Abstract
Background
Real-world data from different regions are needed to support the external validity of controlled trials and assess the impact of new oral anticoagulants (NOAC) in clinical practice.
Methods
“GLORIA-AF” is a large, ongoing, multicenter, global, prospective registry program in patients with newly diagnosed non-valvular atrial fibrillation (NVAF) at risk of stroke. Newly diagnosed patients with NVAF (within 4.5 months) and a CHA2DS2-VASc score ≥ 1 were consecutively enrolled. The study objective was to estimate the incidence rate of stroke and major bleeding after a two year follow up of patients on dabigatran that participated in the “GLORIA-AF” study (Phase II) in Latin America.
Results
Latin America included 378 eligible patients that received dabigatran in eight countries (Argentina, Brazil, Chile, Colombia, Ecuador, Mexico, Perú, and Venezuela): 56.3% were male; mean age was 70.3 ± 10.8 years; 43.4% had paroxysmal AF; 36.0% persistent AF and 20.6% permanent AF. Mean CHA2DS2-VASc score was 3.2 ± 1.4; mean HAS-BLED score was 1.2 ± 0.8. Incidence rates for clinical events after 2-years of follow-up per 100 patient-years were as follows: stroke 0.33 (95% CI: 0.04–1.17), major bleeding 0.49 (95% CI: 0.10–1.42) and all-cause death 4.06 (95% CI: 2.63–6.00). Persistence with dabigatran at 6, 12 and 24 months was 91%, 86%, and 80%, respectively.
Conclusion
These regional data shows the sustained safety and effectiveness of dabigatran over two years of follow-up, consistent with already available evidence. An increase in accessibility and incorporation of NOAC to anticoagulant treatment strategies could potentially have a positive impact on AF stroke prevention in Latin America.
Keywords: Non-valvular atrial fibrillation (NVAF), New oral anticoagulants (NOAC), Dabigatran, Latin America (LA)
1. Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia with a lifetime risk for development of 1 in 4 for the population with 40 years of age and older [1].
Data from local databases and their respective national healthcare systems in seven Latin American (LA) countries showed a range of prevalence from 1.4% to 2.0% for AF in the general population over 40 years old, with non-valvular AF (NVAF) accounting for over 85% of cases [2]. Prevalence increases with age, ranging from 2.2% to 2.3% in people aged 60 to 69 years to 8.2% to 8.5% in those aged ≥ 80 years [2].
Vitamin K antagonists (VKA) have been the standard of care for stroke prevention in AF, with reductions in stroke by 64% and mortality by 26% [3]. Despite the evidence showing its efficacy, anticoagulation with VKA is widely underused in LA [2], [4]. Depending on the country, 18.3% to 24.6% of the AF population [2], and up to 51.2% of the patients with CHADS2 score ≥ 2 (Realise AF study), received no VKA treatment [4]. Moreover, underuse tends to increase with age [2], [5]. Also, a study in Argentina that evaluated consecutive patients with ischemic stroke and prior diagnosis of atrial fibrillation showed that only 54% of patients receiving VKA had an international normalized ratio (INR) within therapeutic ranges [6]. VKA has several drawbacks, such as slow onset of action, variable dose–response, and significant food and drug interactions with frequent monitoring requirements.
Non-VKA oral anticoagulants (NOAC) are changing the current management of AF stroke prevention. Current guidelines recommend NOAC over warfarin in patients eligible to NOAC therapy, including all patients except those with moderate-to-severe mitral stenosis or a mechanical heart valve [7], [8].
Dabigatran was the first NOAC approved by the FDA in 2010 based on the results of phase III RE-LY study that included patients who had NVAF at risk of stroke. In RE-LY, dabigatran 150 mg twice daily was superior to warfarin for preventing all stroke/systemic embolism and ischemic stroke, with rates of major bleeding similar to warfarin. The dabigatran 110 mg twice-daily dose was non-inferior to warfarin for preventing stroke/systemic embolism, with considerably less major bleeding [9], [10]. Both dosages of dabigatran were associated with significantly lower rates of intracranial hemorrhage (ICH) compared to warfarin [10].
Additional regional data are needed to support the external validity of randomized controlled trials (RCT) and to better understand the use of newly approved drugs outside the context of RCT.
The GLORIA-AF Registry was designed to provide additional information in different regions on patients with recent-onset NVAF at risk of stroke with a special focus on effectiveness and safety of dabigatran [11]. Herein we report the analysis of the LA patients included in Phase II of the GLORIA AF that were treated with dabigatran.
2. Methods
2.1. Design and study population
The design and rationale of the three phased GLORIA-AF global non-interventional registry has been previously published [11].
GLORIA-AF included consecutive adult patients with newly diagnosed NVAF (within the last 4.5 months) at risk of stroke with a CHA2DS2-VASc score ≥ 1. Patients with mechanical valves, prior VKA therapy for > 60 days, other medical indications for chronic VKA therapy, AF due to a reversible cause, and life expectancy < 1 year were excluded. The HAS-BLED bleeding score assessed bleeding risk. Centers were selected to reflect those that typically manage new AF in LA.
Phase II of this global registry started when dabigatran was approved in each of the participating countries with the collection of data on the baseline characteristics (including demographics, comorbid disease, and characteristics of AF). In patients prescribed dabigatran, a two year follow up was performed to assess effectiveness and safety. Follow up visits after baseline assessment took place at 3, 6, 12 and 24 months. Dabigatran was prescribed according to the local approved label and judgment of the treating physician (150 and 110 mg twice daily-BID). One patient that received 75 mg BID was excluded from the study population eligible for analysis.
Data were collected from medical records obtained in clinical practice setting and was recorded in a specifically designed web-based electronic data collection form to ensure confidentiality, completeness, and integrity. Additional quality measures included automatic, manual and independent data checks.
2.2. Study outcomes
Two-year effectiveness outcomes included stroke (ischemic or hemorrhagic or unknown type/uncertain classification stroke), and the composite outcome of stroke, systemic embolism, myocardial infarction, life-threatening bleed, and vascular death. Additional outcomes analyzed were myocardial infarction (MI), vascular death, and all-cause death (including vascular, non-vascular and death of unknown cause). Safety outcome was major bleeding defined as reduction in hemoglobin level of ≥ 2 g/L or transfusion of ≥ 2 units (U) of blood or symptomatic bleeding in a critical area or organ. Persistence was analyzed through the proportion of patients discontinuing dabigatran (suspension of treatment longer than 30 days or switch to another OAC) at 6, 12 and 24 months.
2.3. Ethics
The study was approved by local applicable ethics committees and conducted according to good pharmacoepidemiology practice guidelines and local country regulations for observational clinical research. All patients signed a written informed consent before entering the study.
2.4. Statistical analysis
Descriptive results are presented with data summarized by means and standard deviations for continuous variables and by frequencies and percentages for categorical variables. Crude incidence rates are shown per 100 patient-years with two-sided 95% confidence intervals (CI) based on the Poisson distribution and its relation to Chi-square distribution and are based on the period of index dabigatran exposure (on-treatment analysis). This period is from the commencement of dabigatran until permanent discontinuation (that is treatment stop > 30 days, switch to another treatment, or study completion/discontinuation). Treatment interruptions < 30 days were disregarded for the outcome analysis and these patients were considered to be on continuous treatment. Only events occurring while the patient was on the first treatment regimen was considered, in case of recurrent events for one patient, only the first event was considered. The probability of treatment persistence was estimated from Kaplan Meier curve analysis. No statistical hypothesis tests were performed. Statistical analysis was performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
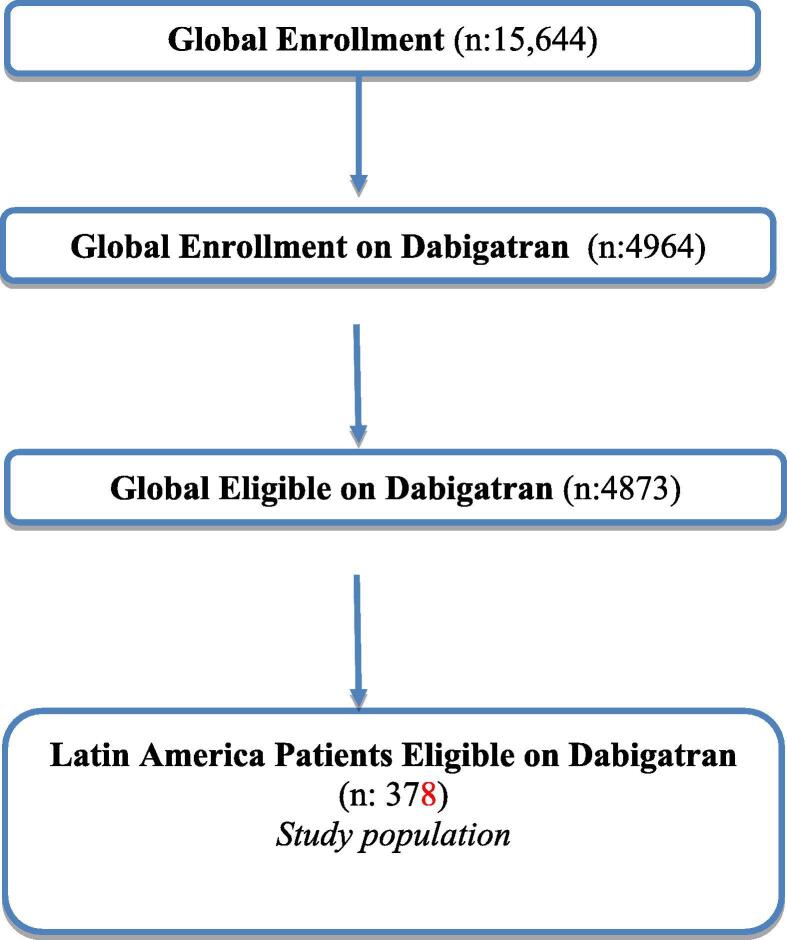
From November 2011 to December 2014, a total of 15,644 patients were globally enrolled in phase II at 984 centers across 44 countries. Of these, 4964 were prescribed dabigatran, of whom 4873 were eligible for analysis. Among them 378 (7.8%) were from eight LA countries (Argentina, Brazil, Chile, Colombia, Ecuador, Mexico, Peru, and Venezuela). They received dabigatran (150 mg or 110 mg twice a day) and were eligible for analysis, see Fig. 1.
Fig. 1.
GLORIA AF, Phase 2 Flow Chart.
3.1. Patient population
For the LA population, the mean age at enrollment was 70.3 ± 10.8 years, and 56.3% were male. Paroxysmal AF was present in 43.4% of the patients, 36.0% had persistent AF, and 20.6% had permanent AF. The most prevalent comorbidity was history of hypertension in 77.5% of the patients followed by congestive heart failure in 31.2% and diabetes in 20.1%. Coronary artery disease was present in 11.6% of the patients, 8.5% had prior myocardial infarction, and 8.2% prior stroke. Mean CHA2DS2-VASc score was 3.2 ± 1.4 and mean HAS-BLED score was 1.2 ± 0.8. The mean creatinine clearance was 76.7 ± 31.6 ml/min and history of prior bleeding was reported in 5.3% of LA patients. Table 1 shows the baseline characteristics of the LA population.
Table 1.
Eligible patients in Phase 2 from Latin America. Baseline characteristics by dabigatran dose group.
| Total Population | Dabigatran 150 mg BID | Dabigatran 110 mg BID | |
|---|---|---|---|
| Patients, n* | 378 | 202 | 176 |
| Age, mean (SD), years | 70.3 (10.8) | 66.0 (11.0) | 75.2 (8.3) |
| BMI, mean (SD), kg/m2 | 28.36 (5.01) | 29.44 (4.85) | 27.11 (4.92) |
| Sex, male, n (%) | 213 (56.3) | 122 (60.4) | 91 (51.7) |
| Type of AF, n (%) | |||
| Paroxysmal | 164 (43.4) | 83 (41.1) | 81 (46.0) |
| Persistent | 136 (36.0) | 82 (40.6) | 54 (30.7) |
| Permanent | 78 (20.6) | 37 (18.3) | 41 (23.3) |
| Categorization of AF, n (%) | |||
| Symptomatic | 113 (29.9) | 67 (33.2) | 46 (26.1) |
| Minimally symptomatic | 143 (37.8) | 82 (40.6) | 61 (34.7) |
| Asymptomatic | 122 (32.3) | 53 (26.2) | 69 (39.2) |
| Medical history, n (%) | |||
| Previous stroke | 31 (8.2) | 17 (8.4) | 14 (8.0) |
| MI | 32 (8.5) | 17 (8.4) | 15 (8.5) |
| Coronary artery disease | 44 (11.6) | 21 (10.4) | 23 (13.1) |
| Congestive heart failure | 118 (31.2) | 59 (29.2) | 59 (33.5) |
| History of hypertension | 293 (77.5) | 164 (81.2) | 129 (73.3) |
| Diabetes mellitus | 76 (20.1) | 47 (23.3) | 29 (16.5) |
| Prior bleeding | 20 (5.3) | 6 (3.0) | 14 (8.0) |
| Creatinine clearance, mean (SD), mL/min | 76.7 (31.6) | 88.1 (32.4) | 62.5 (23.8) |
| CHA2DS2-VASc, mean (SD) | 3.2 (1.4) | 2.9 (1.5) | 3.6 (1.3) |
| HAS-BLED score, mean (SD) | 1.2 (0.8) | 1.0 (0.9) | 1.4 (0.7) |
| Medications, n (%) | |||
| Antiplatelet* | 77 (20.4) | 44 (21.8) | 33 (18.8) |
| Other cardiovascular** | 351 (92.9) | 190 (94.1) | 161 (91.5) |
| PPI | 81 (21.4) | 50 (24.8) | 31 (17.6) |
Acronyms: AF, atrial fibrillation; BID, twice a day; BMI, body mass index; MI, myocardial infarction; PPI, proton pump inhibitor; SD, standard deviation.
Antiplatelet use on the baseline visit date.
Antihypertensive/heart failure and antiarrhythmic therapy.
3.2. Dabigatran treatment
For the LA population, the prescribed dose of dabigatran was 150 mg BID in 53.4% of the patients, 110 mg BID in 46.6%, (Table 1). Antiplatelet treatment was used together with dabigatran in 20.4% of the patients, mostly aspirin (18.3% of patients), see Table 1. Dabigatran was prescribed by cardiologists (90.7%), other treating physicians included general practitioners (5.6%) and internists (3.7%). Patients taking low doses of dabigatran (110 mg BID) were older, with lower creatinine clearances, higher CHA2DS2-VASc, and HAS-BLED scores, and more often with prior bleeding, see Table 1. A total of 47 patients with creatinine clearances<50 ml/min were included. Of these patients 72.3% received 110 mg BID, and 27.7% 150 mg BID.
3.3. Effectiveness and safety outcomes
Incidence rate for stroke after 2-years of follow up was 0.33 (95% CI: 0.04–1.17) per 100 patient-years. Incidence rate for myocardial infarction was 0.98 (95% CI: 0.36–2.13) per 100 patient-years. The incidence rate for major bleeding was 0.49 (95% CI: 0.10–1.42) per 100 patient-years. The incidence rate for all-cause death was 4.06 (95% CI: 2.63–6.00). The incidence rate for the composite outcome of SSE, myocardial infarction, life-threatening bleeding, and vascular death was 2.45 (95% CI: 1.37–4.03) per 100 patient-years. (Table 2).
Table 2.
Effectiveness and safety outcomes in treated patients from Latin America. Crude incidence rates.
|
Latin America (n = 376) |
|||
|---|---|---|---|
| Patients with event | Patient years |
Crude IR |
|
| per 100 PY (95% CI) | |||
| Stroke | 2 | 615 | 0.33 (0.04–1.17) |
| -Ischemic stroke | 1 | ||
| -Unknown type/uncertain classification | 1 | ||
| Major bleeding | 3 | 615 | 0.49 (0.10–1.42) |
| -Life-threatening | 2 | ||
| -Transfusion (2 + units of blood/red cells) | 2 | ||
| -Fall in haemoglobin of 2 g/dL | 2 | ||
| -Fatal bleeds | 2 | ||
| -Gastrointestinal | 3 | ||
| -Intracranial | 0 | ||
| Myocardial Infarction | 6 | 614 | 0.98 (0.36–2.13) |
| All-cause death | 25 | 615 | 4.06 (2.63–6.00) |
| -Vascular death | 8 | ||
| -Nonvascular death | 11 | ||
| -Unknown | 6 | ||
| Composite outcome (Stroke, systemic embolism, myocardial infarction, life-threatening bleed, vascular death) | 15 | 613 | 2.45 (1.37–4.03) |
Acronyms: CI-Confidence interval; IR-Incidence rate; PY-Patient-years.
3.4. Dabigatran persistence
The probability of treatment persistence (estimated from Kaplan Meier curves) with dabigatran at 6 months was 90.6% (95% CI: 87.1%-93.1%), at 12 months it was 85.7% (95% CI: 81.6%-88.9%) and at 24 months it was 80.2% (95% CI: 75.7%-84.0%).
4. Discussion
The current global landscape of AF stroke prevention has changed since NOAC regulatory approval [12]. GLORIA-AF is a prospective global real-world data registry providing long term follow-up data of patients with newly diagnosed NVAF treated with dabigatran. Even though the burden of AF is high in LA, with up to 51.2% of patients at high risk of stroke not receiving adequate antithrombotic therapy, there is limited information on modern AF antithrombotic management and outcomes in this region [4]. Exploring similarities and differences in efficacy and safety across regions in the controlled trial and real-world setting is of great importance for healthcare management.
In the LA patients in GLORIA-AF, the overall incidence of stroke for dabigatran treated patients was 0.33 (95% CI: 0.04–1.17) per 100 patient-years after two years of follow up. These low numbers are consistent and even lower in comparison to the main results at the global level for the registry, where the rate of stroke was 0.65 (95% CI: 048–0.87) [13], [14].
The LA subset of RE-LY compared to the patients in the current study was characterized by having a greater percent of permanent AF (70.7% vs. 20.6%) and comorbidities such as hypertension (82.3% vs. 77.5%), congestive heart failure (41.1% vs 31.2%) and prior stroke (11.5% vs. 8.2%) with a higher mean CHA2DS2-VASc score (3.5 vs 3.2) and aspirin use (48.8% vs. 18.3%).
The overall Phase II dabigatran population of GLORIA-AF compared with the LA specific dabigatran patients was characterized by a higher prevalence of prior stroke (11.9% vs. 8.2%) and a history of diabetes (22.7% vs. 20.1%), however the mean CHA2DS2-VASc score of 3.2 was the same [13].
The observed differences in baseline clinical characteristics with a lower risk population in our study may explain the lower rates of stroke and bleeding complications that we observed in this study.
Evidence from RE-LY and meta‐analysis of studies on NOAC indicate that the effectiveness of NOAC for prevention of SSE may differ between geographic regions similar to differences in stroke rates observed with warfarin [15], [16]. Less effective management of care with VKA therapy in Asia and LA patients may explain the greatest benefit observed with NOAC therapy in these regions [15], [16].
In the eligible LA patients in GLORIA-AF, the overall incidence of major bleeding for dabigatran patients was 0.49 (95% CI: 0.10–1.42) per 100 patient-years after two years of follow up. Comparative risks of major bleeding among newly diagnosed NVAF patients who initiate VKA or NOAC in real-world clinical practice have been recently published [17], [18], [19]. In a Danish registry that included patients with NVAF who were naive to oral anticoagulants, annual rates of any bleeding for dabigatran (2.4%) were significantly lower than for warfarin (5.0%) (HR 0.62, 95%CI: 0.51 to 0.74), [17]. In a Norwegian registry that included NVAF patients with the first prescription of oral anticoagulants, use of dabigatran (HR 0.74, 95% CI 0.66–0.84, P < 0.001) was associated with a lower risk of major or clinically relevant non-major bleeding compared with warfarin [18]. A US-based study used propensity score matching to balance age, sex, region, baseline comorbidities, and co-medications. Compared to matched warfarin initiators, dabigatran (HR: 0.69; 95% CI: 0.50–0.96) initiators had a significantly lower risk of major bleeding [19]. These data are supported by other population-based propensity cohort studies including NOAC, and well-managed VKA matched cohort, showing lower bleeding risk with NOACs [20], [21].
Our study showed a very high treatment persistence with dabigatran in LA patients at 12 and 24 months, which was 85.7% and 80.2%, respectively. In the Phase II of GLORIA-AF, the probability of dabigatran treatment persistence was 76.6% at 12 months and 69.2% at 24 months [22]. In the global GLORIA-AF registry, patients in the LA region were at lower risk for non-persistence (HR 0.61; 95% CI: 0.40 to 0.90), and patients with asymptomatic or minimally symptomatic AF, permanent AF and those not on proton pump inhibitors were more likely to be persistent [22]. The observed differences in persistence with a higher rate in our patients compared with the overall dabigatran patients in Phase II of GLORIA may explain the lower rates of stroke that we observed in this study. Persistence is of great relevance as treatment discontinuation with VKAs is high with only 45–53.5% of patients remaining on treatment after one year [23], [24].
A US-based study in newly diagnosed patients with NVAF naive-to-treatment, identified by propensity score matching 1775 pairs of patients on dabigatran and warfarin . The persistence rates were higher for dabigatran than for warfarin at both six months (72% versus 53%) and one year (63% versus 39%). Patients on dabigatran with a low-to-moderate risk of stroke (CHADS2 score < 2) or with a higher bleed risk (HEMORR2HAGES > 3) had a higher likelihood of non-persistence (hazard ratios, 1.37; 95% CI, 1.17–1.60; P < 0.001; and hazard ratios, 1.24; 95% CI, 1.04–1.47; P = 0.016) respectively, [25].
Importantly, in our study, dabigatran was prescribed and managed mostly by cardiologists, which is in contrast with usual management of VKA in anticoagulation clinics in most LA countries, similar to what is seen in countries like The Netherlands, England and Spain [26]. This additional factor needs to be further investigated as a potential contributor to widespread treatment and adherence to stroke prevention therapy in NVAF in our region.
4.1. Limitations
Our study has limitations related to the observational design and, therefore, subject to potential selection bias and unmeasured confounding. The small size of LA patient subset within the global GLORA-AF registry is a second limitation that may limit the generalizability of results.
In conclusion, the effectiveness and safety results, consistency with final Phase II of GLORIA-AF, and other real-world registries provide evidence of the benefit and safety of dabigatran in standard clinical practice in our region. The epidemiological burden of AF in LA and the suboptimal treatment with VKAs leads to great room for improvement in the prevention of stroke and SE among LA population. An increase in accessibility and incorporation of NOAC to treatment strategy may have a positive health impact on AF stroke prevention in the LA region.
Acknowledgments
Acknowledgements
GLORIA-AF LA investigators: List of all investigators, country and sites that have participated in the study is provided in the supplementary appendix. We also acknowledge the contribution of Raúl Bozzo (IC-Projects) in the medical writing of this article.
Funding: GLORIA-AF study was funded by Boehringer Ingelheim.
Conflict of interest or disclosures: Barón-Esquivias, has received honoraria for presentations and/or consultancy fees and/or research grants from Boehringer Ingelheim, Bayer, Daiichi-Sankyo, Pfizer-Bristol-Meier-Squib, and Biotronik. Christine Teutsch, Venkatesh Kumar Gurusamy, Sabrina Marler and Cecilia Zeballos: Are Boehringer Ingelheim employees. Menno V. Huisman: Has received honoraria for research grants, consultation, and presentations from Actelion, Bayer HealthCare Pharmaceuticals, Boehringer Ingelheim, GlaxoSmithKline, and Pfizer. Gregory Y.H. Lip: Consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi-Sankyo. Speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received personally.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100666.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lloyd-Jones D.M., Wang T.J., Leip E.P., Larson M.G., Levy D., Vasan R.S., D'Agostino R.B., Massaro J.M., Beiser A., Wolf P.A., Benjamin E.J. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Cubillos L., Haddad A., Kuznik A., Mould-Quevedo J. Burden of disease from atrial fibrillation in adults from seven countries in Latin America. Int. J. Gen. Med. 2014;2(7):441–448. doi: 10.2147/IJGM.S62819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart R.G., Pearce L.A., Aguilar M.I. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 4.Gamra H., Murin J., Chiang C.E., Naditch-Brûlé L., Brette S., Steg P.G. RealiseAF investigators. Use of antithrombotics in atrial fibrillation in Africa, Europe, Asia and South America: insights from the International RealiseAF Survey. Arch. Cardiovasc. Dis. 2014 Feb;107(2):77–87. doi: 10.1016/j.acvd.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Cantú-Brito C., Silva G.S., Ameriso S.F. Use of Guidelines for Reducing Stroke Risk in Patients With Nonvalvular Atrial Fibrillation: A Review From a Latin American Perspective. Clin. Appl. Thromb. Hemost. 2018;24(1):22–32. doi: 10.1177/1076029617734309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pujol Lereis V.A., Ameriso S., Povedano G.P., Ameriso S.F. Ischemic stroke in patients with atrial fibrillation receiving oral anticoagulation. J. Neurol. Sci. 2013;334(1–2):139–142. doi: 10.1016/j.jns.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B., Castella M., Diener H.C., Heidbuchel H., Hendriks J., Hindricks G., Manolis A.S., Oldgren J., Popescu B.A., Schotten U., Van Putte B., Vardas P. ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016;37(38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 8.January C.T., Wann L.S., Calkins H., Chen L.Y., Cigarroa J.E., Cleveland J.C., Jr, Ellinor P.T., Ezekowitz M.D., Field M.E., Furie K.L., Heidenreich P.A., Murray K.T., Shea J.B., Tracy C.M., Yancy C.W. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019 doi: 10.1016/j.jacc.2019.01.011. pii: S0735-1097(19)30209-8. [DOI] [PubMed] [Google Scholar]
- 9.Blair H.A., Keating G.M. Dabigatran Etexilate: A Review in Nonvalvular Atrial Fibrillation. Drugs. 2017;77(3):331–344. doi: 10.1007/s40265-017-0699-z. [DOI] [PubMed] [Google Scholar]
- 10.Connolly S.J., Ezekowitz M.D., Yusuf S., Eikelboom J., Oldgren J., Parekh A., Pogue J., Reilly P.A., Themeles E., Varrone J., Wang S., Alings M., Xavier D., Zhu J., Diaz R., Lewis B.S., Darius H., Diener H.C., Joyner C.D., Wallentin L. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 11.Huisman M.V., Lip G.Y., Diener H.C., Dubner S.J., Halperin J.L., Ma C.S., Rothman K.J., Teutsch C., Zint K., Ackermann D., Clemens A., Bartels D.B. Design and rationale of Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation: a global registry program on long-term oral antithrombotic treatment in patients with atrial fibrillation. Am. Heart J. 2014;167(3):329–334. doi: 10.1016/j.ahj.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Huisman M.V., Rothman K.J., Paquette M., Teutsch C., Diener H.C., Dubner S.J., Halperin J.L., Ma C.S., Zint K., Elsaesser A., Bartels D.B., Lip G.Y. GLORIA-AF Investigators. The Changing Landscape for Stroke Prevention in AF: Findings From the GLORIA-AF Registry Phase 2. J. Am. Coll. Cardiol. 2017;69(7):777–785. doi: 10.1016/j.jacc.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 13.Huisman M.V., Rothman K.J., Paquette M., Teutsch C., Diener H.C., Dubner S.J., Halperin J.L., Ma C.S., Zint K., Elsaesser A., Lu S., Bartels D.B., Lip G.Y.H. GLORIA-AF Investigators. Two-year follow-up of patients treated with dabigatran for stroke prevention in atrial fibrillation: Global Registry on Long-Term Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF) registry. Am. Heart J. 2018;198:55–63. doi: 10.1016/j.ahj.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Mazurek M., Teutsch C., Diener H.C., Dubner S.J., Halperin J.L., Ma C.S., Rothman K.J., Paquette M., Zint K., França L.R., Lu S., Bartels D.B., Huisman M.V., Lip G.Y.H. GLORIA-AF Investigators. Safety and effectiveness of dabigatran at 2 years: Final outcomes from Phase II of the GLORIA-AF registry program. Am. Heart J. 2019 Dec;218:123–127. doi: 10.1016/j.ahj.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Avezum A., Oliveira G.B.F., Diaz R., Hermosillo J.A.G., Oldgren J., Ripoll E.F., Noack H., Piegas L.S., Connolly S.J. Efficacy and safety of dabigatran versus warfarin from the RE-LY trial. Open Heart. 2018;5(1) doi: 10.1136/openhrt-2018-000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gómez-Outes A., Terleira-Fernández A.I., Calvo-Rojas G., Suárez-Gea M.L., Vargas-Castrillón E. Direct oral anticoagulants for stroke prevention in patients with atrial fibrillation: meta-analysis by geographic region with a focus on European patients. Br. J. Clin. Pharmacol. 2016;82(3):633–644. doi: 10.1111/bcp.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen T.B., Skjøth F., Nielsen P.B., Kjældgaard J.N., Lip G.Y. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;16(353) doi: 10.1136/bmj.i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halvorsen S., Ghanima W., Fride Tvete I., Hoxmark C., Falck P., Solli O., Jonasson C. A nationwide registry study to compare bleeding rates in patients with atrial fibrillation being prescribed oral anticoagulants. Eur. Heart J. Cardiovasc. Pharmacother. 2017;3(1):28–36. doi: 10.1093/ehjcvp/pvw031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lip G.Y., Keshishian A., Kamble S., Pan X., Mardekian J., Horblyuk R., Hamilton M. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. A propensity score matched analysis. Thromb. Haemost. 2016;116(5):975–986. doi: 10.1160/TH16-05-0403. [DOI] [PubMed] [Google Scholar]
- 20.Denas G., Gennaro N., Ferroni E., Fedeli U., Saugo M., Zoppellaro G., Padayattil Jose S., Costa G., Corti M.C., Andretta M., Pengo V. Effectiveness and safety of oral anticoagulation with non-vitamin K antagonists compared to well-managed vitamin K antagonists in naïve patients with non-valvular atrial fibrillation: Propensity score matched cohort study. Int. J. Cardiol. 2017;15(249):198–203. doi: 10.1016/j.ijcard.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Esteve-Pastor M.A., Rivera-Caravaca J.M., Roldán V., Vicente V., Romiti G.F., Romanazzi I., Proietti M., Valdés M., Marín F., Lip G.Y.H. Estimated absolute effects on efficacy and safety outcomes of using non-vitamin K antagonist oral anticoagulants in 'real-world' atrial fibrillation patients: A comparison with optimally acenocoumarol anticoagulated patients. Int. J. Cardiol. 2018;1(254):125–131. doi: 10.1016/j.ijcard.2017.11.087. [DOI] [PubMed] [Google Scholar]
- 22.Paquette M., Riou França L., Teutsch C., Diener H.C., Lu S., Dubner S.J., Ma C.S., Rothman K.J., Zint K., Halperin J.L., Huisman M.V., Lip G.Y.H., Nieuwlaat R. Persistence With Dabigatran Therapy at 2 Years in Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2017;70(13):1573–1583. doi: 10.1016/j.jacc.2017.07.793. [DOI] [PubMed] [Google Scholar]
- 23.Spivey C.A., Qiao Y., Liu X., Mardekian J., Parker R.B., Phatak H., Claflin A.B., Kachroo S., Abdulsattar Y., Chakrabarti A., Wang J. Discontinuation/Interruption of Warfarin Therapy in Patients with Nonvalvular Atrial Fibrillation. J. Manag. Care Spec. Pharm. 2015;21(7):596–606. doi: 10.18553/jmcp.2015.21.7.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song X., Sander S.D., Varker H., Amin A. Patterns and predictors of use of warfarin and other common long-term medications in patients with atrial fibrillation. Am. J. Cardiovasc. Drugs. 2012;12(4):245–253. doi: 10.1007/BF03261833. [DOI] [PubMed] [Google Scholar]
- 25.Zalesak M., Siu K., Francis K., Yu C., Alvrtsyan H., Rao Y., Walker D., Sander S., Miyasato G., Matchar D., Sanchez H. Higher persistence in newly diagnosed nonvalvular atrial fibrillation patients treated with dabigatran versus warfarin. Circ. Cardiovasc. Qual. Outcomes. 2013;6(5):567–574. doi: 10.1161/CIRCOUTCOMES.113.000192. [DOI] [PubMed] [Google Scholar]
- 26.Pengo V., Pegoraro C., Cucchini U., Iliceto S. Worldwide management of oral anticoagulant therapy: the ISAM study. J Thromb. Thrombol. 2006;21(1):73–77. doi: 10.1007/s11239-006-5580-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.