Abstract
Study Design:
Retrospective, single institution, multisurgeon case control series.
Objective:
To determine whether there are differences in reoperation rates or outcomes for patients undergoing 2-level posterolateral fusion (PLF) augmented by a transforaminal lumbar interbody fusion (TLIF) at only one of the levels or at both.
Methods:
A total of 416 patients were identified who underwent 2-level PLF with a TLIF at either one of those levels (n = 183) or at both (n = 233) with greater than 1-year follow-up. Demographic, surgical, radiographic, and clinical data was reviewed for each patient. These included age, sex, race, body mass index, smoking status, Charleston Comorbidity Index, operative time, estimated blood loss, length of stay, and patient-reported outcome measures.
Results:
Each cohort underwent 24 reoperations. Although the number of overall reoperations was not significantly different (P > .05), among the reoperation types, there were significantly more reoperations for adjacent segment disease in the 2-level group compared to the 1-level group (19 vs 12, P = .04). There was no difference in reoperation for pseudarthrosis between the groups (P > .05). Although both groups experienced significant improvements in Oswestry Disability Index (P < .001) and Short Form–12 health questionnaire (P < .001), there were no differences between improvements for 1- versus 2-level cohorts.
Conclusions:
For patients undergoing 2-level PLF in the setting of a TLIF, using a TLIF at one versus both levels does not seem to influence reoperation rates or outcomes. However, reoperation rates for adjacent segment disease are increased in the setting of a 2-level PLF augmented by a 2-level TLIF.
Keywords: transforaminal lumbar interbody fusion, posterolateral fusion, adjacent segment disease, pseudarthrosis
Introduction
Fusion for spondylolistheses, both isthmic and degenerative, leads to improved clinical and radiographic outcomes compared with nonoperative management or with decompression procedures alone.1-4 Over the past decade, interbody devices have been used to augment fusions with reported fusion rates of greater than 90%.5-7 Specifically, transforaminal lumbar interbody fusions (TLIF) have been shown to increase neuroforaminal height,8 improve sagittal alignment9 and increase fusion rates5-7 all leading to improved outcomes, such as in back and leg pain and overall global outcome measures.10-12 In turn, the use of TLIFs has been increasing.
These improvements have translated into reduced reoperation rates following single-level TLIF. In a study of 103 patients undergoing posterolateral fusion (PLF) versus PLF + TLIF with greater than 2-year follow-up, Macki et al13 showed a significantly higher reoperation rate for PLF alone compared with PLF + TLIF (29.3% vs 8.89%, P = .011) and a significantly decreased risk of pseudarthrosis/instrumentation failure for the TLIF cohort (P = .043). Furthermore, they were able to demonstrate that the addition of an interbody decreased the odds of reoperation for adjacent segment disease (ASD) by 82%. A recent meta-analysis also demonstrated improved fusion rates (P = .0007) and decreased reoperation rates (P = .004) for the addition of an interbody fusion to PLF.14
Although excellent outcomes have been demonstrated in the setting of single-level TLIF, outcomes of single-level TLIF in the setting of multiple level PLF are unknown (ie, the case of a 2-level PLF with one of the levels augmented with a TLIF). In fact, there is a dearth of literature addressing multiple levels TLIF in general. Several surgeons in our practice have anecdotally identified an increased risk of pseudarthrosis at the level without an interbody in the setting of a 2-level PLF with a 1-level TLIF. We surmise that the increased stiffness at the interbody level adjacent to a nascent and evolving fusion at the level above leads to increased motion at that second level and an increased risk for pseudarthrosis. Additionally, it has been widely reported that single-level PLF has inferior fusion rates compared to TLIF. Therefore, many surgeons in our practice now place an interbody either at both levels or at neither level when performing a 2-level PLF.
However, the effects of a 2-level TLIF in the setting of a 2-level PLF are also unknown. In this setting, although a fusion may be more likely, the increased overall stiffness may alter spinal biomechanics and may lead to an increased risk of ASD (similar to the case of adjacent level disease following anterior cervical discectomy and fusion). Therefore, it is unclear whether the revision rates for a single-level TLIF in the setting of a 2-level PLF would be different than the revision rate for a 2-level TLIF in the setting of a 2-level PLF.
In an effort to explore these issues, we reviewed the records of 416 patients who underwent primary 2-level PLF augmented by either single- or 2-level TLIF to examine whether there were any clinical or radiographic differences between the cohorts (Figures 1 and 2).
Figure 1.
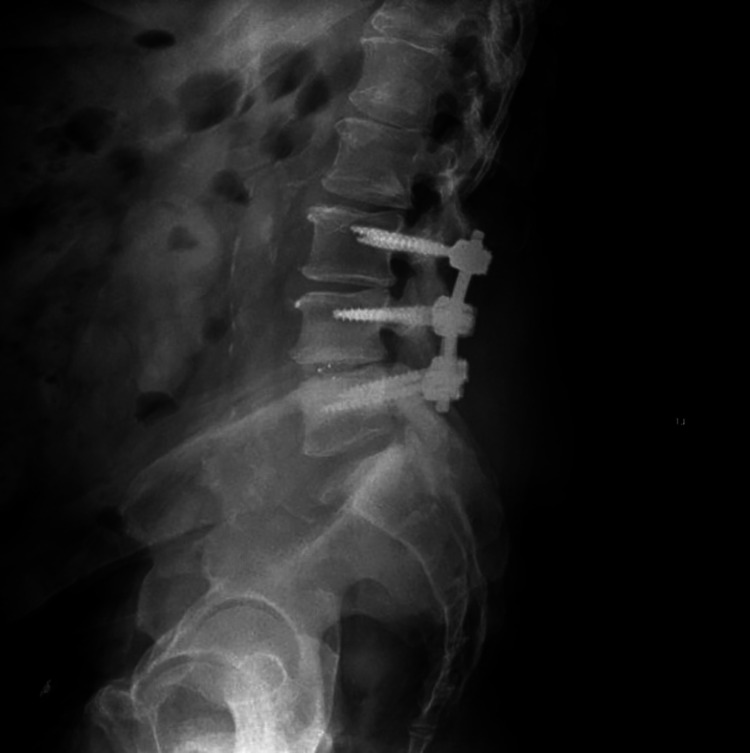
Lateral X-ray of lumbar spine for typical patient with a 1-level transforaminal lumbar interbody fusion (TLIF) and 2-level posterolateral fusion.
Figure 2.
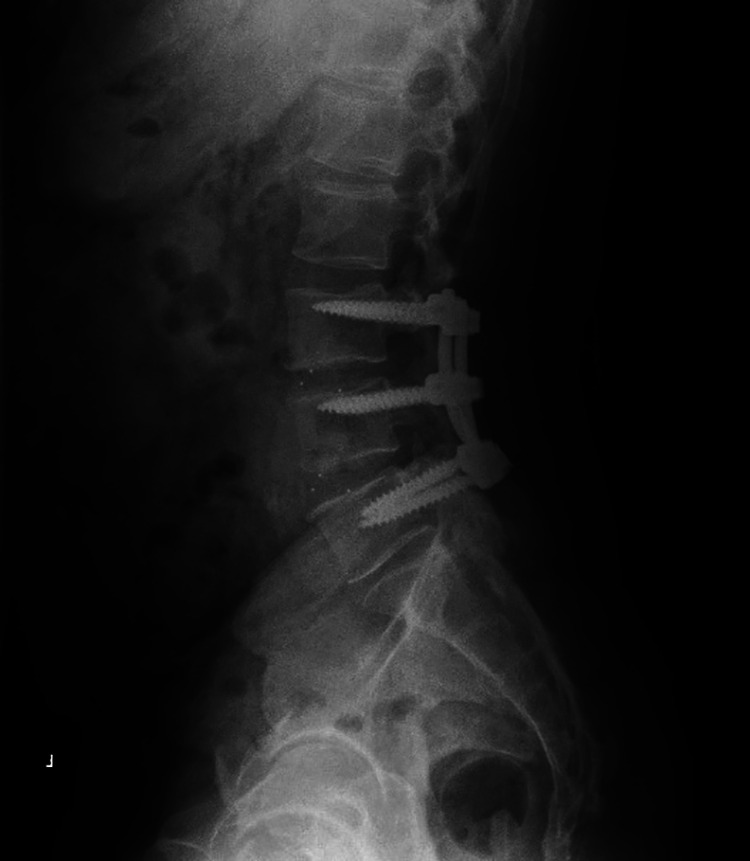
Lateral X-ray of lumbar spine for typical patient with a 2-level transforaminal lumbar interbody fusion (TLIF) and posterolateral fusion.
Materials and Methods
Study Design and Data Source
This is a retrospective analysis of data collected from a single institution database. At our institution, all of the data, including demographic, comorbidity, preoperative, intraoperative, and postoperative data from patients undergoing a variety of surgical procedures was recorded. This study was conducted under the supervision of our institutional review board.
Data Collection
From September 2012 through September 2 016 416 patients were identified who underwent a primary 2-level PLF with a TLIF at either one of those levels (n = 183) or at both (n = 233) with greater than 1-year follow-up. Patients were identified by reviewing our clinical database and searching for patients who had undergone either a 2-level TLIF (with PLF), identified by searching for CPT (Current Procedural Terminology) code 22 630 and 22 632 or patients who had undergone a TLIF at one level (CPT 22 630/2) and an additional level with a PLF (22 612/4).
Demographic, surgical, radiographic, and clinical data was reviewed for each patient. Demographic data included age, sex, race, body mass index (BMI), smoking status, and Charleston Comorbidity index (CCI). Surgical parameters included level of interbody, operative time, and estimated blood loss. Clinical data included length of stay (LOS), need for revision, and preoperative and postoperative patient reported outcome measures, including Oswestry Disability Index (ODI) and quality of life (Short Form 12 physical and mental component score (SF-12 PCS and MCS).
In this study, the primary outcome measure of interest was revision rate with secondary outcome measures including the aforementioned surgical and clinical parameters.
Statistical Analysis
Statistical analysis was done via the Statistical Package for the Social Science 24.0 (IBM, Armonk, NY). The distribution of the quantitative variables was given by mean, SD, and range. We compared 1- and 2-interbody cohorts. Shapiro-Wilk test was used to test the normality of the data. For comparisons between the groups, Student’s t-test was used to compare continuous variables and the chi-square test was used for categorical variables. For comparisons between pre- and postoperative results, paired t-test was used. A multivariate logistic regression was performed to correlate the number of the interbody cages used and all other studies variables. All P values <.05 were defined as having statistical significance.
Results
Demographics
A total of 183 patients underwent a single-level TLIF/2-level PLF with an average follow-up of 3.57 years (range 1.00-5.01 years) and 233 patients underwent a 2-level TLIF/2-level PLF with an average follow-up of 4.17 years (range 1.00-5.17 years). The 2-level TLIF group was significantly younger than the single-level TLIF group (average age 60.2 vs 63.7 years, P = .001) and had a significantly lower average Charleston Comorbidity Index (0.53 vs 2.80, P < .001). There were also significantly more smokers (P < .001) and women (P = .048) in the single-level versus 2-level cohort. No other demographic differences were identified (Table 1).
Table 1.
Demographic for Patients Undergoing 1-Interbody or 2-Interbody Spinal Fusion.
| 1-Interbody, n (%) or Mean [SD] | 2-Interbody, n (%) or Mean [SD] | P | |
|---|---|---|---|
| Case year | |||
| 2012 | 14 (7.3) | 11 (4.7) | |
| 2013 | 43 (22.3) | 29 (12.3) | |
| 2014 | 47 (24.5) | 44 (18.7) | N/A |
| 2015 | 41 (21.4) | 55 (23.4) | |
| 2016 | 38 (19.8) | 94 (40.0) | |
| Age, y | 63.7 [11.2] | 60.2 [10.9] | .001 |
| Sex | |||
| % Female | 56.3 | 47.3 | .048 |
| Race | |||
| % Caucasian | 78.2 | 80.6 | .620 |
| BMI, kg/m2 | 30.1 [6.1] | 29.5 [5.9] | .363 |
| Smoking | |||
| %Nonsmoker | 79.7 | 89.3 | <.001 |
| %Former smoker | 4.4 | 10.7 | .016 |
| %Current smoker | 15.9 | 0.0 | .048 |
| CCI | 2.8 [1.7] | 0.533 [0.83] | <.001 |
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; N/A, not applicable.
Surgical Parameters
Expectedly, average estimated blood loss was less for the single-level TLIF cohort compared with 2-level (643 vs 790 mL, P = .002). Operative time was equivalent between the groups (222 vs 234 minutes, P = .147; Table 2).
Table 2.
Surgical and Clinical Parameters for Patients in 1-Interbody and 2-Interbody Cohorts.
| 1-Interbody, n (%) or Mean [SD] | 2-Interbody, n (%) or Mean [SD] | P | |
|---|---|---|---|
| Estimated blood loss (mL) | 643.8 [530.3] | 790.2 [402.5] | .002 |
| Operative time (min) | 222.2 [55.6] | 234.9 [67.9] | .147 |
| Length of stay (days) | 4.0 [2.1] | 3.3 [1.7] | .003 |
| Revisions | 24 (13.1) | 24 (10.3) | .440 |
Patient-Reported and Clinical Outcomes
The 1- and 2-level TLIF groups underwent 24 reoperations for 27 complication types and 24 reoperations for 25 complication types, respectively. Average time to reoperation was 1.23 years (range 7-1260 days) for the single-level TLIF cohort and 1.91 years (range 31-1575 days) for the 2-level TLIF cohort. For the 1-level TLIF group, there were 12 reoperations for ASD, 6 reoperations for infection, 5 reoperations for pseudarthrosis, and 4 reoperations for postoperative fractures. Among the 2-level TLIF cohort, there were 19 reoperations for ASD, 3 reoperations for pseudarthrosis, 2 reoperations for infection, and 1 reoperation for instrumentation failure. Although the number of overall reoperations was not significantly different (P = .44), among the reoperation types, there were significantly more reoperations for adjacent segment disease in the 2-level group compared with the 1-level group (P = .04). Otherwise, there were no differences for reoperations for other subtypes, including no difference in reoperation for pseudarthrosis (P > .05; Table 3).
Table 3.
Revision Reason for 1-Interbody and 2-Interbody Transforaminal Lumbar Interbody Fusion.
| Complications | 1-Interbody, No. of Patients (%) | 2-Interbody, No. of Patients (%) | P |
|---|---|---|---|
| Adjacent level disease | 12 (44.4) | 19 (76.0) | .040 |
| Vertebrae fracture | 4 (14.8) | 0 (0.0) | .154 |
| Instrumentation failure | 0 (0.0) | 1 (4.0) | .562 |
| Infection | 6 (22.2) | 2 (8.0) | .124 |
| Pseudoarthrosis | 5 (18.5) | 3 (12.0) | .077 |
| Total | 27 (100.0) | 25 (100.0) | .440 |
Complete (pre- and postoperative) patient-reported outcome measures, including ODI and SF-12 were available for 106 patients in the single-level TLIF cohort with an average of 1.56-year follow-up (SD ±0.97) and for 119 patients in the 2-level cohorts with an average follow-up of 1.34 years (SD ±1.1; P = .12). Although both groups experienced significant improvements in ODI (P < .001) and SF-12 (P < .001), there were no differences between improvements for 1- versus 2-level cohorts (Table 4). Regarding other clinical outcomes, the 2-level cohort experienced an overall shorter LOS (3.31 vs 4.05 days, P = .003).
Table 4.
Patient-Reported Outcome Measures for Patients in 1-Interbody and 2-Interbody Cohorts.
| 1-Interbody, Mean [SD] | 2-Interbody, Mean [SD] | P | |
|---|---|---|---|
| Pre-ODI | 41.9 [16.7] | 45.2 [15.7] | .134 |
| Post-ODI | 28.4 [19.8] | 27.5 [20.8] | .781 |
| Pre-SF-12-PCS | 30.3 [8.1] | 31.8 [8.7] | .176 |
| Post-SF-12-PCS | 37.0 [10.3] | 35.8 [10.7] | .316 |
| Pre-SF-12-MCS | 47.9 [12.1] | 47.6 [11.4] | .871 |
| Post-SF-12-MCS | 49.2 [11.8] | 50.7 [10.4] | .259 |
Abbreviations: ODI, Oswestry Disability Index; SF-12, Short Form–12 health questionnaire; PCS, physical component summary; MCS, mental component summary.
Multivariate Regression
A multivariate logistic regression was performed to account for any potential confounding and correlated the number of the interbody cages used and all other outcomes. No significant differences were identified for any of the variables, including surgery time, estimated blood loss, age, BMI, smoking status, sex, ASA (American Society of Anesthesiologists grade), LOS, and pre- and postoperative ODI and SF-12 scores (Table 5).
Table 5.
Multivariate Logistic Regression Using Single- Versus 2-Level TLIF Independent Variable.
| P | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|
| Pre-ODI | .314 | 0.974 | −0.081, 0.024 |
| Post-ODI | .735 | 1.007 | −0.033, 0.046 |
| Pre-SF-12-MCS | .196 | 0.955 | −0.120, 0.021 |
| Post-SF-12-MCS | .280 | 1.082 | 0.015, 0.157 |
| Pre-SF-12-PCS | .382 | 0.962 | −0.133, 0.046 |
| Post-SF-12-PCS | .091 | 0.936 | −0.150, 0.006 |
| OR time | .434 | 1.005 | −0.007, 0.017 |
| EBL | .249 | 1.001 | 0.000, 0.002 |
| Age | .783 | 0.990 | −0.085, 0.064 |
| BMI | .139 | 0.940 | −0.153, 0.018 |
| Smoking | .082 | 0.273 | −2.996, 0.018 |
| Sex | .567 | 0.679 | −1.746, 0.939 |
| ASA | .387 | 1.768 | −0.704, 1.935 |
| LOS | .365 | 1.257 | −0.270, 0.751 |
| Race | .442 | 2.536 | −1.389, 3.546 |
Abbreviations: ODI, Oswestry Disability Index; SF-12, Short Form–12 health questionnaire; PCS, physical component summary; MCS, mental component summary; OR, operating room; EBL, estimated blood loss; BMI, body mass index; ASA, American Society of Anesthesiologists; LOS, length of stay.
Discussion
This is the first study to specifically examine whether a 2-level posterolateral fusion in the setting of a TLIF performed at either one of both the levels influences either reoperation rates or patient outcomes. In this retrospective review of 5 years of consecutive patients, there was no significant difference in reoperation rate regardless of technique. Moreover, clinically, there were no differences in patient-reported outcomes.
Given the reported increased fusion rates when using an interbody graft as opposed to a posterolateral fusion alone,5 -71 015 and the potential for differential in adjacent level strains/stiffness, leading to theoretically suboptimal conditions for fusion (with increased relative stresses at the levels adjacent to a TLIF16-18 in a native fusion, we expected that in the case of a 2-level PLF where only one of the levels was supplemented with an interbody, as opposed to both, that increased risk for reoperation for pseudarthrosis may have been observed. However, we were unable to demonstrate this clinically, even though there were actually statistically more smokers in the single-level TLIF cohort.
Instead, we found regarding revisions, that the 2-level TLIF group had statistically more revisions for ASD compared with the single-level group. Several biomechanical studies have documented increased relative motion at adjacent segments16 as well as increased intradiscal pressures at levels adjacent to a TLIF.17,18 So it was not completely unexpected that the 2-level interbody cohort experienced relatively greater rates of ASD requiring reoperation, compounding the effects seen in single-level TLIFs. Directions for further study may involve biomechanical or finite element analyses of single and 2-level TLIF constructs in the setting of PLF to assess whether there are in fact significantly increased stresses at adjacent levels, which may predispose to ASD and ultimately revision.
Other differences were noted between the cohorts as well. As would be expected for a less extensive procedure, there was significantly less average blood loss in the single- versus 2-level cohort. Interestingly, LOS was actually shorter for the 2-level cohort but this was likely because these patients were generally younger (average age 60.2 years for 2-level vs 63.7 years for single-level group, P = .001) and healthier (CCI index of 0.32 vs 1.21, P < .001) to start. Patient-specific rather than technique-specific factors likely drove this finding.
Both groups experienced significant improvements in ODI and SF-12 scores although these were not statistically different from one another. Many previous studies have demonstrated the positive effects of TLIFs as well as PLF regarding patient-reported outcomes. Although the average follow-up for patients with complete patient-reported outcome measures (both preoperative and postoperative scores) was one and a half years, several recent studies have confirmed that patient-reported outcome measures for functional disability and pain severity at 12 months accurately reflect those at 24 months.19-21
Ghasemi10 reviewed 145 patients, 80 patients undergoing TLIF and 65 undergoing PLF for the management of degenerative spondylolisthesis. Although both groups experienced improvements in PROMs such as ODI, visual analogue scale, and global outcome, the TLIF group experienced significantly greater improvements at final follow-up.
Similarly, in a randomized trial of PLF (n = 25) or PLF + TLIF (n = 25) for the management of patients with isthmic spondylolistheses, Etemadifar et al22 found that at 2 years, both groups had significantly improved in terms of visual analogue scale back and leg and ODI (P < .001) but the PLF + TLIF group reported significantly greater improvement (P < .05).
A recently published meta-analysis15 comparing TLIF and PLF concluded that TLIF offers advantages in terms of achieving radiographic fusion (P = .02) and of experiencing greater improvement in ODI (P = .03) and back pain (P = .002).
Alternatively, Kim et al23 recently reviewed their 2-year results from a 99-patient cohort treated for degenerative spondylolisthesis, 62 who underwent TLIF and 37 who underwent PLF, and concluded that while both groups experienced significant improvements in measures such as EQ-5D, ODI, SF-12 MCS/PCS, and Numerical Rating Scale–back and leg pain, there were no statistical differences between the cohorts. Similarly, we were unable to identify a benefit for a PLF augmented with 1 versus 2 TLIFs.
One complication type, fracture, was only identified in the single-level TLIF cohort. Two of those occurred in the setting of nonunion and may be better reflected as representing part of the spectrum of pseudarthrosis. One of the fractures, fracture of L3 body and right L5 pedicle, occurred following a fall after an L3-5 decompression and posterolateral fusion with an L4-5 interbody. The last patient was an elderly woman who sustained a sacral U type fracture about 2 weeks after her initial L3-5 decompression and PLF with a TLIF at L4-5. This complication was included as an instance of ASD as well, representing distal junctional failure. Regarding postoperative fracture (other than traumatic) after 2-level posterolateral fusion with or without interbody, Tan et al24 published the only other account we could find in the literature. The authors reported 2 cases of distal junctional failure, both at L5. One case involved a fracture of L5 6 months after undergoing an instrumented PLF from T12-L5 with interbody at L2-3 and L4-5. The second case involved an L5 vertebral body fracture 6 months after undergoing an L3-4 and L4-5 TLIF.
Limitations
We performed a retrospective, single-institution, multisurgeon review, which is limited by the usual biases of a retrospective study. Specifically, we could not reliably account for the specific surgical and procedural indications by review of the medical record. As the study was not randomized, it is subject to selection bias. It is likely that the surgeons specifically chose augmentation with a TLIF at one versus both levels for particular reasons, such as the need for greater foraminal decompression at just one of the levels or significant instability at one versus both of the levels, and so on. It is possible that using one technique over the other for all patients, rather than tailoring treatment per patient, would have led to different results. Additionally, while a multisurgeon series may entail more variability in technique, all surgeons in the series performed a traditional open approach for TLIF (as opposed to minimally invasive access), including a formal bilateral decompression and posterolateral fusion ± TLIF at one or more levels.
Although this study included over 400 patients each with greater than 1-year follow-up, given the relatively low incidence of postoperative complications, it is possible that an even larger study could have identified greater differences. For example, there is a trend toward significance for reoperation for pseudarthrosis in the single-level TLIF versus two-level TLIF cohorts (P = .077). Perhaps review of more patients would reveal significance.
Conversely, given the low reoperation rate, specifically considering subtypes individually (eg, pesudarthrosis, ASD, fracture, etc), an appropriate model (logistic regression) could not be performed. Therefore, potential confounders may exist which could influence complication types such as the development of pseudarthrosis or ASD but have not adequately identified here.
Conclusions
For patients undergoing 2-level PLF in the setting of a TLIF, using a TLIF at one versus both levels does not seem to influence reoperation rates or outcomes. However, reoperation rates for adjacent segment disease are increased in the setting of a 2-level PLF augmented by 2 TLIFs. We are currently working on a biomechanical model to replicate these conditions and evaluate whether changes in intraconstruct loads or in adjacent segment forces can explain our findings of increased reoperations for adjacent segment disease in the 2-level TLIF cohort. There are no other biomechanical or in vivo studies in the literature specifically comparing these 2 populations and likely even larger, potentially prospective studies are needed to confirm our findings before widespread adoption of one technique over the other.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: I. David Kaye, MD  https://orcid.org/0000-0002-0797-8760
https://orcid.org/0000-0002-0797-8760
Alex R. Vaccaro, MD, PhD, MBA  https://orcid.org/0000-0002-8073-0796
https://orcid.org/0000-0002-8073-0796
References
- 1. Pearson AM, Lurie JD, Blood EA, et al. Spine patient outcomes research trial: radiographic predictors of clinical outcomes after operative or nonoperative treatment of degenerative spondylolisthesis. Spine (Phila Pa 1976). 2008;33:2759–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tosteson AN, Tosteson TD, Lurie JD, et al. Comparative effectiveness evidence from the spine patient outcomes research trial: surgical versus nonoperative care for spinal stenosis, degenerative spondylolisthesis, and intervertebral disc herniation. Spine (Phila Pa 1976). 2011;36:2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356:2257–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am. 2009;91:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Potter BK, Freedman BA, Verwiebe EG, Hall JM, Polly DW, Jr, Kuklo TR. Transforaminal lumbar interbody fusion: clinical and radiographic results and complications in 100 consecutive patients. J Spinal Disord Tech. 2005;18:337–346. [DOI] [PubMed] [Google Scholar]
- 6. Wong AP, Smith ZA, Stadler JA, 3rd, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF): surgical technique, long-term 4-year prospective outcomes, and complications compared with an open TLIF cohort. Neurosurg Clin N Am. 2014;25:279–304. [DOI] [PubMed] [Google Scholar]
- 7. Wu RH, Fraser JF, Härtl R. Minimal access versus open transforaminal lumbar interbody fusion: meta-analysis of fusion rates. Spine (Phila Pa 1976). 2010:15;35:2273–2281. [DOI] [PubMed] [Google Scholar]
- 8. Hawasli AH, Khalifeh JM, Chatrath A, Yarbrough CK, Ray WZ. Minimally invasive transforaminal lumbar interbody fusion with expandable versus static interbody devices: radiographic assessment of sagittal segmental and pelvic parameters. Neurosurg Focus. 2017;43:E10. [DOI] [PubMed] [Google Scholar]
- 9. Jagannathan J, Sansur CA, Oskouian RJ, Jr, Fu KM, Shaffrey CI. Radiographic restoration of lumbar alignment after transforaminal lumbar interbody fusion. Neurosurgery. 2009;64:955–964. [DOI] [PubMed] [Google Scholar]
- 10. Ghasemi AA. Transforaminal lumbar interbody fusion versus instrumented posterolateral fusion in degenerative spondylolisthesis: an attempt to evaluate the superiority of one method over the other. Clin Neurol Neurosurg. 2016;150:1–5. [DOI] [PubMed] [Google Scholar]
- 11. Fujimori T, Le H, Schairer WW, Berven SH, Qamirani E, Hu SS. Does transforaminal lumbar interbody fusion have advantages over posterolateral lumbar fusion for degenerative spondylolisthesis? Global Spine J. 2015;5:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jalalpour K, Neumann P, Johansson C, Hedlund R. A randomized controlled trial comparing transforaminal lumbar interbody fusion and uninstrumented posterolateral fusion in the degenerative lumbar spine. Global Spine J. 2015;5:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Macki M, Bydon M, Weingart R, et al. Posterolateral fusion with interbody for lumbar spondylolisthesis is associated with less repeat surgery than posterolateral fusion alone. Clin Neurol Neurosurg. 2015;138:117–123. [DOI] [PubMed] [Google Scholar]
- 14. Liu XY, Qiu GX, Weng XS, Yu B, Wang YP. What is the optimum fusion technique for adult spondylolisthesis-PLIF or PLF or PLIF plus PLF? A meta-analysis from 17 comparative studies. Spine (Phila Pa 1976). 2014;39:1887–1898. [DOI] [PubMed] [Google Scholar]
- 15. Levin JM, Tanenbaum JE, Steinmetz MP, Mroz TE, Overley SC. Posterolateral fusion (PLF) vs transforaminal lumbar interbody fusion (TLIF) for spondylolisthesis: a systematic review and meta-analysis. Spine J. 2018;18:1088–1098. [DOI] [PubMed] [Google Scholar]
- 16. Sim HB, Murovic JA, Cho BY, Lim TJ, Park J. Biomechanical comparison of single-level posterior versus transforaminal lumbar interbody fusions with bilateral pedicle screw fixation: segmental stability and the effects on adjacent motion segments. J Neurosurg Spine. 2010;12:700–708. [DOI] [PubMed] [Google Scholar]
- 17. Tang S. Comparison of posterior versus transforaminal lumbar interbody fusion using finite element analysis. Influence on adjacent segmental degeneration. Saudi Med J. 2015;36:993–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang S. Does TLIF aggravate adjacent segmental degeneration more adversely than ALIF? A finite element study. Turk Neurosurg. 2012;22:324–328. [DOI] [PubMed] [Google Scholar]
- 19. Staartjes VE, Siccoli A, de Wispelaere MP, Schröder ML. Patient-reported outcomes unbiased by length of follow-up after lumbar degenerative spine surgery: do we need 2 years of follow-up? Spine J. 2019;19:637–644. [DOI] [PubMed] [Google Scholar]
- 20. Adogwa O, Elsamadicy AA, Han JL, Cheng J, Karikari I, Bagley CA. Do measures of surgical effectiveness at 1 year after lumbar spine surgery accurately predict 2-year outcomes? J Neurosurg Spine. 2016;25:689–696. [DOI] [PubMed] [Google Scholar]
- 21. Parker SL, Asher AL, Godil SS, Devin CJ, McGirt MJ. Patient-reported outcomes 3 months after spine surgery: is it an accurate predictor of 12-month outcome in real-world registry platforms? Neurosurg Focus. 2015;39:E17. [DOI] [PubMed] [Google Scholar]
- 22. Etemadifar MR, Hadi A, Masouleh MF. Posterolateral instrumented fusion with and without transforaminal lumbar interbody fusion for the treatment of adult isthmic spondylolisthesis: a randomized clinical trial with 2-year follow-up. J Craniovertebr Junction Spine. 2016;7:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim E, Chotai S, Stonko D, Wick J, Sielatycki A, Devin CJ. A retrospective review comparing two-year patient-reported outcomes, costs, and healthcare resource utilization for TLIF vs PLF for single-level degenerative spondylolisthesis. Eur Spine J. 2018;27:661–669. [DOI] [PubMed] [Google Scholar]
- 24. Tan JH, Tan KA, Hey HWD, Wong HK. Distal junctional failure secondary to L5 vertebral fracture—a report of two rare cases. J Spine Surg. 2017;3:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


