Abstract
Background:
Decision support smartphone applications integrated with continuous glucose monitors may improve glycemic control in type 1 diabetes (T1D). We conducted a survey to understand trends and needs of potential users to inform the design of decision support technology.
Methods:
A 70-question survey was distributed October 2017 through May 2018 to adults aged 18-80 with T1D from a specialty clinic and T1D Exchange online health community (myglu.org). The survey responses were used to evaluate potential features of a diabetes decision support tool by Likert scale and open responses.
Results:
There were 1542 responses (mean age 46.1 years [SD 15.2], mean duration of diabetes 26.5 years [SD 15.8]). The majority (84.2%) have never used an app to manage diabetes; however, a large majority (77.8%) expressed interest in using a decision support app. The ability to predict and avoid hypoglycemia was the most important feature identified by a majority of the respondents, with 91% of respondents indicating the highest level of interest in these features. The task that respondents find most difficult was management of glucose during exercise (only 47% of participants were confident in glucose management during exercise). The respondents also highly desired features that help manage glucose during exercise (85% of respondents were interested). The responses identified integration and interoperability with peripheral devices/apps and customization of alerts as important. Responses from participants were generally consistent across stratified categories.
Conclusions:
These results provide valuable insight into patient needs in decision support applications for management of T1D.
Keywords: decision support, multiple daily injections, type 1 diabetes, smartphone app, survey
Introduction
Type 1 diabetes (T1D) is a complex condition characterized by the loss of endogenous insulin secretion. The focus of care in T1D is achieving near normal glucoses which can prevent the long-term consequences of this disease. Improvements in the care of people with diabetes over the last 100 years have turned T1D from a debilitating illness into a chronic condition with life expectancy nearing that of the general population.1,2 These advancements have sometimes come at the cost of increasing burden on the patient. Improving glucose control with a therapy like multiple daily injections typically requires fastidious attention to insulin dose, timing, glucose trends, diet, activity level, and more. As this field advances, careful attention should be paid to how we can improve care without increasing burden.
People with T1D have benefited from advancements of consumer technology over the last several decades. Commercially available hybrid closed-loop insulin delivery systems and accurate continuous glucose monitors (CGM) are leading to substantial improvements in glycemic control for people using these technologies.3-5 Still the burden of diabetes lies in the frequent and high-stakes management decisions that people with T1D have to make. The current model to empower patients to sustain a lifetime of continuous self-management includes diabetes education and frequent follow-up with a diabetes provider for adjustment of the insulin regimen, coaching on behaviors, and screening for complications. Decision support technology may hold the key for a paradigm shift in this construct.
Clinical decision support refers to a system “designed to be a direct aid to clinical decision-making, in which the characteristics of an individual patient are matched to a computerized clinical knowledge base and patient-specific assessments or recommendations are then presented to the clinician or the patient for a decision.”6 The computational capacity of smartphone technology can now power a decision support tool. Smartphones accept inputs (ie, CGM, physical activity tracker, insulin dose tracker) to generate the data needed for personalized recommendations. They can run complex algorithms to provide insight to the user on how to act on this data. Lastly, their ubiquity in modern daily life allows for acceptance of their use.
Reviews of currently available diabetes apps highlight the need for ongoing innovation and study of these systems.7-12 Current decision support apps in T1D have been criticized for poor usability, lack of integration with peripheral technologies (meters, CGM, etc.), and lack of properly designed control trials to evaluate benefits and challenges with sustained use. The majority of apps with evidence for clinical benefit (A1c lowering of ~0.5%) are intended for use in type 2 diabetes.9 The study of these technologies is limited by the rapid rate of evolution; most of the apps with available evidence in the literature are no longer in use today. In the recent meta-analyses9,12,13 several apps for T1D were reviewed. These studies dated from 2010 to 2018, with the older studies evaluating apps that required manual entry of data. Two studies showed statistically significant improvement in A1c and four showed no statistically significant improvement. Beyond the variable outcomes in glycemic improvements, there is concern for accuracy and applicability of currently available apps for short-acting insulin dosing. In 2015, Huckvale et al reviewed 46 apps that provided short-acting insulin dose calculators.14 Notably, 91% did not have numeric input validation, 67% gave inappropriate dose recommendations, 59% allowed dose calculation despite missing values, and only 30% provided the calculation formula. A recent evidence review by Veazie et al reviewed five apps for T1D; only one app scored “acceptable” on usability testing.15
We are designing a decision support smartphone application for use by those patients with T1D on multiple daily injections of insulin (MDI) that will incorporate CGM trends, physical activity tracker data, and insulin dosing from a dose capture device into one user-friendly interface. The algorithms powering the decision support app will account for insulin-on-board, upcoming or past exercise events, as well as making predictions of future hyper- or hypoglycemia based on historical trends. The system will analyze trends weekly and provide recommendations for adjustment of basal insulin, correction factors, and carbohydrate ratios with the goal to improve glycemic control. We hypothesize that a well-designed decision support application can optimize an individual’s insulin regimen and intelligently alert the user to predicted trends to improve glycemic control.
Given limitations of the currently available apps for T1D, there is a clear need to rigorously design and clinically evaluate decision support applications. The main objective of this survey was to ascertain patient needs to guide design of a decision support tool that reflects the knowledge and experience of people with T1D. In this paper, we discuss results of this survey and briefly discuss how this informed the design of our decision support smartphone application.
Methods
Survey Design and Description
We conducted a 70-question (24 header questions with 46 subquestions) online survey that assessed the needs and preferences assessment for the design of decision support smartphone applications to support management of T1D. Questions to assess respondent’s self-perceptions of health and motivation for the care of diabetes were based upon the approach from published questionnaires.16,17 Additional questions were developed by consensus by physician scientists, biomedical engineers, and software developers who have extensive experience with T1D in both clinical and research arenas. Duration of the survey was approximately 20 minutes (survey questions in Supplemental Table S1).
Survey Instrument
Opinion questions were rated on a five-point Likert scale (1 = strongly disagree/not confident, 5 = strongly agree/very confident). An additional coded response of “Decline to answer” was included for each question; these responses were excluded from the analysis. Three open response questions collected additional input on desired features.
Survey Distribution
The survey was released during October 2017 to January 2018 to adults aged 18-80 with T1D from the academic medical center’s diabetes clinic at Oregon Health & Science University (OHSU) via email to 132 people (targeting MDI users) resulting in 46 survey responses. Respondents were compensated with a $10 gift card for the pilot survey. The second phase was through email in collaboration with myglu.org, an online health community associated with T1D Exchange for those with T1D and their caregivers. Myglu.org has over 16 700 members with T1D (30% male and 70% female, average age 43 years, myglu.org community statistics, May 2019). The email distribution was from February to May 2018, resulting in 1496 survey responses. All survey responses were collected anonymously through REDCap database (supported through grant UL1TR002369). Given the nature of the distribution, repeat survey participation could not be prevented. All survey materials and procedures were approved by the Institutional Review Board at OHSU (Portland, OR).
Statistical Analysis
The survey responses were stratified by method of insulin administration (pump, MDI), use of CGM, gender, age (≥50, <50 years old), and origin of recruiting (OHSU, myglu.org). The data were analyzed and presented using R-software (www.r-project.org) packages “Likert” and “createtableone.” Frequency tables summarized Likert scale and categorical responses (frequent use ≥50% of the time; agreement = Likert 4 or 5; confidence = Likert 4 or 5; interest = Likert 4 or 5). Comparisons among stratification groups were performed using Mann-Whitney hypothesis testing for ranked and nonparametric distributions, with alpha equal to 0.005. LMW and NT independently reviewed open responses and categorized by theme.
Responses (n = 1542) were weighted according to gender, CGM use, and pump use reflecting our knowledge of the T1DM population as previously reported18 (50% F, 15% CGM use, 60% pump use). Poststratification weighting was accomplished in the R Survey package19,20 through “raking,” a method of iteratively sampling survey observations to match known population characteristics. Weights were constrained from 0.3 to 3. The weighted population demographics are reported in Table 1.
Table 1.
Survey Population Characteristics.
| Before weighting | Overall |
Stratified by insulin administration |
||||
|---|---|---|---|---|---|---|
| SD/% | MDI | SD/% | Pump | SD/% | ||
| n | 1542 | 390 | 25.3% | 1152 | 74.7% | |
| Age (mean) | 46.1 | 15.2 | 46.7 | 15.9 | 45.7 | 14.9 |
| Duration of T1D (mean) | 26.5 | 15.8 | 24.1 | 15.9 | 27.4 | 15.7 |
| Male (n) | 498 | 32.3% | 159 | 40.8% | 339 | 29.4% |
| Female (n) | 1039 | 67.4% | 230 | 59.0% | 809 | 70.2% |
| CGM users (n) | 1163 | 75.4% | 191 | 49.0% | 972 | 84.4% |
| After weighting | Overall |
Stratified by insulin administration |
||||
| SD/% | MDI | SD/% | Pump | SD/% | ||
| n | 1542 | 580 | 37.8% | 955 | 62.2% | |
| Age (mean) | 46.9 | 15.4 | 47.1 | a | 46.8 | a |
| Duration of T1D (mean) | 26.8 | 15.8 | 24.4 | a | 28.3 | a |
| Male (n) | 620 | 40.3% | 275 | 47.3% | 345 | 36.1% |
| Female (n) | 915 | 59.6% | 305 | 52.7% | 610 | 63.9% |
| CGM users (n) | 478 | 31.2% | 57 | 9.8% | 420 | 44.1% |
Characteristics of survey respondents overall and stratified by insulin administration type, multiple daily injection users (MDI), and insulin pump users (Pump). For the question about gender 0.3% (n = 5) respondents reported “Decline to answer.” Survey population characteristics varied from known T1D population statistics, so survey weighting was used to align with reported population parameters (50% F, 15% CGM use, 60% pump use).
Not available due to method of analysis.
Results
Subject Characteristics
We received 1542 responses to the survey. See Table 1 for summary of characteristics. We did not collect information regarding race/ethnicity or disability status. All responses were included in data analysis. Survey population characteristics varied from recently reported T1D population statistics,18 so poststratification weighting was used to align the survey population with the following population parameters: 50% F, 15% CGM use, 60% pump use (Table 1, “After weighting”). All following results are consistent before and after weighting unless otherwise stated.
Motivation and Confidence in Diabetes Management
For all motivation questions to assess motivation and confidence in their T1D management, the majority of respondents reported high levels of motivation/confidence. The lowest level of confidence was seen for management of glucose with exercise (Supplemental Figure S1). Confidence with exercise glucose management was higher in MDI users, those ≥50 years, and in men (P < .005 for each).
Current Diabetes Management Characteristics
The most common frequency of adjustment of short-acting insulin doses was every three months. Men, MDI users, and those ≥50 years were more likely to report that they change their short-acting insulin ratios daily (P < .005 for each). MDI users tended to adjust long-acting insulin infrequently, with the majority adjusting every three months. Most MDI users (76.9%) reported interest in an app that adjusts basal insulin. A majority of MDI users were interested in insulin adjustment weekly or more frequently (54.9%).
Current Use of Adjunctive Technology for Diabetes Management
Stratification groups based on age and gender showed similar frequencies of CGM use. Frequent use of an expert meter (ie, bolus advisor) to calculate insulin doses was reported by 39% overall; this increased to 46.8% after weighting. Those without CGM were more likely to use expert meters (non-CGM 50.1% vs. CGM 35.7%, P < .005). Only 15.8% of all respondents and 20% of MDI users reported prior or current use of a smartphone application to calculate insulin doses. The top three applications were Dexcom, MyFitnessPal, and Loop/LoopKit (Table 2). The top four reasons for discontinuation were (1) pump use, (2) app use being cumbersome or taking too much time, (3) ability to do calculations without app, and (4) technical issues/poor user experience.
Table 2.
Summary of Open Response Questions.
| Smartphone applications used | |
|---|---|
| Application | n |
| Dexcom | 27 |
| MyFitnessPal | 12 |
| Loop/LoopKit | 12 |
| RapidCalc | 8 |
| Diabetes:M | 6 |
| CalorieKing | 5 |
| PredictBGL | 5 |
| One Drop | 5 |
| AndroidAPS | 5 |
| xDrip | 4 |
| Nightscout | 3 |
| Other apps with ≦ 2 respondents | 29 |
| Why did you stop using the phone application? | |
| Reason | n |
| Pump use | 53 |
| Too long/cumbersome | 23 |
| I can do it in my head | 11 |
| Technical issues/poor user experience | 10 |
| Inaccurate | 10 |
| No longer needed it | 7 |
| Insurance coverage and/or cost | 5 |
| Medicare doesn’t cover Dexcom App | 4 |
| Unavailable on current phone platform | 4 |
| Changed diet | 3 |
| For a study or beta testing | 3 |
| Annoying alarms | 2 |
| No value provided | 2 |
| Fell out of the routine | 1 |
| Geared for younger age group | 1 |
| Additional features to include in a phone app | |
| Suggestion | n |
| App compatibility | 159 |
| Customizable alert settings | 93 |
| Data review | 91 |
| Insulin dose adjustments | 57 |
| Data sharing | 55 |
| Carb counting | 48 |
| Predicted trends in glucose | 45 |
| Decreased user input | 30 |
| Insurance coverage and/or cost | 28 |
| App specs: battery, memory, GUI | 16 |
| Emergency alert | 14 |
| Insulin-on-board | 13 |
| Low vision compatibility | 2 |
Abbreviation: GUI, graphical user interface.
There were 112, 132, and 595 unique respondents for each question respectively. App compatibility included many mentions of a phone app to perform insulin pump functions as well as the desire for an app to automatically import data from peripheral devices.
Use of Other Technologies
Nearly all respondents had their smartphone nearby >75% of the time. Physical activity monitor (ie, Fitbit, Apple watch) use showed a dichotomous use pattern; the majority reported that they do not use these devices, while >30% reported frequent use (Supplemental Table S1, question 7). Frequent use of physical activity monitors was higher in pump users and CGM users (P < .05 for each).
Patterns of CGM Use
CGM use questions are reported in Supplemental Table S1, questions 15a-f. The vast majority wear the CGM device more than 75% of the time and look at their CGM more than six times per day. The vast majority review CGM trends before eating, before exercise, before bedtime, and frequently modify insulin dosing in response to CGM data. CGM use behavior did not generally vary for the stratifications analyzed (pump/MDI, gender, age).
Interest in a Smartphone App
Overall, 79.4% reported they were interested in using a smartphone application to help manage their diabetes (Supplemental Table S1, question 5) with similar findings in CGM and MDI users. Most respondents (78.9%) were interested in an app to provide suggestions on short-acting insulin doses. Most MDI users (64.9%) were interested in using an app to provide suggestions on long-acting insulin dosing.
Application Features and Alerts
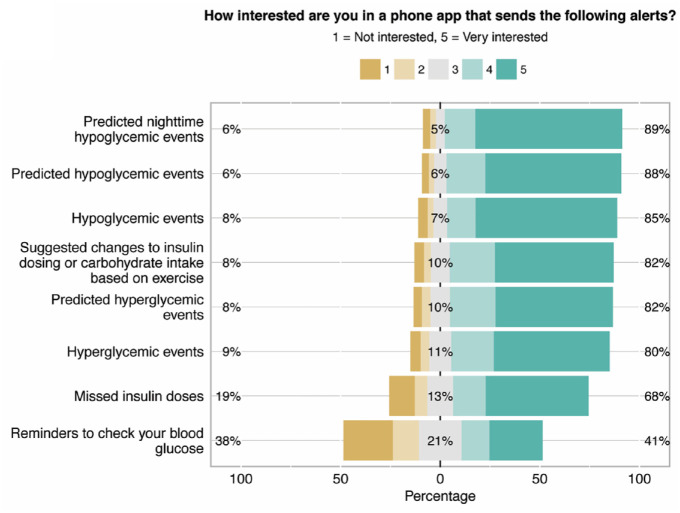
There was a high level of interest in the features proposed with >70% of respondents expressing interest in 14 out of 16 features (Figure 1a). The features with the highest levels of interest were those related to hypoglycemia. Men and women had similar levels of interest in proposed features.
Figure 1.
Likert responses for features and alerts questions. Responses about (a) features and (b) alerts proposed for a decision support smartphone application. The percentages listed are for scores of 1, 3, and 5 from left to right (1 = not interested, 3 = neutral, 5 = very interested).
The preferred frequency of alerts was one to three times per day (Supplemental Table S1, question 23). For hypoglycemia alerts, most expressed a preference for alert >30 minutes prior to the event. The strongest preferences were for hypoglycemia alerts (Figure 1a) The least popular alerts were for missed insulin doses and reminders to check CBGs. Preferences of alerts did not generally vary for stratifications analyzed (pump/MDI, use of CGM, gender); however, those ≥50 have stronger preferences for alerts across all categories
Open-Ended Responses
A total of 595 respondents answered an open-ended question about additional features for a decision support system (Table 2). Many indicated the importance of avoidance of manual data entry. Another common request was for full and detailed control over alert settings (profiles for different times of the day, days of the week, ability to set volume/vibration/silence based on their preferences). Many desired data review for personal use and to share with healthcare providers.
Discussion
This survey provides important insight into the desired features of a decision support system for T1D. Respondents were very interested in features to prevent hypoglycemia, especially features that can predict future hypoglycemia (Figure 1). Based on this information, we have developed several hypoglycemia prediction algorithms for incorporation into our decision support system. The vast majority of respondents were open to a decision support system that would provide recommendations for insulin regimen adjustments. This will be essential for optimum functioning of our decision support system as users will need to accept recommendations to alter their bolus calculator settings. From the qualitative analysis, one essential feature was repeatedly mentioned: compatibility and automated import of data from peripheral devices. If we are asking people with diabetes to carry/wear a smartphone, CGM, physical activity monitor, and an insulin dose tracking device, it is essential to ensure seamless integration of these devices. We are carefully selecting and integrating the peripheral devices and incorporating human factors testing into our development process to provide a satisfactory user experience.
There were varied preferences for management and predictive alerts. Respondents spoke of their frustrations with current CGM alarms and desire for profiles for different times of the day/days of the week and to have full control of volume/vibration/silence based on their preferred glucose levels. There was interest in more physiologic alarms (ie, suppressed hyperglycemia alarm if insulin recently administered). These alarms can be a nuisance and can lead to undesired behaviors such as insulin stacking or discontinued use of CGM. We are designing our decision support system to allow for alert preferences, so users control these alarm elements with minimal hard-coded alerts for patient safety (ie, CGM glucose <54 mg/dL).
This population is clearly a highly motivated diabetes technology using group; yet our survey data indicates that they still tend to wait months between insulin adjustments. The majority of MDI users report changing long-acting insulin doses every three months only, and about half adjust short-acting insulin at this same interval. We suspect that many wait for their quarterly endocrinologist appointments to make adjustments. Despite their current diabetes management behaviors, overall preference was for weekly or daily short-acting changes. Thus, we suspect there will be good acceptance of weekly insulin dose changes with our decision support system.
This survey successfully provided insights into the potential uptake of decision support smartphone applications. Interestingly, even within the myglu.org population of technologically savvy individuals, the vast majority of respondents (84.2%) had never used a smartphone to calculate insulin doses. A large majority (77.8%) expressed interest in using a smartphone app to help manage diabetes. Despite access and frequent use of CGM and pumps, there is still strong interest in new technologies which can help them use pump/CGM technologies more effectively.
There will be multiple components that a person with T1D would need to carry for the decision support application we are designing: smartphone, CGM, physical activity monitor, and an insulin dose tracking device. In our survey, those using CGM wear it very frequently and are in the habit of looking at their display frequently throughout the day. Physical activity trackers may become an important component of automated hormone delivery, especially during exercise,21,22 and these activity trackers may become important in decision support apps as well. Frequent use of a wearable activity tracker was reported in about 30% overall and 21.3% of MDI users, but 80% of respondents reported they would be interested in wearing an activity monitor to improve glucose trends with exercise. Thus, it seems that, at least for our survey population, there is willingness to wear additional sensors if there is possibility of glycemic benefit.
The use of CGM technology in this cohort is much higher than rates published in 2015 from the T1D Exchange database from 2013 to 2014 data.18 In the T1D Exchange cohort of n = 7598 subjects >18 years with T1D, 15% used CGM, whereas 75.4% used CGM in our survey cohort. This likely reflects the high degree of technology fluency of the myglu.org population, and perhaps some expansion CGM use with in the last 5 years. After weighting the survey to more closely match real-world demographics, the percentage of CGM users came down to 31.2%. Even after weighting, MDI users in our survey population were still much less likely to use CGM. This may represent a possible limitation of a technology for MDI users that requires concurrent use of CGM. However, we expect CGM use to increase among MDI users given the recent studies showing the A1c and hypoglycemia reduction benefits of CGM use in patients on MDI.
Our study has several advantages and some limitations. The principal advantage is the large number of respondents from the online community, myglu.org. This community is weighted toward more technologically savvy respondents given its online, self-selecting nature. Of note, a large majority of this community already uses CGM and therefore speak from experience about current use patterns with this technology. We captured responses from a wide breadth of ages and diabetes durations. We saw very similar results for features/alert preferences and attitudes toward technology after weighting our survey to reflect the makeup of the US T1D population. Therefore, we believe these results can apply to the larger population of people with T1D. It is difficult to draw any conclusions from OHSU clinic population given the small number of respondents.
The major limitation of this study is the nature of the myglu.org population which made up the majority of survey respondents. Due to this, we have undersampled those with lower technology fluency and people with T1D with lower engagement with their disease. Future studies of decision support applications in this population are needed to ensure ease of use even by those who are less technologically savvy. Similar to starting pumps or CGM, there will need to be robust on-boarding protocols so all users can learn to use decision support apps effectively.
Conclusions
We surveyed a large number of respondents, including almost 400 representative of target users of our decision support application; people with T1D using MDI. These respondents expressed high levels of interest in the concepts and features proposed for a decision support app, and in particular predictive algorithms to avoid hypoglycemia. Special care in designing apps will be essential to establish and maintain user acceptability, as well as to ensure peripheral device compatibility and fully customizable alarms. Given the highly technologically savvy nature of the surveyed population, special efforts will need to be made to solicit input and test our decision support system in people with T1D not currently using technology.
Supplemental Material
Supplemental material, Supplementary for Patient Input for Design of a Decision Support Smartphone Application for Type 1 Diabetes by Leah M. Wilson, Nichole Tyler, Peter G. Jacobs, Virginia Gabo, Brian Senf, Ravi Reddy and Jessica R. Castle in Journal of Diabetes Science and Technology
Acknowledgments
The guarantor of this research is Leah M. Wilson who takes responsibility for the contents of this article. LMW, NT, JRC, and PGJ contributed to writing, literature search, survey design, data collection, data analysis, data interpretation, and design of figures/tables. VG, BS, and RR contributed to survey design, data collection, and data analysis.
Footnotes
Authors’ Note: The material in this manuscript was previously presented in part in poster format at the 2018 American Diabetes Association Scientific Sessions in Orlando, FL, on June 24, 2018.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JRC and PGJ have a financial interest in Pacific Diabetes Technologies Inc., a company that may have a commercial interest in automated insulin delivery technology. JRC and PGJ have received honorarium for consulting and research support from Dexcom. LMW, NT, VG, BS, and RR have no competing financial interests to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Helmsley Charitable Trust Grant #2018PG-T1D001.
ORCID iDs: Leah M. Wilson  https://orcid.org/0000-0003-3634-481X
https://orcid.org/0000-0003-3634-481X
Peter G. Jacobs  https://orcid.org/0000-0001-9897-4783
https://orcid.org/0000-0001-9897-4783
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Livingstone SJ, Levin D, Looker HCet al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015;313(1):37-44. doi: 10.1001/jama.2014.16425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller RG, Mahajan HD, Costacou T, Sekikawa A, Anderson SJ, Orchard TJ. A contemporary estimate of total mortality and cardiovascular disease risk in young adults with type 1 diabetes: the Pittsburgh epidemiology of diabetes complications study. Diabetes Care. 2016;39(12):2296-2303. doi: 10.2337/dc16-1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beck RW, Riddlesworth T, Ruedy Ket al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371-378. doi: 10.1001/jama.2016.19975 [DOI] [PubMed] [Google Scholar]
- 4. Garg SK, Weinzimer SA, Tamborlane WVet al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155-163. doi: 10.1089/dia.2016.0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lind M, Polonsky W, Hirsch IBet al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379-387. doi: 10.1001/jama.2016.19976. [DOI] [PubMed] [Google Scholar]
- 6. Sim I, Gorman P, Greenes RAet al. Clinical decision support systems for the practice of evidence-based medicine. J Am Med Inform Assoc. 2001;8(6):527-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arnhold M, Quade M, Kirch W. Mobile applications for diabetics: a systematic review and expert-based usability evaluation considering the special requirements of diabetes patients age 50 years or older. J Med Internet Res. 2014;16(4):e104. doi: 10.2196/jmir.2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Demidowich AP, Lu K, Tamler R, Bloomgarden Z. An evaluation of diabetes self-management applications for Android smartphones. J Telemed Telecare. 2012;18(4):235-238. doi: 10.1258/jtt.2012.111002 [DOI] [PubMed] [Google Scholar]
- 9. Hou C, Carter B, Hewitt J, Francisa T, Mayor S. Do mobile phone applications improve glycemic control (HbA1c) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials. Diabetes Care. 2016;39(11):2089-2095. doi: 10.2337/dc16-0346 [DOI] [PubMed] [Google Scholar]
- 10. Klonoff DC, Kerr D. Overcoming barriers to adoption of digital health tools for diabetes. J Diabetes Sci Technol. 2018;12(1):3-6. doi: 10.1177/1932296817732459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Payne HE, Lister C, West JH, Bernhardt JM. Behavioral functionality of mobile apps in health interventions: a systematic review of the literature. JMIR Mhealth Uhealth. 2015;3(1):e20. doi: 10.2196/mhealth.3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun C, Malcolm JC, Wong B, Shorr R, Doyle MA. Improving glycemic control in adults and children with type 1 diabetes with the use of smartphone-based mobile applications: a systematic review. Can J Diabetes. 2019;43(1):51-8.e3. doi: 10.1016/j.jcjd.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 13. Wu Y, Yao X, Vespasiani Get al. Correction: mobile app-based interventions to support diabetes self-management: a systematic review of randomized controlled trials to identify functions associated with glycemic efficacy. JMIR Mhealth Uhealth. 2018;6(1):e20. doi: 10.2196/mhealth.8789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huckvale K, Adomaviciute S, Prieto JT, Leow MK, Car J. Smartphone apps for calculating insulin dose: a systematic assessment. BMC Med. 2015;13:106. doi: 10.1186/s12916-015-0314-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Veazie S, Winchell K, Gilbert Jet al. Rapid evidence review of mobile applications for self-management of diabetes. J Gen Intern Med. 2018;33:1167-1176. doi: 10.1007/s11606-018-4410-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brod M, Hammer M, Christensen T, Lessard S, Bushnell DM. Understanding and assessing the impact of treatment in diabetes: the Treatment-Related Impact Measures for Diabetes and Devices (TRIM-Diabetes and TRIM-Diabetes Device). Health Qual Life Outcomes. 2009;7:83. doi: 10.1186/1477-7525-7-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmitt A, Gahr A, Hermanns N, Kulzer B, Huber J, Haak T. The Diabetes Self-Management Questionnaire (DSMQ): development and evaluation of an instrument to assess diabetes self-care activities associated with glycaemic control. Health Qual Life Outcomes. 2013;11:138. doi: 10.1186/1477-7525-11-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller KM, Foster NC, Beck RWet al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971-978. doi: 10.2337/dc15-0078 [DOI] [PubMed] [Google Scholar]
- 19. Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9(8):1-19. [Google Scholar]
- 20. Lumley T. Package ‘survey’ 2013. https://cran.r-project.org/web/packages/survey/survey.pdf. Accessed January 29, 2019.
- 21. Castle JR, El Youssef J, Wilson LMet al. Randomized outpatient trial of single- and dual-hormone closed-loop systems that adapt to exercise using wearable sensors. Diabetes Care. 2018;41:1471-1477. doi: 10.2337/dc18-0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacobs PG, El Youssef J, Reddy Ret al. Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy. Diabetes Obes Metab. 2016;18(11):1110-1119. doi: 10.1111/dom.12707 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary for Patient Input for Design of a Decision Support Smartphone Application for Type 1 Diabetes by Leah M. Wilson, Nichole Tyler, Peter G. Jacobs, Virginia Gabo, Brian Senf, Ravi Reddy and Jessica R. Castle in Journal of Diabetes Science and Technology