Highlights
-
•
Expeditious germline testing is a vital component of epithelial ovarian cancer treatment.
-
•
Uninsured and minority patients are at risk for lower germline testing rates.
-
•
Physician performed germline testing shortens the interval to results reporting in a safety net clinic population.
-
•
The majority of patients tested were uninsured or underinsured at the time of testing.
Keywords: Ovarian cancer, Germline testing, Health disparities
Abstract
Germline genetic mutations occur in approximately 25% of women with epithelial ovarian cancers (EOC). We sought to determine whether newly initiated in-office oncologist-led germline testing improved time to testing and dissemination of results compared with historical controls.
Patients with epithelial ovarian cancer seen between 4/1/2018 and 12/31/2019 were identified. Patients treated before genetic testing kits were made available in the gynecologic oncology clinics were compared to those treated after. Categorical variables were compared using Chi Squared and Fisher’s Exact test. Cox proportional hazards model was used to compare elapsed time from testing to results.
73 patients were identified, and 502 clinic visits were analyzed. 56 (76.7%) patients were White Hispanic, 15 (20.5%) were Black, and 2 (2.7%) were White non-Hispanic. 55 (75.7%) underwent germline testing. Median time to genetic testing in the intervention group was shorter than in the control group (5, vs 24.3 weeks, 95% CI = 0–10.8 vs 14.9–33.7, p < 0.001). Among the 51 patients with genetic tests completed; results were recorded in a clinic note at 14 weeks (95% CI = 0–28.1) from first visit in the intervention group compared with 47 weeks (95% CI = 30.7–63.3) in the control group (p < 0.001). The majority of patients tested had county charity care insurance or were uninsured.
Genetic testing in a safety net gynecologic oncology clinic is feasible. By initiating in-office testing, time to testing and receipt of results were meaningfully shortened. This allowed for timely identification of patients who would most benefit from PARP inhibitor maintenance therapy.
1. Introduction
Epithelial ovarian cancer (EOC) is the most lethal gynecologic cancer, accounting for nearly 14,000 deaths and 22,000 new diagnoses in the United States 2020 (American Cancer Society. Cancer Facts Figures, 2020). Recent advances in poly adenosine diphosphate-ribose polymerase inhibitor (PARPi) maintenance therapy following first-line treatment have dramatically improved survival in patients with germline or somatic BRCA mutations within the homologous recombination pathway (Moore et al., 2018, Gonzalez-Martin et al., 2019, Ray-Coquard et al., 2019). Germline mutations occur in 13–25% of women with EOCs, with 18% BRCA1 or BRCA2 loss-of-function mutations and 3–6% in other genes such as CHEK2, MSH6, PALB2, RAD51C/D or TP53 (Walsh et al., 2011, Pal et al., 2005, Zang et al., 2011, Norquist et al., 2016). Over 30% of women with inherited mutations have no family history of breast or ovarian carcinoma (Society of Gynecologic Oncology. SGO clinical practice statement: Genetic testing for ovarian cancer., 2014).
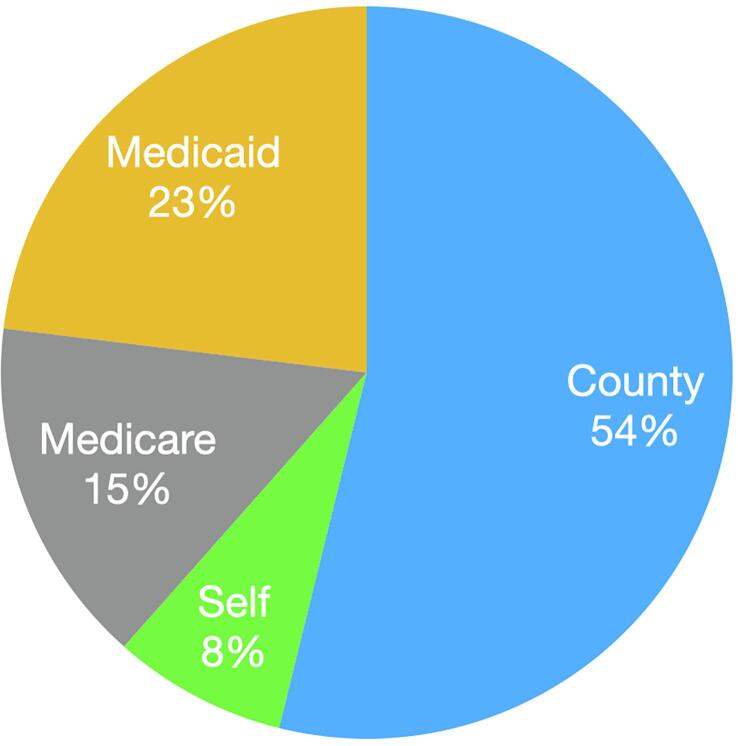
Despite the current recommendations for universal germline screening for patients with EOC by the Society for Gynecologic Oncology (SGO), American Society of Clinical Oncology (ASCO), and National Comprehensive Cancer Network (NCCN) (Society of Gynecologic Oncology. SGO clinical practice statement: Genetic testing for ovarian cancer., 2014, Konstantinopoulos et al., 2020, [11], Paluch-Shimon et al., 2016); germline testing uptake remains low, both locally and nationally. A recent Surveillance, Epidemiology, and End Results (SEER) database study reported an approximate testing rate of 30% in EOC patients, with lower rates reported in Black and uninsured patients (Kurian et al., 2019). A study performed at our institution demonstrated similar results—disproportionate genetic testing between the comprehensive cancer center (60%) and the safety net county hospital (SNH) (38%) (Huang et al., 2019) (see Fig. 1, Fig. 2) .
Fig. 1.
Clinic visits: demonstrating an increase in same-day testing among patients who have not previously undergone testing. GT: Genetic testing.
Fig. 2.
Insurance status of patients undergoing clinic genetic testing.
Possible barriers to genetic testing include inconvenience, time, transportation, and cost to patients, as well as availability of appointments with genetic counselors (Randall et al., 2017). A 2017 SGO white paper recommended physician-performed in office germline testing to streamline results and counseling (Randall et al., 2017). The feasibility of this approach was validated in the single arm ENGAGE study, which demonstrated expeditious test turnaround time and high rates of patient satisfaction (Colombo et al., 2018).
With a goal to improve rates of genetic testing, shortening the interval to specimen collection and obtaining timely results, we began to offer physician performed germline testing to patients in our SNH gynecologic oncology (GO) clinic. The objectives of this study were to (1) determine the rates of genetic testing completion in clinic, (2) examine the time to testing, and (3) compare time to reported results with the prior practice of referral to a genetic counselor.
2. Methods
2.1. Intervention
Prior to initiation of in-clinic germline testing, EOC patients within the SNH were referred to a separate genetics clinic for genetic counseling, testing, and results discussion. Genetic clinic appointments were usually not available for several weeks to months from initial request and often required re-scheduling due to missed appointments (i.e. time off, travel, cancelled clinics). Testing results were unavailable to the GO team until scanned into the electronic medical record by the genetic counseling staff.
After logistical planning and receipt of test materials, in-clinic germline testing was first made available in our clinic on 5/21/2019 utilizing Myriad myRisk saliva collection kits (Myriad Genetics, Salt Lake City, UT). All patients were counseled on the risks and benefits of genetic testing in their native language and provided informed consent for testing. GO fellows and obstetrics and gynecology residents performed the collection and submitted all specimens at the time of scheduled clinic visits. Testing was performed as early as possible after diagnosis. Patients who were uninsured or under-insured were referred to the Myriad patient assistance program for financial support. Test status and results were directly available to fellows and attending physicians through the Myriad online portal. Patients with positive or variant of unknown significance (VUS) test results were then referred to the genetic counselor.
2.2. Data collection and analysis
Institutional review board approval was obtained (UM IRB #20151022). Patients with EOC seen between 4/1/2018 and 12/30/2019 at the SNH GO clinic were identified and included. Patients without EOC, those with only one visit in our clinic, or who were previously tested at an outside facility were excluded. Patients with missing data were excluded from analyses.
Study data were collected and managed using REDCap (Research Electronic Data Capture) tool hosted at the University of Miami (Harris et al., 2009, Harris et al., 2019). Data were abstracted retrospectively for the following variables: Age at first visit, race/ethnicity, primary language, family history of cancer, histology, stage, date of first visit with gynecologic oncology, date genetic testing performed, and date genetic testing results were reported. Each visit during the study period was then abstracted. Visit data variables included: insurance held at the time of visit, whether or not genetic testing had previously been performed, whether or not testing was performed at the current visit, and whether or not results were reported at the current visit. After genetic testing results were noted to be available, future visits were not included in analysis.
Patients were compared between the historical controls who initiated care before the initiation of in-office testing (pre-5/21/2019), and those who initiated care after that date (5/21/2019–12/30/2019). Clinic visit data were similarly compared between pre-intervention (4/1/2018–5/20/2019) and post-intervention (5/21/2019–12/30/2019) encounters.
Data were analyzed using SPSS version 25 (IBM Corp, Armonk, NY). Categorical variables were compared with Chi-squared and Fishers Exact tests where appropriate. Cox Proportional Hazards, and Kaplan Meier models were utilized to compare elapsed time-to-testing and time-to-results. All tests were 2-sided and statistical significance was set at p = 0.05.
3. Results
3.1. Patient data analysis
During the timeframe, 80 patients with ovarian cancer were identified. 73 patients (91%) met inclusion criteria. Seven were excluded. Patient demographic data is summarized in Table 1. 62 patients (84.9% of study population) initiated care prior to the initiation of in-clinic germline testing (earliest: 11/13/2006), and 11 initiated EOC care after that point (15.1%). There were no significant demographic differences between the two cohorts. Overall, 56 patients (76.7%) were White Hispanic (HW), 15 (20.5%) were Black (B), and 2 (2.7%) were White non-Hispanic (NHW). The majority of patients spoke only Spanish.
Table 1.
Demographic information, reported for historical control cohort and intervention cohort.
| Variable (n) | Historical Control (n = 62) | Intervention (n = 11) | p-value |
|---|---|---|---|
| Age (median) | 57.6 (range 25–77) | 60.8 (range 52.9–79) | |
| Race/Ethnicity | |||
|
White non-Hispanic (2) White Hispanic (56) Black (15) |
2 (3.2%) 47 (75.8%) 13 (21%) |
0 9 (81.8%) 2 (18.2%) |
1.00 |
| Language | |||
|
English (14) Spanish (55) Haitian Creole (4) |
13 (21%) 45 (72.6%) 4 (6.5%) |
1 (9.1%) 10 (90.9%) 0 |
0.41 |
| Diagnosis location | |||
|
Within health system (58) Outside facility (14) |
51 (83.6%) 10 (16.4%) |
7 (63.6%) 4 (36.4%) |
0.2 |
| Family history of breast or ovarian cancer | |||
|
Yes (16) No (57) |
13 (21%) 49 (79%) |
3 (27.3%) 8 (72.7%) |
0.697 |
| Stage at diagnosis | |||
|
I-II (26) III-IV (44) |
24 (38.7%) 38 (61.3%) |
2 (25%) 6 (75%) |
0.7 |
| Histology | |||
|
High grade serous (46) Other (27) |
37 (59.7%) 25 (40.3%) |
9 (81.8%) 2 (18.2%) |
0.2 |
Overall, 57 of the 73 patients (78.1%) included had genetic testing performed. Of patients who underwent testing, results were reported within a clinic note in 53 patients (93%). The overall median time from the first visit to the date testing performed was 21.1 weeks (range: 0–525, 95% CI: 16.4–25.9). The overall median time from the first visit to the reporting of results in a gynecologic oncology note was 37 weeks (range: 3–662, 95% CI: 25.8–48.2). The overall median time from testing to the reporting of results was 10.9 weeks (range: 2–267, 95% CI: 7–14.7).
44 patients (71%) of patients in the control group underwent genetic testing prior to the intervention, compared with nine (81.1%) in the intervention group (p = 0.7). Four patients in the control group were tested in-clinic following the intervention. The most commonly identified reason for not receiving genetic testing in the control group was lack of referral to genetic counseling, followed by non-compliance with the scheduled genetic counseling appointment. In the intervention group, the most common reason identified for non-testing was the patient’s desire to avoid a prolonged visit.
In the intervention group, median time from first clinic visit to the initiation of germline testing was 5 weeks versus 24.3 weeks in the control group (Table 2). The hazard ratio for a longer time to testing in the control group was 6.88 (95% CI: 3–15.7, p < 0.001). One patient was able to have testing performed while inpatient prior to her first clinic visit, and two others were tested at the first visit following diagnosis.
Table 2.
Interval to testing, analyzed by Cox Proportional Hazards. Median elapsed time determined by Kaplan Meier regression.
| Variable | Cohort (n) | Median elapsed time in weeks (95% CI) | HR (95% CI) | p-value |
|---|---|---|---|---|
| Time from first visit to test | Control (47) Intervention (9) |
24.3 (14.9–33.7) 5 (0–10.8) |
6.88 (3–15.7) | <0.001 |
| Time from first visit to result | Control (47) Intervention (6) |
47 (30.7–63.3) 14 (0–28.1) |
5.5 (2.2–13.8) | <0.001 |
| Time from test to result | Control (46) Intervention (6) |
10.9 (6.3–15.4) 9 (3.3–14.7) |
1.5 (0.63–3.6) | 0.35 |
Six of the nine patients (67%) tested in the intervention group had results reported at the time of data abstraction. The median time from first clinic visit to the reporting of results in a GO note was 14 weeks in the experimental group versus 47 in the control group. The hazard ratio for prolonged time to results in the control group was 5.5 (95% CI: 2.2–13.8, p < 0.001).
3.2. Visit data analysis
502 clinic encounters were abstracted from the study period. The mean number of visits per patient was 6.9 (range 1–24). During visits where prior testing had not been completed, more notes mentioned a plan to test or refer to genetic counseling in the control group (46.7% vs 19.6%, p = 0.001). If testing had not yet been performed, testing was performed the same day in 26.5% of visits in the intervention group.
Of 13 visits during which testing was performed, 62% of patients either had charity care county insurance or were uninsured. Nine patients (69%) were on active treatment. Ten patients (76.9%) were tested prior to their first recurrence, and the remaining three (23.1%) were during second line treatment.
4. Discussion
Due to recent advances in frontline maintenance therapy using PARP inhibition, expeditious testing is vital in EOC (Moore et al., 2018, Gonzalez-Martin et al., 2019). In this quality improvement project, our goal was to assess the effects of physician lead, in-clinic germline testing for EOC patients in a SNH GO clinic. Even in a short interval, we have been able to demonstrate encouraging advances in the performance of germline testing.
The most encouraging result was the decrease in median time to testing from the first clinic visit. In the historical cohort, Kaplan Meier regression demonstrated a median time to testing of 24.5 weeks from the first clinic visit. Following the initiation of in-office testing, the median elapsed time was 5 weeks.
The results of the SOLO-1, PAOLA-1, and PRIMA trials demonstrated significant improvement in progression free survival in patients with BRCA mutations who receive maintenance PARP inhibitor therapy following frontline chemotherapy (Moore et al., 2018, Gonzalez-Martin et al., 2019, Ray-Coquard et al., 2019). Identifying targetable germline mutations expeditiously will allow for appropriate counseling of patients on their individual risks and benefits of PARP inhibitor therapy in the frontline maintenance setting.
Between the two cohorts, testing uptake was not significantly different. In a recently published study conducted at our institution investigating rates of genetic testing, 38.1% of ovarian cancer patients treated within the SNH GO clinic between 2011 and 2016 underwent germline testing (Huang et al., 2019). Even before offering in-office collection, our testing rate had nearly doubled to 71% of EOCs. We believe this improvement is attributable to standardization amongst our GO division following the advent of PARP inhibitor therapy for germline BRCA-mutated patients starting in 2014 (Kaufman et al., 2015). As mutational status now directly impacts therapeutic planning in frontline therapy, we expect our testing rate to continue to rise.
The insurance status of the patients who did receive in-office testing is encouraging as it relates to improving disparities in access to the standard of care. In our clinical experience, no patient was rejected by the patient assistance program due to inability to pay for testing.
The main strength of our study is the population in whom we were able to implement in-clinic germline testing. It is a majority Hispanic, Spanish speaking population who mostly do not qualify for Medicare or Medicaid, and instead have charity care insurance or are uninsured. This is a patient population which has previously been identified as being at risk for lower testing rates (Kurian et al., 2019, Manrriquez et al., 2018). While the prior study at our institution demonstrated markedly low rates of germline testing in patients with government, charity care/uninsured, or self-pay status, our data demonstrate that these patients are able to be expeditiously tested (Huang et al., 2019).
Our study is limited by the sample size of the intervention group. While small, we were able to demonstrate meaningful decreases in time to testing and results compared to the historical control. As the number of patients tested in the office grows, we expect the process of obtaining and submitting the specimens to run progressively smoother, shortening further the time to testing and the time to results.
Future directions for quality improvement in our population include the feasibility of reflex somatic HRD testing. We are currently beginning to offer this testing in the safety net clinic. Additionally, as the rate of testing increased dramatically between 2011–2016 and 2018–2019, we anticipate the rate of testing to approach 100% in the future.
Aggressive strategies to improve genetic testing uptake and efficiency in all patients, such as in-office germline testing in practice settings where genetic counselors are not readily available should be pursued. Our study demonstrates that in-office testing can be performed in a SNH GO clinic and shortens the time to testing initiation and results, maximizing the clinical benefit for patients at the highest risk to be affected by disparities in oncologic care.
Acknowledgments
Acknowledgement
Myriad Genetics, Inc. provided testing to uninsured and under-insured patients in our practice via its patient Financial Assistance Program.
Declaration of Competing Interest
The authors report no relevant conflicts of interest. No funding was received for this study.
References
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta: American Cancer Society; 2020. Accessed June 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf.
- Moore K., Colombo N., Scambia G. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martin A., Pothuri B., Vergote I. Niraparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2019;381:2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- Ray-Coquard I., Pautier P., Pignata S. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N. Engl. J. Med. 2019;381:2416–2428. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- Walsh T., Casadei S., Lee M.D. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc. Natl. Acad. Sci. U.S.A. 2011;108:18032–18037. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal T., Permuth-Wey J., Betts J.A. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- Zang S., Royer R., Li S. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol. Oncol. 2011;121:353. doi: 10.1016/j.ygyno.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Norquist B.M., Harrell M.I., Brady M.F. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482–490. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Society of Gynecologic Oncology. SGO clinical practice statement: Genetic testing for ovarian cancer. 2014. Accessed June 2020. https://www.sgo.org/resources/genetic-testing-for-ovarian-cancer/.
- Konstantinopoulos P.A., Norquist B., Lacchetti C. Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline. J Clin Oncol. 2020;38:1222–1245. doi: 10.1200/JCO.19.02960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network: Ovarian cancer including fallopian tube cancer and primary peritoneal cancer, version 1.2020. (2020) https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.
- Paluch-Shimon S., Cardoso F., Sessa C. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 2016;27:v103–v110. doi: 10.1093/annonc/mdw327. [DOI] [PubMed] [Google Scholar]
- Kurian A.W., Ward K.C., Howlader N. Genetic testing and results in a population-based cohort of breast cancer patients and ovarian cancer patients. J. Clin. Oncol. 2019;37:1305–1315. doi: 10.1200/JCO.18.01854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Kamath P., Schlumbrecht M. Identifying disparities in germline and somatic testing for ovarian cancer. Gynecol. Oncol. 2019;153:297–303. doi: 10.1016/j.ygyno.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Randall L.M., Pothuri B., Swisher E.M. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology white paper. Gynecol. Oncol. 2017;146:217–224. doi: 10.1016/j.ygyno.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Colombo N., Huang G., Scambia G. Evaluation of a streamlined oncologist-led BRCA mutation testing and counseling model for patients with ovarian cancer. J. Clin. Oncol. 2018;36:1300–1307. doi: 10.1200/JCO.2017.76.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor T., Thielke R. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Minor B.L. The REDCap consortium: Building an international community of software partners. J. Biomed. Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman B., Shapira-Frommer R., Schmutzler R.K. Olaparib monotherapy in patients with advanced cancer and a germline BRCA 1/2 mutation. J. Clin. Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrriquez E., Chapman J.S., Mak J. Disparities in genetics assessment for women with ovarian cancer: can we do better? Gynecol. Oncol. 2018;149:84–88. doi: 10.1016/j.ygyno.2017.10.034. [DOI] [PubMed] [Google Scholar]