Abstract
Background:
The accuracy of flash glucose monitoring (FGM, FreeStyle Libre Pro [FSL-Pro]) remains unclear in patients with type 2 diabetes mellitus (T2DM) undergoing hemodialysis.
Methods:
We assessed 13 patients with T2DM undergoing hemodialysis. They simultaneously underwent FGM, continuous glucose monitoring (CGM, iPro2), and self-monitoring blood glucose (SMBG).
Results:
Parkes error grid analysis against SMBG showed that 49.0% and 51.0% of interstitial fluid glucose (ISFG) levels measured using FGM and 93.3% and 6.7% of those measured using CGM fell into zones A and B, respectively. Mean absolute relative difference (MARD) against SMBG for FGM was significantly higher than that for CGM (19.5% ± 13.2% vs 8.1% ± 7.6%, P < .0001). Parkes error grid analysis of 2496 paired ISFG levels between FGM and CGM showed that 53.6%, 46.2%, and 0.2% of the plots fell into zones A, B, and C, respectively. Mean ISFG levels were lower with FGM than with CGM (143.7 ± 67.2 mg/dL vs 164.6 ± 58.5 mg/dL; P < .0001). Mean absolute relative difference of ISFG levels between FGM and CGM was 19.2% ± 13.8%. Among three groups classified according to CGM ISFG levels (hypoglycemia, <70 mg/dL; euglycemia, 70-180 mg/dL; and hyperglycemia, >180 mg/dL), the MARDs for hypoglycemia (31.9% ± 25.0%) and euglycemia (22.8% ± 14.6%) were significantly higher than MARD for hyperglycemia (13.0% ± 8.5%) (P < .0001 in both).
Conclusions:
Flash glucose monitoring may be clinically acceptable. Average ISFG levels were lower with FGM than with CGM, and MARDs were higher for hypoglycemia and euglycemia in patients with T2DM undergoing hemodialysis.
Keywords: flash glucose monitoring, hemodialysis, interstitial fluid glucose level, iPro2, FreeStyle Libre Pro, type 2 diabetes mellitus
Introduction
Recently, some studies have demonstrated that continuous glucose monitoring (CGM) is useful for detecting hypoglycemia1,2 or glycemic fluctuations1,3,4 and for evaluating the effect of some medications5,6 in patients with diabetes mellitus (DM) undergoing hemodialysis (HD). Therefore, CGM may be suitable for informing diabetes management decisions or recommending treatment in this population. However, CGM has not been widely used owing to certain limitations in CGM devices, such as the need for calibrations by self-monitoring of blood glucose (SMBG) and the shortness of their sensor lifetime (seven days).
Flash glucose monitoring (FGM) systems are novel factory-calibrated interstitial glucose monitoring systems that are currently available as professional, blind to the patient (FreeStyle Libre Pro [FSL-Pro], Abbott Japan, Chiba, Japan), and as personal monitoring systems.7,8 In FGM, calibrations used by SMBG are not required during the 14-day wearing period.7,8 Several studies have reported the accuracy of interstitial glucose measurements9-13 in patients with type 1 DM (T1DM) and type 2 DM (T2DM).
Although FGM has become available to patients with DM undergoing HD in Japan, its accuracy remains unknown in this population. Thus, we simultaneously measured interstitial fluid glucose (ISFG) levels using FGM and CGM (iPro2: Medtronic Japan, Tokyo, Japan) and capillary blood glucose (BG) values using SMBG and investigated their associations in patients with T2DM undergoing HD.
Methods
We evaluated 13 patients with T2DM undergoing HD at the outpatient clinic of Matsunami General Hospital. All patients underwent regular HD with a standard bicarbonate dialysate containing 125 mg/dL of glucose. This study adhered to the principles of the Declaration of Helsinki, and all patients provided informed consent to participate. The ethics committee of Matsunami General Hospital approved the study protocol (No. 336).
All patients were admitted to Matsunami General Hospital in Gifu, Japan, for the management of glycemic control. We obtained blood samples before the HD session. During hospitalization, patients received medical diet therapy (30 kcal/ideal body weight (kg)/day). We attached FGM and CGM devices simultaneously to the upper arm contralateral to the arteriovenous fistula and the abdomen, respectively, on the HD day of hospitalization. The patients performed SMBG using OneTouch Ultra Veriovue device (Johnson & Johnson K.K., Tokyo, Japan) four times a day to calibrate CGM. Flash glucose monitoring and CGM recorded ISFG levels every 15 and 5 minutes, respectively. First, we compared ISFG levels obtained by FGM and CGM with the closest (in time) BG value determined by SMBG. Second, we compared ISFG levels obtained by FGM with the closest (in time) those obtained by CGM. We maintained time lags of less than three minutes between CGM and SMBG measurements, less than eight minutes between FGM and SMBG measurements, and less than three minutes between FGM and CGM measurements, respectively. We used the ISFG levels of day 3 (HD day) and day 4 (non-HD day) for data analysis.
Statistical Analysis
First, we analyzed the associations of ISFG levels obtained by FGM and CGM with BG values determined by SMBG on both HD and non-HD days, HD day, and non-HD day, respectively. We evaluated the associations of ISFG levels obtained by the two systems with BG values of SMBG in clinically meaningful areas from A to E using Parkes error grid analysis.14,15 We also used a Bland-Altman analysis to evaluate the differences between the two systems and SMBG. Moreover, we determined the absolute difference (AD) and absolute relative difference (ARD) between the ISFG levels of FSL-Pro or iPro2 based on BG values of SMBG as follows: AD (mg/dL) = |ISFG − SMBG|, ARD (%) = (|ISFG − SMBG|)/SMBG × 100, where ISFG were the values obtained using either FGM or CGM. We used a paired t-test to compare AD and ARD overall or among the three groups (hypoglycemia, <70 mg/dL; euglycemia, 70-180 mg/dL; and hyperglycemia, >180 mg/dL) classified according to the BG values of SMBG.
Second, we analyzed the associations of paired ISFG levels between FGM and CGM using Parkes error grid and Bland-Altman analyses. Absolute difference and ARD between FGM and CGM were also determined based on CGM ISFG levels as follows: AD (mg/dL) = |FGM − CGM|, ARD (%) = (|FGM − CGM|)/iPro2 × 100. Moreover, we used one-way analysis of variance and the Tukey-Kramer method to evaluate the differences between AD and ARD classified into the three groups according to CGM ISFG levels.
We conducted all statistical analyses using SPSS 21 software (IBM Corp., Armonk, NY, United States), and P < .05 were considered statistically significant.
Results
Patients’ Characteristics
In total, 13 patients with T2DM undergoing HD were enrolled (age: 63.5±11.3 years; men: n = 11; HD duration: 7.3 [4.3-28.4] months; body mass index: 24.5 ± 4.1 kg/m2; hematocrit: 32.1% ± 3.4%; predialysis BG: 213 ± 44 mg/dL; glycated hemoglobin: 7.2% ± 0.8%; and glycated albumin: 23.1% ± 5.5%). They were treated with medical diet therapy (n = 2), dipeptidyl peptidase-4 inhibitor (n = 3), insulin therapy (n = 6; including basal and bolus insulin, 2; basal insulin alone, 3; bolus insulin alone, 1; total insulin: 9.5 [7.0-27.5] U/day, basal insulin: 7.0 [3.0-11.0] U/day, bolus insulin: 4.5 [0-19.5] U/day), basal insulin and dipeptidyl peptidase-4 inhibitor (n = 1), or basal insulin and glucagon-like peptide-1 receptor agonist (n = 1) (Table 1).
Table 1.
Patients’ Characteristics.
| Parameters | N = 13 |
|---|---|
| Age (y) | 63.5 ± 11.3 |
| Gender, men (n) | 11 |
| Hemodialysis duration (mo) | 7.3 (4.3-28.4) |
| Body mass index (kg/m2) | 24.5 ± 4.1 |
| Hemoglobin (g/dL) | 10.8 ±1.1 |
| Hematocrit (%) | 32.1 ± 3.4 |
| Albumin (g/dL) | 3.6 ± 0.3 |
| Predialysis blood glucose (mg/dL) | 213 ± 44 |
| Glycated hemoglobin (%) | 7.2 ± 0.8 |
| Glycated albumin (%) | 23.1 ± 5.5 |
Associations Between ISFG Levels Capillary BG Values on Both HD and Non-HD Days (104 Measurements)
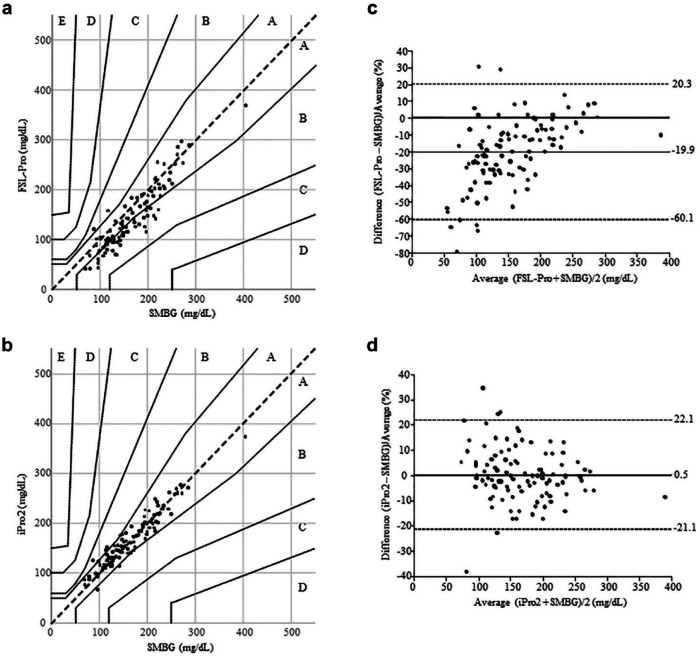
The time lags between CGM and SMBG and between FGM and SMBG were 1.1 ± 0.7 and 4.3 ± 2.2 minutes, respectively. Parkes error grid analysis against SMBG showed that 49.0% and 51.0% (B lower 49.0%) of ISFG levels measured using FGM (Figure 1(a)) and 93.3% and 6.7% of those measured using CGM (Figure 1(b)) fell into zones A and B, respectively.
Figure 1.
Associations between interstitial fluid glucose levels obtained using FreeStyle Libre Pro or iPro2 and capillary blood glucose values determined by self-monitoring blood glucose on both hemodialysis and nonhemodialysis days. The Parkes error grid analysis against self-monitoring blood glucose (104 measurements) showed that 49.0% and 51.0% (B lower 49.0%) of interstitial fluid glucose levels measured using the FreeStyle Libre Pro fell into zones A and B, respectively (a). In contrast, the Parkes error grid analysis against self-monitoring blood glucose showed that 93.3% and 6.7% of interstitial fluid glucose levels determined using iPro2 fell into zones A and B, respectively (b). The mean interstitial fluid glucose levels of FreeStyle Libre Pro decreased significantly by 24.4 mg/dL (19.9%) compared with blood glucose values of self-monitoring blood glucose (P < .0001) (c). Conversely, the mean interstitial fluid glucose levels of iPro2 were comparable to blood glucose values of self-monitoring blood glucose (d).
Compared with BG values of SMBG, mean ISFG levels of FGM were significantly decreased by 24.4 mg/dL (19.9%) (166.9 ± 57.4 mg/dL vs 142.5 ± 63.9 mg/dL, P < .0001) (Figure 1(c) and Table 2). In contrast, the mean ISFG levels of CGM were comparable to the BG values of SMBG (P = .90) (Figure 1(d) and Table 2). Compared with the BG values of SMBG, the mean ISFG levels of FGM were significantly lower; the mean ISFG levels of CGM were comparable to BG values of SMBG during euglycemia and hyperglycemia (Table 2). Because only a single BG measurement was obtained during hypoglycemia, we could not analyze the differences between ISFG levels of FGM or CGM and BG values of SMBG.
Table 2.
Comparison of Interstitial Fluid Glucose Levels Obtained by FreeStyle Libre Pro and iPro2 With the Closest Blood Glucose Values Obtained by Self-monitoring Blood Glucose.
| N | BG values (mg/dL) |
ISFG levels (mg/dL) |
||
|---|---|---|---|---|
| SMBG | FSL-Pro | iPro2 | ||
| HD and non-HD days | ||||
| Overall | 104 | 166.9 ± 57.4 | 142.5 ± 63.9a | 166.7±54.0 |
| Hypoglycemia | 1 | 69.0 | 40.0 | 86.0 |
| Euglycemia | 62 | 129.8 ± 26.7 | 104.7 ± 32.5a | 132.4 ± 27.1 |
| Hyperglycemia | 41 | 225.4 ± 39.2 | 202.2 ± 52.7a | 221.0 ± 37.9 |
| HD day | ||||
| Overall | 52 | 169.5 ± 61.6 | 142.1 ± 61.9a | 169.1 ± 55.0 |
| Hypoglycemia | 1 | 69.0 | 40.0 | 86.0 |
| Euglycemia | 30 | 131.3 ± 28.6 | 107.1 ± 28.0a | 134.7 ± 23.0 |
| Hyperglycemia | 21 | 228.7 ± 45.9 | 196.8 ± 56.1a | 222.1 ± 43.2 |
| Non-HD day | ||||
| Overall | 52 | 164.4 ± 53.5 | 143.0 ± 66.5a | 164.4 ± 53.5 |
| Hypoglycemia | 0 | NA | NA | NA |
| Euglycemia | 32 | 128.4 ± 25.1 | 102.4 ± 36.5a | 130.3 ± 30.6 |
| Hyperglycemia | 20 | 221.9 ± 31.5 | 208.0 ± 49.7a | 218.9 ± 32.5 |
Abbreviations: BG, blood glucose; FSL-Pro, FreeStyle Libre Pro; HD, hemodialysis; NA, not applicable; ISFG, interstitial fluid glucose; SMBG, self-monitoring blood glucose.
Glycemia was classified based on the blood glucose values of self-monitoring blood glucose.
P < .0001 vs self-monitoring blood glucose.
Mean absolute difference (MAD) and mean absolute relative differene (MARD) against SMBG for FGM were significantly higher than those for CGM (28.9 ± 17.9 mg/dL vs 12.4 ± 10.2 mg/dL, P < .0001 and 19.5% ± 13.2% vs 8.1% ± 7.6%, P < .0001) (Table 3). In addition, MAD and MARD were significantly higher for FGM than for CGM during euglycemia and hyperglycemia (Table 3). Because only a single BG value was determined using SMBG as hypoglycemia, we also could not evaluate the differences of MARD in the classified three groups based on SMBG.
Table 3.
Mean Absolute Difference and Mean Absolute Relative Difference Against Self-monitoring Blood Glucose for FreeStyle Libre Pro and iPro2.
| N | MAD against SMBG (mg/dL) |
MARD against SMBG (%) |
|||
|---|---|---|---|---|---|
| FSL-Pro | iPro2 | FSL-Pro | iPro2 | ||
| HD and non-HD days | |||||
| Overall | 104 | 28.9 ± 17.9a | 12.4 ± 10.2 | 19.5 ± 13.2b | 8.1 ± 7.6 |
| Hypoglycemia | 1 | 29.0 | 17.0 | 42.0 | 24.6 |
| Euglycemia | 62 | 28.9 ± 15.3a | 11.9 ± 10.1 | 23.2 ± 13.1b | 9.3 ± 8.6 |
| Hyperglycemia | 41 | 28.8 ± 21.7a | 13.0 ± 10.5 | 13.3 ± 10.8b | 5.8 ± 4.7 |
| HD day | |||||
| Overall | 52 | 30.0 ± 19.6a | 15.1±10.0 | 19.4 ± 12.7 b | 9.8 ± 7.8 |
| Hypoglycemia | 1 | 29.0 | 17.0 | 42.0 | 24.6 |
| Euglycemia | 30 | 27.9 ± 15.0a | 13.5 ± 9.7 | 21.6 ± 11.8 b | 10.8 ± 8.9 |
| Hyperglycemia | 21 | 33.0 ± 25.2a | 17.2 ± 10.3 | 15.2 ± 12.4b | 7.7 ± 4.7 |
| Non-HD day | |||||
| Overall | 52 | 27.7 ± 16.3a | 9.7 ± 9.7 | 19.5 ± 13.9b | 6.4 ± 7.1 |
| Hypoglycemia | 0 | NA | NA | NA | NA |
| Euglycemia | 32 | 29.8 ± 15.8a | 10.3 ± 10.3 | 24.6 ± 14.3b | 8.0 ± 8.2 |
| Hyperglycemia | 20 | 24.4 ± 16.9a | 8.6 ± 8.9 | 11.4 ± 8.7b | 3.8 ± 3.9 |
Abbreviations: BG, blood glucose; FSL-Pro, FreeStyle Libre Pro; HD, hemodialysis; ISFG, interstitial fluid glucose; MAD, mean absolute difference; MARD, mean absolute relative difference; NA, not applicable; SMBG, self-monitoring blood glucose.
Glycemia was classified based on the blood glucose values of self-monitoring blood glucose.
P < .0001 vs iPro2 for mean absolute difference.
P < .0001 vs iPro2 for mean absolute relative difference.
Associations Between ISFG Levels and Capillary BG Values on HD Day vs Non-HD Day
Parkes error grid analysis against SMBG for FGM and CGM showed almost same results on HD day and non-HD day, respectively (FGM: zone A 48.1%, B 51.9% and zone A 50%, B 50%; CGM: zone A 90.4%, B 9.6% and zone A 96.2%, B 3.8%, respectively). As for the differences between ISFG levels of FGM or CGM and BG values of SMBG, similar results were obtained on HD and non-HD days (Tables 2 and 3).
Average BG values by SMBG and ISFG levels by FGM and CGM on HD day were comparable to those on non-HD day (P = .65, P = .94, and P = .66, respectively). Mean absolute relative difference against SMBG for FGM on HD day was comparable to that on non-HD day (P = .96). Conversely, MARD against SMBG for CGM on HD day was significantly higher than that on non-HD day (9.8% ± 7.8% vs 6.4% ± 7.1%, P = .022).
Associations of Paired ISFG Levels Between FGM and CGM on Both HD and Non-HD Days (2496 Measurements)
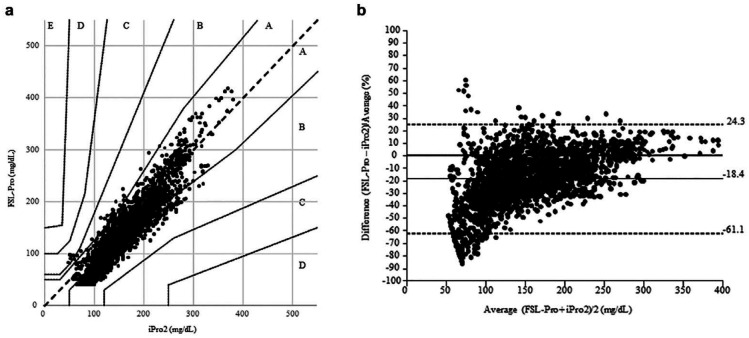
The time lag between FGM and CGM measurements was 1.7 ± 0.8 minutes. The Parkes error grid analysis showed that 53.6%, 46.2%, and 0.2% of the plots fell into zones A, B, and C, respectively (Figure 2(a)). Especially, 45.0% of those were within the lower zone B, and 78.8% of ISFG levels were lower with FGM than with CGM. The mean ISFG levels were significantly decreased by 20.9 mg/dL (18.4%) when measured using FGM compared with CGM (143.7 ± 67.2 mg/dL vs 164.6 ± 58.5 mg/dL, P < .0001; Figure 2(b)). The MAD of ISFG levels between FGM and CGM was 28.1 ± 17.5 mg/dL. The MARD of ISFG levels between FGM and CGM was 19.2% ± 13.8%. When classified into three groups based on CGM ISFG levels, the MADs of euglycemia (27.7 ± 16.4 mg/dL) and hyperglycemia (29.1 ± 19.3 mg/dL) were significantly higher than that of hypoglycemia (18.9 ± 13.2 mg/dL) (P = .0041 and P = .016, respectively). Conversely, the MARDs of hypoglycemia (31.9% ± 25.0%) and euglycemia (22.8% ± 14.6%) were significantly higher than that of hyperglycemia (13.0% ± 8.5%) (both P-values <.0001) (Table 4).
Figure 2.
The associations of paired interstitial fluid glucose levels between FreeStyle Libre Pro and iPro2 on both hemodialysis and nonhemodialysis days. The Parkes error grid analysis of 2496 paired interstitial fluid glucose levels between FreeStyle Libre Pro and iPro2 showed that 53.6% of the plots were within zone A, 46.2% within zone B (B lower: 45.0%), and 0.2% within zone C (a). The mean interstitial fluid glucose levels were significantly decreased by 20.9 mg/dL (18.4%) when measured using the FreeStyle Libre Pro compared with the iPro2 (P < .0001) (b).
Table 4.
Comparison of Interstitial Fluid Glucose Levels Obtained by FreeStyle Libre Pro and iPro2.
| N | FSL-Pro (mg/dL) | iPro2 (mg/dL) | MAD (mg/dL) | MARD (%) | |
|---|---|---|---|---|---|
| HD and non-HD days | |||||
| Overall | 2496 | 143.7 ± 67.2a | 164.6 ± 58.5 | 28.1 ± 17.5 | 19.2 ± 13.8 |
| Hypoglycemia | 31 | 66.4 ± 19.6 | 61.9 ± 5.7 | 18.9 ± 13.2 | 31.9 ± 25.0 |
| Euglycemia | 1537 | 104.7 ± 36.6a | 128.2 ± 27.9 | 27.7 ± 16.4 | 22.8 ± 14.6 |
| Hyperglycemia | 928 | 210.7 ± 52.2a | 228.3 ± 36.2 | 29.1 ± 19.3 | 13.0 ± 8.5 |
| HD day | |||||
| Overall | 1248 | 142.5 ± 63.8a | 163.5 ± 57.4 | 28.2 ± 18.4 | 18.7±13.1 |
| Hypoglycemia | 8 | 81.1 ± 5.7a | 61.4 ± 7.2 | 19.8 ± 11.8 | 34.6 ± 24.6 |
| Euglycemia | 807 | 107.3 ± 33.2a | 129.4 ± 26.5 | 27.0 ± 16.7 | 21.4 ± 13.8 |
| Hyperglycemia | 433 | 209.3 ± 53.2a | 228.9 ± 39.3 | 30.5 ± 21.1 | 13.4 ± 9.1 |
| Non-HD day | |||||
| Overall | 1248 | 144.8 ± 70.5a | 165.7 ± 59.5 | 28.1 ± 16.6 | 19.8 ± 14.4 |
| Hypoglycemia | 23 | 61.3 ± 20.2 | 62.1 ± 5.2 | 18.7 ± 13.9 | 31.0 ± 25.6 |
| Euglycemia | 730 | 102.0 ± 39.8a | 126.9 ± 29.3 | 28.5 ± 15.9 | 24.3 ± 15.2 |
| Hyperglycemia | 495 | 211.9 ± 51.4a | 227.7 ± 33.3 | 27.9 ± 17.5 | 12.6 ± 8.1 |
Abbreviations: FSL-Pro, FreeStyle Libre Pro; HD, hemodialysis; MAD, mean absolute difference; MARD, mean absolute relative difference.
Glycemia was classified based on interstitial fluid glucose levels of iPro2.
P < .0001 vs iPro2.
Safety of FGM and CGM Devices
We found no device-associated complications, such as bleeding, erythema, itching, pain, or infection, on insertion sites with either device.
Discussion
In this study, Parkes error grid analysis showed that 100% of ISFG levels measured using both FGM and CGM against the BG values of SMBG fell into zone A or B. However, the ratio of plots occupying zone B (altered clinical action with little or no effect on clinical outcome) for FGM against SMBG was higher than that for CGM against SMBG. Bland-Altman analysis showed that the average ISFG levels of CGM were comparable to the average BG values of SMBG, however, those of FGM were significantly lower than the average BG values of SMBG. Some previous studies have reported similar results: the average ISFG levels of FGM were 5 to 9.2 mg/dL lower than the average BG values of SMBG in patients with DM who were not undergoing HD.13,16 Moreover, MARD against SMBG for FGM (19.5%) was significantly higher than that for CGM (8.1%). Some previous studies found different MARDs when comparing FGM with capillary BG values (10%-16.6%).7,9,11,16,17 In this study, we used SMBG device, which met the ISO15197:2013 accuracy standards for system accuracy,18 to monitor capillary BG values. Therefore, based on the Parkes error grid analysis, FGM may be clinically acceptable in patients with T2DM undergoing HD. However, it appears that the results are much better for CGM in this population.
Few studies have investigated paired ISFG levels between FGM and CGM directly. Bonora et al9 performed a Clarke error grid analysis showing 62.4%, 29.2%, 4.1%, and 4.3% of paired ISFG levels between FGM and CGM (Dexcom G4 Platinum) falling within zones A, B, C, and D, respectively, in patients with T1DM. Sato et al12 reported a Parkes error grid analysis showing 84.8% and 15.1% of paired ISFG levels between FGM and CGM falling within zones A and B, respectively, in insulin-treated patients with T2DM. Our results showed that 99.8% of the plots were within zone A or B, but the ratio of plots occupying zone B lower (45.0%) was higher than that of previous studies.9,12 Moreover, Bland-Altman analysis revealed that the ISFG levels of FGM were 1.1, 13.6, and 20.9 mg/dL lower than those of CGM in the study of Bonora et al,9 Sato et al,12 and ours, respectively. The reason why FGM measurements result in somewhat low ISFG levels is uncertain in the present study. However, the location where FGM and CGM are attached may be associated with the ISFG level discrepancy. According to a report evaluating the FGM insertion site, the accuracy and precision of FGM sensors placed on the abdomen is unacceptably poor.19 Because we did not investigate the ISFG level agreements obtained by FGM and CGM sensors placed on the same site, we cannot confirm this hypothesis. However, in patients undergoing HD, the extent of edema in the arm and the abdomen might be different. In contrast, SMBG calibration may have also affected the results. The hematocrit may affect blood glucose meter performance in patients undergoing HD: low hematocrit values (<35%) result in high readings.20 However, we used a hematocrit-corrected SMBG device based on ISO15197:2013,18 and the SMBG calibration may have caused only a marginal effect, at least from the perspective of hematocrit interference. Furthermore, we think that a possible explanation for the low bias of FGM is that the algorithm was purposely established to display low readings in the hypoglycemia and euglycemia ranges to minimize missed hypoglycemia.
Mean absolute relative difference between FGM and CGM (19.2%) in our study was somewhat poorer than that reported by Bonora et al9 (18.1%). Moreover, studies have shown that the accuracy seems to be lower within the lower glucose ranges.9-11,16 In this study, MARDs for hypoglycemia and euglycemia were significantly higher than MARD for hyperglycemia; therefore, the accuracy seems to be lower during euglycemia as well as hypoglycemia. Therefore, when FGM indicates hypoglycemia, BG values should be confirmed with SMBG. Furthermore, even if FGM does not indicate hypoglycemia, SMBG should be performed if hypoglycemic symptoms appear. However, any time lags between FGM and CGM measurements should also be considered when the glucose rate of change is high.
On the other hand, MARD against SMBG for FGM was significantly higher than that for iPro2 on HD day and non-HD day, respectively. Although the MARD against SMBG for FGM on HD day was comparable to that on non-HD day, the MARD against SMBG for CGM on HD day was significantly higher than that on non-HD day. The results of CGM might be expected because it is reported that the glycemic excursion on HD day is larger than that on non-HD day.1,3 Therefore, whether HD itself can affect ISFG measurement differences obtained between FGM and CGM remains unclear. Further, more detailed studies are needed to evaluate the comparison between ISFG levels of FGM or CGM and BG values of SMBG during and after hemodialysis.
There are some limitations to this study. First, the number of study participants and the numbers of compared ISFG levels and BG values were small. Second, we did not use a venous glucose level as a reference for comparison. However, we think it was difficult to acquire frequent venous blood samples considering the burden on the patients undergoing HD. Third, we could not evaluate the ISFG levels of FGM or CGM and BG values of SMBG during and after the hemodialysis session, when the glycemic excursion may be large. Additional studies are needed to clarify the accuracy of FGM with larger sample size and frequent BG measurements using venous glucose as the reference glucose in this population.
Conclusion
Flash glucose monitoring may be clinically acceptable; however, ISFG levels of FGM seem to be lower than those of CGM, and MARDs may be higher during hypoglycemia and euglycemia (<180 mg/dL) in patients with T2DM undergoing HD.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Takahiro Yajima  https://orcid.org/0000-0002-8180-5614
https://orcid.org/0000-0002-8180-5614
References
- 1. Kazempour-Ardebili S, Lecamwasam VL, Dassanyake T, et al. Assessing glycemic control in maintenance hemodialysis patients with type 2 diabetes. Diabetes Care. 2009;32(7):1137-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vos FE, Schollum JB, Coulter CV, Manning PJ, Duffull SB, Walker RJ. Assessment of markers of glycaemic control in diabetic patients with chronic kidney disease using continuous glucose monitoring. Nephrology. 2012;17(2):182-188. [DOI] [PubMed] [Google Scholar]
- 3. Jin YP, Su XF, Yin GP, et al. Blood glucose fluctuations in hemodialysis patients with end stage diabetic nephropathy. J Diabetes Complications. 2015;29(3):395-399. [DOI] [PubMed] [Google Scholar]
- 4. Yajima T, Yajima K, Hayashi M, Takahashi H, Yasuda K. Serum albumin-adjusted glycated albumin as a better indicator of glycemic control in Type 2 diabetes mellitus patients with short duration of hemodialysis. Diabetes Res Clin Pract. 2017;130:148-153. [DOI] [PubMed] [Google Scholar]
- 5. Yajima T, Yajima K, Hayashi M, Takahashi H, Yasuda K. Efficacy and safety of teneligliptin in addition to insulin therapy in type 2 diabetes mellitus patients on hemodialysis evaluated by continuous glucose monitoring. Diabetes Res Clin Pract. 2016;122:78-83. [DOI] [PubMed] [Google Scholar]
- 6. Yajima T, Yajima K, Hayashi M, Takahashi H, Yasuda K. Improved glycemic control with once-weekly dulaglutide in addition to insulin therapy in type 2 diabetes mellitus patients on hemodialysis evaluated by continuous glucose monitoring. J Diabetes Complications. 2018;32(3):310-315. [DOI] [PubMed] [Google Scholar]
- 7. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17(11):787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoss U, Budiman ES. Factory-calibrated continuous glucose sensors: the science behind the technology. Diabetes Technol Ther. 2017;19(S2):S44-S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonora B, Maran A, Ciciliot S, Avogaro A, Fadini GP. Head-to-head comparison between flash and continuous glucose monitoring systems in outpatients with type 1 diabetes. J Endocrinol Invest. 2016;39(12):1391-1399. [DOI] [PubMed] [Google Scholar]
- 10. Aberer F, Hajnsek M, Rumpler M, et al. Evaluation of subcutaneous glucose monitoring systems under routine environmental conditions in patients with type 1 diabetes. Diabetes Obes Metab. 2017;19(7):1051-1055. [DOI] [PubMed] [Google Scholar]
- 11. Boscari F, Galasso S, Facchinetti A, et al. FreeStyle Libre and Dexcom G4 Platinum sensors: accuracy comparisons during two weeks of home use and use during experimentally induced glucose excursions. Nutr Metab Cardiovasc Dis. 2018;28(2):180-186. [DOI] [PubMed] [Google Scholar]
- 12. Sato T, Oshima H, Nakata K, et al. Accuracy of flash glucose monitoring in insulin-treated patients with type 2 diabetes.J Diabetes Investig. 2019;10(3):846-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumagai R, Muramatsu A, Fujii M, et al. Comparison of glucose monitoring between FreeStyle Libre Pro and iPro2 in patients with diabetes mellitus. J Diabetes Investig. 2019;10(3):851-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143-1148. [DOI] [PubMed] [Google Scholar]
- 15. Pfutzner A, Klonoff DC, Pardo S, et al. Technical aspects of the Parkes error grid. J Diabetes Sci Technol. 2013;7(5):1275-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ólafsdóttir AF, Attvall S, Sandgren U, et al. A clinical trial of the accuracy and treatment experience of the flash glucose monitor FreeStyle Libre in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):164-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ji L, Guo X, Guo L, Ren Q, Yu N, Zhang J. A multicenter evaluation of the performance and usability of a novel glucose monitoring system in Chinese adults with diabetes. J Diabetes Sci Technol. 2017;11(2):290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katz LB, Grady M, Setford SJ, Levy BL. OneTouch blood glucose monitoring systems: impact of new technologies on the efficacy of self-monitoring blood glucose. US Endocrinol. 2018;14(suppl 1):2-8. [Google Scholar]
- 19. Charleer S, Mathieu C, Nobels F, Gillard P. Accuracy and precision of flash glucose monitoring sensors inserted into the abdomen and upper thigh compared with the upper arm. Diabetes Obes Metab. 2018;20(6):1503-1507. [DOI] [PubMed] [Google Scholar]
- 20. Ramljak S, Lock JP, Schipper C, et al. Hematocrit interference of blood glucose meters for patient self-measurement. J Diabetes Sci Technol. 2013;7(1):179-189. [DOI] [PMC free article] [PubMed] [Google Scholar]