Zoonotic Diseases. 2005;5(2):101–109. doi: 10.1089/vbz.2005.5.101. [DOI] [PubMed] [Google Scholar]
- Eng T.R., Wilson M.L., Spielman A., Lastavica C.C. Greater risk of Borrelia burgdorferi infection in dogs than in people. The Journal of Infectious Diseases. 1988;158(6):1410–1411. doi: 10.1093/infdis/158.6.1410. http://www.ncbi.nlm.nih.gov/pubmed/3198950 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Fahrer H., Sauvain M.J., Zhioua E., Van Hoecke C., Gern L.E. Longterm survey (7 years) in a population at risk for Lyme borreliosis: What happens to the seropositive individuals? European Journal of Epidemiology. 1998;14(2):117–123. doi: 10.1023/A:1007404620701. [DOI] [PubMed] [Google Scholar]
- Falco R.C., Smith H.A., Fish D., Mojica B.A., Bellinger M.A., Harris H.L. The distribution of canine exposure to Borrelia burgdorferi in a Lyme-Disease endemic area. American Journal of Public Health. 1993;83(9):1305–1310. doi: 10.2105/ajph.83.9.1305. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1694954%26tool=pmcentrez%26rendertype=abstract%0Ahttp://www.ncbi.nlm.nih.gov/pubmed/8363007%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1694954 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulde M., Freise J. Public health pests. Arthropods and rodents as causative disease agents as well as reservoirs and vectors of pathogens. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2014;57(5):495–503. doi: 10.1007/s00103-013-1919-7. http://www.ncbi.nlm.nih.gov/pubmed/24781905 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Goossens H.A.T. Vol. 39. 2001. pp. 844–848. (Dogs as sentinels for human Lyme Borreliosis in The Netherlands). 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutacker M., Valsangiacomo C., Balmelli T., Bernasconi M.V., Bouras C., Piffaretti J.-C. Arguments against the involvement of Borrelia burgdorferi sensu lato in Alzheimer’s disease. Research in Microbiology. 1998;149(1):31–37. doi: 10.1016/S0923-2508(97)83621-2. [DOI] [PubMed] [Google Scholar]
- Guy E.C., Bateman D.E., Martyn C.N., Heckels J.E., Lawton N.F. Lyme disease: Prevalence and clinical importance of Borrelia burgdorferi specific IgG in forestry workers. Lancet. 1989;1(8636):484–486. doi: 10.1016/s0140-6736(89)91377-9. http://www.ncbi.nlm.nih.gov/pubmed/2563850 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Hebert L.E., Weuve J., Scherr P.A., Evans D.A. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley A.F., Connally N.P., Meek J.I., Johnson B.J., Kemperman M.M., Feldman K.A. Clinical infectious Diseases. An Official Publication of the Infectious Diseases Society of America; 2014. Lyme disease testing by large commercial laboratories in the United States. ciu397- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käsbohrer A., Schönberg A. Serologic studies of the occurrence of Borrelia burgdorferi in domestic animals in Berlin (West) Berliner und Münchener Tierärztliche Wochenschrift. 1990;103(11):374–378. http://www.ncbi.nlm.nih.gov/pubmed/2268252 Retrieved from. [PubMed] [Google Scholar]
- Kuiper H., de Jongh B.M., Nauta A.P., Houweling H., Wiessing L.G., van Charante A.W. Lyme borreliosis in Dutch forestry workers. Journal of Infection. 1991;23(3):279–286. doi: 10.1016/0163-4453(91)92936-y. http://www.ncbi.nlm.nih.gov/pubmed/1753136 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Kuiper H., van Dam A.P., Moll van Charante A.W., Nauta N.P., Dankert J., Dam A.P. One year follow-up study to assess the prevalence and incidence of Lyme borreliosis among Dutch forestry workers. European Journal of Clinical Microbiology & Infectious Diseases. 1993;12(6):413–418. doi: 10.1007/BF01967434. [DOI] [PubMed] [Google Scholar]
- Lindenmayer J.M., Marshall D., Onderdonk A.B. Dogs as sentinels for Lyme disease in Massachusetts. American Journal of Public Health. 1991;81(11):1448–1455. doi: 10.2105/AJPH.81.11.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren E., Jaenson T.G.T. Lyme borreliosis in Europe : Influences of climate and climate change , epidemiology, ecology and adaptation measures. In: Menne B., Ebi K.L., editors. Vol. EU/04/5046. 2006. http://www.euro.who.int/__data/assets/pdf_file/0006/96819/E89522.pdf (World health organization). World Health Organization Europe. Retrieved from. [Google Scholar]
- MacDonald A.B. Borrelia in the brains of patients dying with dementia. Journal of the American Medical Association. 1986;256(16):2195–2196. [PubMed] [Google Scholar]
- MacDonald A.B. Plaques of Alzheimer’s disease originate from cysts of Borrelia burgdorferi, the Lyme disease spirochete. Medical Hypotheses. 2006;67(3):592–600. doi: 10.1016/j.mehy.2006.02.035. [DOI] [PubMed] [Google Scholar]
- Mannelli A., Cerri D., Buffrini L., Rossi S., Rosati S., Arata T. Low risk of Lyme borreliosis in a protected area on the Tyrrhenian coast, in central Italy. European Journal of Epidemiology. 1999;15(4):371–377. doi: 10.1023/a:1007535313763. http://www.ncbi.nlm.nih.gov/pubmed/10414379 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Marquard R., Kurz A., Bremer D., Dose M. Borrelia burgdorferi: Risk factor in alzheimer’s DiseasePoster session 2 , monday 12 september. European Journal of Neurology. 2011;18(Suppl):345. doi: 10.1111/j.1468-1331.2011.03552.x. [DOI] [Google Scholar]
- Marques A.R., Weir S.C., Fahle G.A., Fisher S.H. Lack of evidence of Borrelia involvement in alzheimer ’ s disease. Journal of Infectious Diseases. 2000;182:1999–2000. doi: 10.1086/315792. [DOI] [PubMed] [Google Scholar]
- McLaughlin R., Ng Ying Kin N.M.K., Chen M.F., Nair N.P.V., Chan E.C.S. Alzheimer’s disease may not be a spirochetosis. NeuroReport. 1999;10(7):1489–1491. doi: 10.1097/00001756-199905140-00018. [DOI] [PubMed] [Google Scholar]
- Mead P., Goel R., Kugeler K. Canine serology as adjunct to human surveillance. Emerging Infectious Diseases. 2011;17(9):1710–1712. doi: 10.3201/1709.110210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy J. Alzheimer disease -- A spirochetosis? Alzheimer Disease. 1994:41–45. doi: 10.1007/978-1-4615-8149-9_7. [DOI] [Google Scholar]
- Miró G., Montoya A., Roura X., Gálvez R., Sainz A. Seropositivity rates for agents of canine vector-borne diseases in Spain: A multicentre study. Parasites & Vectors. 2013;6(1):117. doi: 10.1186/1756-3305-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Capner P., Cutler S., Wright D.J., Hamlet N., Nathwani D., Ho-Yen D. Borrelia burgdorferi infection in UK Workers at risk of tick bites. The Lancet. 1989:789. doi: 10.1016/S0140-6736(89)92612-3. April 8. [DOI] [PubMed] [Google Scholar]
- Muhlemann M.F., Wright D.J.M. Emerging pattern of Lyme disease in the United Kingdom and Irish republic. The Lancet. 1987;329(8527):260–262. doi: 10.1016/S0140-6736(87)90074-2. [DOI] [PubMed] [Google Scholar]
- Müller I., Freitag MH., Poggensee G., Scharnetzky E., Straube E., Schoerner Ch.…Hunfeld KP. Evaluating frequency , diagnostic quality and cost of Lyme borreliosis testing in Germany : A retrospective model analysis. Molecular Microbiology. 2012:2–26. doi: 10.1155/2012/595427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., Saha S., Kugeler K.J., Delorey M.J., Shankar M.B., Hinckley A.F. Incidence of clinician-diagnosed Lyme disease. Emerging Infectious Diseases. 2015;21(9):1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappolla M.A.A., Omar R., Saran B., Andorn A., Suarez M., Pavia C. Concurrent neuroborreliosis and alzheimer’s disease: Analysis of the evidence. Human Pathology. 1989;20(8):753–757. doi: 10.1016/0046-8177(89)90068-3. [DOI] [PubMed] [Google Scholar]
- Piantedosi D., Neola B., D’Alessio N., Di Prisco F., Santoro M., Pacifico L. Seroprevalence and risk factors associated with Ehrlichia canis, Anaplasma spp., Borrelia burgdorferi sensu lato, and D. immitis in hunting dogs from southern Italy. Parasitology Research. 2017;116(10):2651–2660. doi: 10.1007/s00436-017-5574-z. [DOI] [PubMed] [Google Scholar]
- Riviere G.R., Riviere K.H., Smith K.S. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiology and Immunology. 2002;17(2):113–118. doi: 10.1046/j.0902-0055.2001.00100.x. [DOI] [PubMed] [Google Scholar]
- Seifert D. Borreliose - die unterschätzte Zeckeninfection. Pressekonferenz Borreliose und FSME Bund Deutschland. Die Deutsche Borreliose-Gesellschft e.v; Berlin and Jena: 2010. Inzidenz der Lyme-borrliose 2009 in deutschland. [Google Scholar]
- Shope R.E., Causey C.E., Causey O.R. Itaqui virus, a new member of {Arthropod-Borne} group C. The American Journal of Tropical Medicine and Hygiene. 1961;10(2):264–265. [Google Scholar]
- Smith F.D., Ballantyne R., Morgan E.R., Wall R. Estimating Lyme disease risk using pet dogs as sentinels. Comparative Immunology, Microbiology and Infectious Diseases. 2012;35(2):163–167. doi: 10.1016/j.cimid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Steere A.C., Malawista S.E., Snydman D.R., Shope R.E., Andiman W.A., Ross M.R. Lyme Arthritis: An epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis & Rheumatism. 1977;20(1):7–17. doi: 10.1002/art.1780200102. [DOI] [PubMed] [Google Scholar]
- Stone E.G., Lacombe E.H., Rand P.W. Antibody testing and Lyme disease risk. Emerging Infectious Diseases. 2005;11(5):722–724. doi: 10.3201/eid1105.040381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R.A., Makiello P. An estimate of Lyme borreliosis incidence in western Europe. Journal of Public Health. 2016;1(1):1–8. doi: 10.2218/resmedica.v22i1.743. [DOI] [PubMed] [Google Scholar]
- Töpfer K. Charakterisierung der humoralen immunantwort im hund nach impfung mit verschiedennen Impfstoffen gegen den erreger der Lyme-borreliose, Borrelia burgdorferi, unter berücksichtigung zweier verschiedener impfstrategien. Inaugural-Dissertation. 2005 Veterinärmedizinische Fakultät. Univ Leipzig. Univ Leipzig. [Google Scholar]
- Valdez A.R., Hancock E.E., Adebayo S., Kiernicki D.J., Proskauer D., Attewell J.R. Estimating prevalence, demographics, and costs of ME/CFS using large scale medical claims data and machine learning. Frontiers in Pediatrics. 2019;6(JAN):1–14. doi: 10.3389/fped.2018.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove O., De Buck E., Van Wijngaerden E. Lyme disease in western Europe: An emerging problem? A systematic review. Acta Clinica Belgica: International Journal of Clinical and Laboratory Medicine. 2019 doi: 10.1080/17843286.2019.1694293. Taylor and Francis Ltd. [DOI] [PubMed] [Google Scholar]
- Vos T., Bell B, Bertozzi-Villa A. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study. The Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin M.T., Culpepper W.J., Campbell J.D., Nelson L.M., Langer-Gould A., Marrie R.A. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 2019;92(10):E1029–E1040. doi: 10.1212/WNL.0000000000007035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson P., Fryland L., Lindblom P., Sjöwall J., Ahlm C., Berglund J. A prospective study on the incidence of Borrelia burgdorferi sensu lato infection after a tick bite in Sweden and on the Åland Islands, Finland (2008-2009) Ticks and Tick-Borne Diseases. 2016;7(1):71–79. doi: 10.1016/j.ttbdis.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Zeman P., Januška J. Epizootiologic background of dissimilar distribution of human cases of Lyme borreliosis and tick-borne encephalitis in a joint endemic area. Comparative Immunology, Microbiology and Infectious Diseases. 1999;22(4):247–260. doi: 10.1016/S0147-9571(99)00015-6. [DOI] [PubMed] [Google Scholar]