Abstract
Study Design:
Retrospective cohort study.
Objectives:
The learning curve associated with the implementation of minimally invasive spinal surgery (MIS) has been the center of attention in numerous publications. So far, these studies referred to a single MIS procedure. In our view, minimally invasive surgical skills are acquired simultaneously through a variety of procedures that share common features. The aim of this study was to analyze the skills progression of a single surgeon implementing diverse minimally invasive techniques.
Methods:
We retrospectively collected all patients who underwent spinal surgery for thoracic or lumbar pathology by a single surgeon between 2012 and 2015 at a single institute. Both minimally invasive as well as open surgical techniques were analyzed; these groups were compared on the basis of surgical indications and outcomes. Skills progression analysis in reference to minimally invasive technique was performed.
Results:
A total of 230 patients met the inclusion criteria for this study. MIS group included higher percentage of lumbar discectomy and the open-surgery group included higher percentage of tumor resection surgery. Learning curve evaluation demonstrated increased surgical complexity, evaluated by number of levels treated, over the 4-year period, which corresponded with decreased complication rates.
Discussion:
A gradual increase in surgical complexity over 4 years, together with careful patient selection, enables the surgeon to maintain the rate of complication within acceptable limits. The main challenge facing the MIS community is constructing an education program for MIS surgeons in order to reduce the learning curve–induced complications.
Conclusion:
Advancement of educational aids for MIS surgical skill improvement, including spine models, virtual and augmented reality aids and surgical simulators may reduce the learning curve of spine surgeons.
Keywords: spine surgery, minimally invasive surgery, learning curve, education
Introduction
Over the past decade, spinal surgery is constantly shifting toward minimally invasive procedures. In 2010, approximately 15% of all spine operations in the United States were performed using minimally invasive surgical (MIS) technique. Within 6 years, that number has doubled, and it is expected that by 2020 more than 50% of all spine operations in the United States would be performed in a minimally invasive approach.1 This trend is partly explained by a wider range of indications that are now considered operable by MIS technique, and by the increasing number of surgeons who have begun implementing spine MIS in their clinical practice. Since the integration of minimally invasive technique involves the acquisition of new skills, a learning curve is expected. To date, numerous publications have portrayed learning curves for a single type of operation, such as MIS discectomy,2-4 MIS laminectomy,2-4 MIS transforaminal lumbar interbody fusion,5-8 and MIS screw placement.9 However, in our view, these studies do not faithfully represent real-life clinical practice, since surgical skills are acquired simultaneously through a variety of MIS procedures that share common features, such as minimal exposure, identification of anatomical landmarks in a limited surgical field and development of microsurgery skills. The aim of this study was to analyze the skills progression of a single surgeon during the initial 4 years of implementing diverse minimally invasive techniques for the treatment of various spinal pathologies.
Materials and Methods
After obtaining the institution’s review board approval, we retrospectively collected patients who underwent spinal surgery for thoracic or lumbar pathology by a single senior neurosurgeon (RH) between 2012 and 2015 at the Department of Neurosurgery, Sheba Medical Center. Minimally invasive approach surgery was initiated in our department in 2012. The surgeon’s training in minimally invasive technique until 2012 was based on a 2-year general spine surgery fellowship that included only limited exposure to MIS approaches. Throughout the analyzed period, the decision on MIS versus open approach was determined according to surgeon’s discretion. Surgical goals included discectomy, decompression (laminectomy, laminotomy and foraminotomy), instrumented stabilization, fusion and tumor resection. MIS discectomies, laminectomies, and foraminotomies were performed under microscopic vision using the METRx System (Medtronic, Minneapolis, MN, USA).10 MIS transforaminal lumbar interbody fusion procedure was performed utilizing expandable X-TUBE retractor for facetectomy and bilateral laminectomy, and instrumented fusion with Cresent PEEK (polyetherether ketone) cage and Sextant percutaneous screws (Medtronic, Minneapolis, MN, USA).11 MIS tumor resection procedure was performed utilizing expandable X-TUBE retractor for trans-pedicular approach, hemicorpectomy, and instrumentation with longitude-FNS screws and PMMA (polymethylmethacrylate) augmentation into the vertebral bodies (Medtronic, Minneapolis, MN, USA).12 Lateral trans-psoas approach was performed with XLIF (extreme lateral interbody fusion) retractor, electrophysiologic monitor, cages, and lateral screws and plates (Nuvasive, San Diego, CA, USA). Full surgical descriptions are beyond the scope of this article and are detailed in previously published literature. Preoperative data was acquired by reviewing records of admission files. Radiographic evaluation prior to operation was based on all available imaging modalities. Patient records were analyzed retrospectively and compared for demographics, preoperative clinical status, surgical goals, surgical technique, postoperative complications, and clinical outcome.
Skills progression analysis in reference to minimally invasive technique was performed by dividing the MIS cohort into 4 chronological groups; each group was analyzed separately for complication rate and clinical outcome. In addition, the proportion of operations involving multiple spinal levels (as opposed to single-level disease) was assessed. We thus aimed to plot a learning curve associated with MIS technique in view of the growing complexity of the procedures and the expanding experience of the surgeon.
Statistical Analysis
Data is expressed as mean ± SD for parametric variables and frequencies, and percentages for nonparametric variables. Univariate analysis was performed using independent Student’s t test /chi-square test/Fisher’s exact test (where appropriate) to identify significant variables (P < .05). Multivariate analysis was performed using forward stepwise logistic regression in order to control for potential confounders and to determine independent predictors for major, minor, and total complications in each of the surgical techniques. Potential confounders included age, gender, chronic diseases, American Society of Anesthesiologists score, symptoms/signs at presentation, indication for surgery, goal of surgery, spinal level of surgery, number of operated levels, and surgeon’s experience. In this model, highly intercorrelated independent variables (r > 0.7) were avoided. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated. Methods were performed using SPSS v.22 software (IBM Corp, Armonk, NY, USA).
Results
Patient Population
A total of 230 patients met the inclusion criteria for this study; 115 in each group. Population characteristics and demographics are displayed in Table 1. There were no significant differences in age, gender, and comorbidities between the groups. Patients’ presenting symptoms are displayed as well. The proportion of thoracic myelopathic patients was significantly higher in the open-surgery group compared with the MIS group (34.8% and 5.2%, respectively, P < .001).
Table 1.
Patients’ Demographic Data.
| MIS, Absolute Number (%) | Open Surgery, Absolute Number (%) | P | |
|---|---|---|---|
| N | 115 (50) | 115 (50) | N/A |
| Age, years, mean ± SD | 54.7 ± 16.1 | 57.0 ± 14.3 | .254 |
| Sex | |||
| Male | 63 (54.8) | 61 (53.0) | .791 |
| Female | 52 (45.2) | 54 (47.0) | |
| ASA score | |||
| I | 53 (46.1) | 52 (45.2) | .966 |
| II | 30 (26.1) | 33 (28.7) | |
| III | 18 (15.7) | 16 (13.9) | |
| IV | 14 (12.2) | 14 (12.2) | |
| Diabetes mellitus | 25 (21.7) | 22 (19.1) | .624 |
| Hypertension | 45 (39.1) | 36 (31.3) | .214 |
| Ischemic heart disease | 8 (7.0) | 10 (8.7) | .623 |
Abbreviations: MIS, minimally invasive surgery; N/A, not applicable; ASA, American Society of Anesthesiologists.
Operative Features and Patient Selection
The distribution of surgical goals within each group is displayed in Table 2. The rate of discectomy operations was significantly higher in the MIS group compared with the open-surgery group (27.8% and 4.3%, respectively, P < .001). Tumor resections and instrumented stabilization procedures were more prevalent in the open surgery group. The number of treated spinal segments and their levels are summarized in Table 2. In the open-surgery group, 2.3 average spinal levels were treated, compared with 1.3 spinal levels in the MIS group (P < .001). Estimated blood loss (EBL) was 340 ± 589 mL for open surgery compared with 40 ± 169 mL for minimal invasive surgery (P < .001).
Table 2.
Preoperative Symptoms, Operative Goals, and Spinal Levels.
| MIS, Absolute Number (%) | Open Surgery, Absolute Number (%) | P | |
|---|---|---|---|
| Preoperative symptoms | |||
| Radiculopathy | 80 (57.6) | 59 (42.4) | .005 |
| Myelopathy | 6 (5.2) | 40 (34.8) | <.001 |
| Back pain | 57 (46.9) | 70 (60.9) | .080 |
| Neurogenic claudication | 40 (34.8) | 42 (51.2) | .783 |
| Surgical goals | |||
| Decompression | 35 (30.4) | 27 (23.5) | .235 |
| Stabilization | 29 (25.2) | 45 (39) | .024 |
| Discectomy | 32 (27.8) | 5 (4.3) | <.001 |
| Tumor resection | 19 (16.5) | 38 (33) | .004 |
| Number of levels, mean | 1.31 | 2.31 | <.001 |
| 1 level | 81 (70.4) | 30 (26.3) | <.001 |
| 2 levels | 32 (27.8) | 44 (38.6) | |
| ≥3 levels | 2 (1.7) | 40 (35.1) | |
| Thoracic | 11 (9.6) | 40 (34.8) | <.002 |
| Lumbar | 101 (87.8) | 74 (64.3) |
Abbreviation: MIS, minimally invasive surgery.
Complications
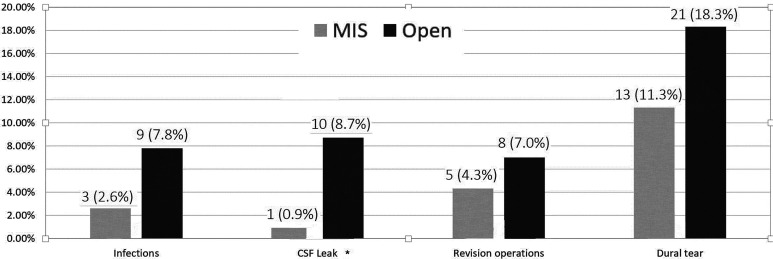
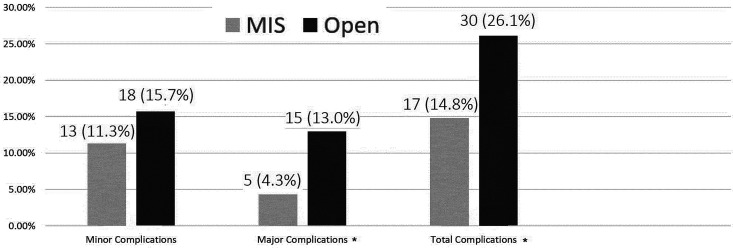
Overall postoperative complication rate was 26.1% for the open-surgery group compared with 14.8% for the MIS group (P = .03). Univariate analysis of complications is displayed in Figures 1 and 2. Multivariate analysis revealed the following variables as significant predictors for postoperative complications: diabetes mellitus (P < .005; OR 4.19, CI 1.89-9.30), myelopathy (P = .001; OR 4.27, CI 1.87-9.77), and instrumented stabilization surgeries (P = .006; OR 2.72, CI 1.33-5.56).
Figure 1.
Minimally invasive surgery (MIS) versus open postoperative complications. Rate of postoperative infections, cerebrospinal fluid (CSF) leaks, revision operations, and witnessed dural tears over time; univariate analysis. *Statistically significant (P < .005).
Figure 2.
Minimally invasive surgery (MIS) versus open postoperative complications, overall analysis (univariate analysis). Minor complications included urinary tract infection (UTI), pneumonia, neurological deterioration to a minor extent, cerebrospinal fluid (CSF) leak that resolved without revision surgery, superficial wound infection, need for postoperative inhalations of steroids and bronchodilators. Major complications included death, score drop on ASIA (American Spinal Injury Association) impairment scale, postoperative revision surgery, deep wound infection, meningitis, operated epidural hematoma, prolonged ventilation. *Statistically significant (P < .005).
Outcome
Table 3 presents the various postoperative outcomes. The symptomatic change (improvement/deterioration) was not significantly different between the groups (P = .47). Average postoperative stay at the hospital following open surgery was 7.1 days compared with 2.9 days following MIS (P < .001).
Table 3.
Outcomes.
| MIS, Absolute Number (%) | Open Surgery, Absolute Number (%) | P | |
|---|---|---|---|
| Neurologic outcome | |||
| Improved | 57 (54.8) | 54 (49.5) | .470 |
| Stable | 38 (36.5) | 40 (36.7) | |
| Deteriorated | 9 (8.7) | 15 (13.8) | |
| Length of admission, days, mean ± SD | 2.9 ± 3.2 | 7.1 ± 6.2 | <.001 |
| Estimated blood loss, mL, mean ± SD | 39.1 ± 169.5 | 339.1 ± 589.9 | <.0001 |
| Discharged to home | 104 (92) | 82 (73.2) | .002 |
| Follow-up period, months, mean ± SD | 6.2 ± 10.1 | 8.0 ± 9.5 | .017 |
Abbreviation: MIS, minimally invasive surgery.
Learning Curve
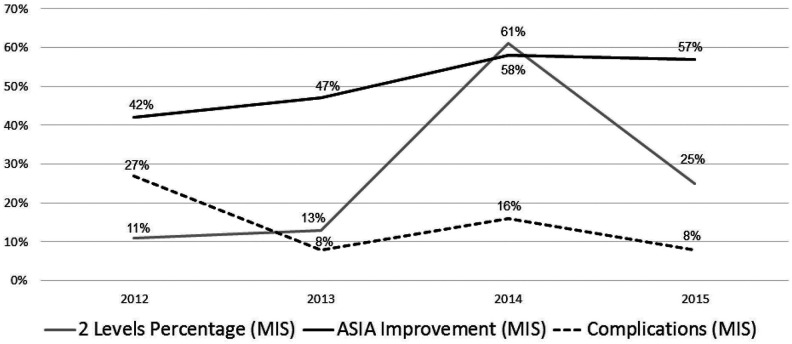
Learning curve was initially evaluated by inspecting surgical complexity of cases in itself. Figure 3 demonstrates the gradual increase in 2-level surgeries over the first 2-year period. While surgical complexity is on the rise, complication level decreases. Over the third year, frequency of 2-level surgeries increased to 61% of cases with a slight rise of complication rate. During the fourth study year, the rate of 2-level surgeries was reduced to 25% with further reduction of complication rates.
Figure 3.
Minimally invasive skills analysis over time. Surgical learning curve demonstrating the surgeon’s skills progression over a 4-year period. Rate of operations involving 2 spinal levels, rate of patients who improved by at least 1 ASIA (American Spinal Injury Association) score following surgery, and rate of overall complications are presented.
Discussion
Despite numerous previous publications of learning curves associated with minimally invasive spinal surgery, what constitutes an acceptable learning curve has not been agreed upon as yet.2-9,13-15 Epstein,16 for instance, proposed a slightly vague definition: “the number of cases required to become proficient for performing various MIS spinal procedures.” A review of recent literature reveals that authors include different parameters in their learning curve analyses. Doherty et al8 included measurements of EBL, fluoroscopy time, and length of postoperative stay in their analysis. Silva et al13 emphasized the gradual decrease over time in the duration of surgery. Staartjes et al14 recently published the learning curve for microdiscectomy at its later stages and measured the rate of disc reherniation as an indicator of skills progression. The purpose of our analysis is to describe the learning curve of versatile MIS surgeries with increasing complexity by a single surgeon. We will propose that this learning curve can be reproduced by others and hence should be the base for constructing education programs for surgeons performing MIS.
In the current study the MIS patient group had similar risk factors to those found in the control open group; yet the number of treated levels and their pathologies were significantly different between the two groups. That difference is derived from the meticulous patient selection for MIS procedures and explains the insignificant impact of MIS surgery on complication rate once multivariable analysis is performed. As described in other articles regarding MIS surgery, a significant reduction can be witnessed on EBL and length of stay. Figure 3 demonstrates an initial steep decline in complications followed by a plateau of approximately 8% to 16% of overall complication rate, despite the trend toward an increment in the complexity of the procedures that is demonstrated by the increase in the rate of 2-level operations. Importantly, a continuing increase in the percentage of patients who improved neurologically is seen throughout the curve. The sharp rise in 2-level surgeries over the third year followed by a decrease in the fourth year might represent the pendulum swing in favor of new technologies followed by the back swing after evaluation of the technology limitation. Our interpretation of the data is that a gradual increase in surgical complexity over 4 years, together with careful patient selection, enables the surgeon to maintain the rate of complication within acceptable limits. Possible interpretations of the rapid increase of 2 levels surgery on the third year, followed by a decline on the fourth year includes surgeon’s understanding of MIS limitations at approaching multilevel pathologies. To date, previous publications portrayed learning curves of spine MIS that are associated with a single type of procedure.3-7 We believe that different surgical interventions share common qualities when performed in a minimally invasive approach. We therefore grouped in our analysis the entire diversity of spine MIS, which takes into account the accumulative experience of the surgeon and shows his skills progression over time as the operations were originally performed. This type of eclectic learning curve has not yet been published.
As the demand for spine minimally invasive procedures is constantly growing by patients and surgeons alike, the need for designated training programs is now more prominent than ever before.
It is safe to assume that spine surgeons graduating in the coming years would benefit from gaining proficiency, or at least acquaintance with minimally invasive techniques. Spine minimally invasive approaches pose unique challenges, such as the limited access and orientation, the need to rely on fluoroscopy and navigation, the use of microsurgical techniques and the limited ability of the tutor to assist the trainee in a small surgical field. In order to reduce learning curve–associated complications, there is a need for an innovative training setup that would consider the unique necessities of spine MIS. Developing educational programs with gradual increase in complexity, assisted by such learning aids as simulators, microsurgical training setups, cadaver workshops, and instructional case presentations is pivotal in order to minimize the learning curve associated complications.
Limitations
This study carries the limitations of retrospective data collection. The control group is significantly different in terms of number of levels treated, symptoms, and surgical goals. We attributed these differences to selection bias, since meticulous patient selection for MIS is crucial for patients’ safety and complication avoidance. However, as this study aims to describe the learning curve of a single surgeon, the comparison with the control group serves only as a base line for complications, hence does not impede this study’s strength.
Conclusion
This article demonstrates that gradual increase in surgical complexity over four years was associated with favorable outcomes and lower complication rates in the MIS group in comparison to the open-surgery group. The main challenge facing the MIS community is finding the optimal tools to teach MIS techniques in order to reduce the learning curve–induced complications.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ran Harel, MD  https://orcid.org/0000-0002-0165-7114
https://orcid.org/0000-0002-0165-7114
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
References
- 1. Phillips FM, Cheng I, Rampersaud YR, et al. Breaking through the “glass ceiling” of minimally invasive spine surgery. Spine (Phila Pa 1976). 2016;41(suppl 8):S39–S43. [DOI] [PubMed] [Google Scholar]
- 2. McAfee PC, Garfin SR, Rodgers WB, Allen RT, Phillips F, Kim C. An attempt at clinically defining and assessing minimally invasive surgery compared with traditional “open” spinal surgery. SAS J. 2011;5:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mannion RJ, Guilfoyle MR, Efendy J, Nowitzke AM, Laing RJ, Wood MJ. Minimally invasive lumbar decompression: long-term outcome, morbidity, and the learning curve from the first 50 cases. J Spinal Disord Tech. 2012;25:47–51. [DOI] [PubMed] [Google Scholar]
- 4. Ahn J, Iqbal A, Manning BT, et al. Minimally invasive lumbar decompression—the surgical learning curve. Spine J. 2016;16:909–916. [DOI] [PubMed] [Google Scholar]
- 5. Lau D, Lee JG, Han SJ, Lu DC, Chou D. Complications and perioperative factors associated with learning the technique of minimally invasive transforaminal lumbar interbody fusion (TLIF). J Clin Neurosci. 2011;18:624–627. [DOI] [PubMed] [Google Scholar]
- 6. Nandyala SV, Fineberg SJ, Pelton M, Singh K. Minimally invasive transforaminal lumbar interbody fusion: one surgeon’s learning curve. Spine J. 2014;14:1460–1465. [DOI] [PubMed] [Google Scholar]
- 7. Khan NR, Clark AJ, Lee SL, Venable GT, Rossi NB, Foley KT. Surgical outcomes for minimally invasive vs open transforaminal lumbar interbody fusion: an updated systematic review and meta-analysis. Neurosurgery. 2015;77:847–874. [DOI] [PubMed] [Google Scholar]
- 8. Doherty P, Welch A, Tharpe J, Moore C, Ferry C. Transforaminal lumbar interbody fusion with rigid interspinous process fixation: a learning curve analysis of a surgeon team’s first 74 cases. Cureus. 2017;9:e1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parikh K, Tomasino A, Knopman J, Boockvar J, Härtl R. Operative results and learning curve: microscope-assisted tubular microsurgery for 1- and 2-level discectomies and laminectomies. Neurosurg Focus. 2008;25:E14. [DOI] [PubMed] [Google Scholar]
- 10. Armin SS, Holly LT, Khoo LT. Minimally invasive decompression for lumbar stenosis and disc herniation. Neurosurg Focus. 2008;25:E11. [DOI] [PubMed] [Google Scholar]
- 11. Park P, Foley KT. Minimally invasive transforaminal lumbar interbody fusion with reduction of spondylolisthesis: technique and outcomes after a minimum of 2 years’ follow-up. Neurosurg Focus. 2008;25:E16. [DOI] [PubMed] [Google Scholar]
- 12. Harel R, Doron O, Knoller N. Minimally invasive spine metastatic tumor resection and stabilization: new technology yield improved outcome. Biomed Res Int. 2015;2015:948373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silva PS, Pereira P, Monteiro P, Silva PA, Vaz R. Learning curve and complications of minimally invasive transforaminal lumbar interbody fusion. Neurosurg Focus. 2013;35:E7. [DOI] [PubMed] [Google Scholar]
- 14. Staartjes VE, de Wispelaere MP, Miedema J, Schröder ML. Recurrent lumbar disc herniation after tubular microdiscectomy: analysis of learning curve progression. World Neurosurg. 2017;107:28–34. [DOI] [PubMed] [Google Scholar]
- 15. Wood MJ, McMillen J. The surgical learning curve and accuracy of minimally invasive lumbar pedicle screw placement using CT based computer-assisted navigation plus continuous electromyography monitoring—a retrospective review of 627 screws in 150 patients. Int J Spine Surg. 2014;8:27 doi:10.14444/1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Epstein NE. Learning curves for minimally invasive spine surgeries: are they worth it? Surg Neurol Int. 2017;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]