The use of continuous glucose monitoring (CGM) has increased steadily over the past decade.1 As these devices become more pervasive in the outpatient setting, there is increasing interest in using CGM for inpatient management of diabetes. This raises concerns regarding safety in the setting of radiology imaging. Current industry guidelines recommend removal of devices when undergoing certain radiology procedures due to potential interference with CGM system functioning.2 Expert opinion has suggested placing a lead apron over the device prior to x-ray, CT scan, or catheterization procedures.3,4 However, there is no published data on the impact of radiation and contrast exposure on CGM precision and accuracy to support these recommendations.
We monitored 49 adult medicine and surgery patients with type 1 and type 2 diabetes who used Dexcom G6 CGM (Dexcom, San Diego, CA, USA) devices during hospitalization. Glycemic data were collected before and after radiology procedures (x-rays, n = 28; CT scan, n = 13; catheterization/angiography, n = 8) without lead apron protection. Patients undergoing magnetic resonance imaging (MRI) were excluded. The primary outcome was glycemic variability by coefficient of variation (%CV) measured by CGM two hours pre- and post-imaging. Secondary outcome was the mean absolute relative difference (MARD) between matched capillary point-of-care (POC) and CGM glucose values pre- and post-imaging.
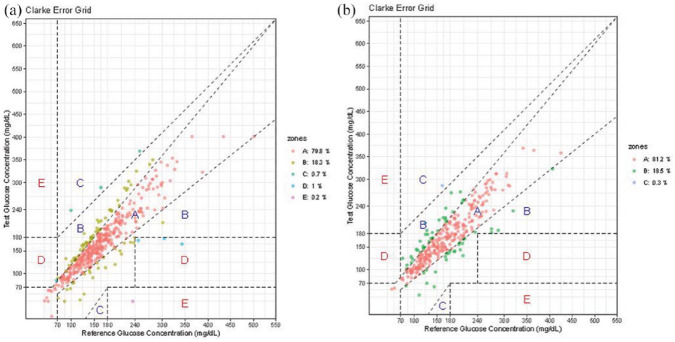
There was no significant difference in mean CGM blood glucose (BG) or %CV for all imaging procedures combined (delta mean BG: −7.7 ± 26.0, P = .051; %CV 18.0% pre vs 19.1% post, P = .65) or when stratified by x-ray only (delta mean BG: −8.8 ± 29.9, P = 0.15, %CV 20.1% pre vs 21.5% post, P = .60) vs CT scan/catheterization/angiography (delta mean BG: −6.3 ± 20.6, P = .19, %CV 15.2% pre vs 15.9% post, P = .97). The overall MARD was 13.3% pre-imaging and 12.7% post-imaging. The overall percentage of glucose values within ±15%/15 mg/dL, ±20%/20 mg/dL, and ±30%/30 mg/dL of the POC reference value was 69%, 80%, and 94%, respectively, pre-imaging, and 68%, 82%, and 93% post-imaging. Clarke Error Grid analysis showed 98.1% of glucoses falling into Zones A and B pre-imaging and 99.7% post-imaging (Figure 1).
Figure 1.
(a) Clark error grid pre-imaging and (b) Clark error grid post-imaging.
This is the first report to describe the effects of radiology imaging on CGM functionality. Our data indicate that ongoing usage during radiology procedures is safe and reliable without interference in CGM data transmission. Recommendations to remove devices prior to imaging may lead to increased resource utilization and health care costs, and findings here suggest that this may be unnecessary. The ability to continue CGM use while undergoing radiology studies may support broader use and acceptance of CGM in the inpatient setting. This may be particularly relevant to the medical community utilizing CGM technology during the COVID-2019 pandemic as the US Food and Drug Administration has allowed the inpatient use of CGM devices during the current public health care crisis.5 Importantly, MRI imaging was excluded from this analysis as it is likely that magnetic exposure will have differential effects on functionality of CGM technology compared to radiation exposure. Future studies should evaluate the effect of cumulative radiation exposure and location of imaging on impact of CGM accuracy and precision.
Footnotes
Disclaimer: The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: EKS has received unrestricted research support from Dexcom (to Baltimore VA Medical Center and to University of Maryland) for the conduction of clinical trials. FJP has received research support from Merck and Dexcom and consulting fees from Sanofi, Merck, Boehringer Ingelheim, Lilly, and AstraZeneca. RJG has received research grants from Novo Nordisk (to Emory University) and consulting fees from Abbott Diabetes, Sanofi, Novo Nordisk, and Valeritas. GEU has received unrestricted research support for inpatient studies (to Emory University) from Novo Nordisk, Sanofi, and Dexcom. No other potential conflicts of interest relevant to this article were reported.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dexcom (San Diego, CA, USA). This work was supported in part by the VA MERIT award (#1I01CX001825) from the United States (US) Department of Veterans Affairs Clinical Sciences Research and Development Service.
ORCID iDs: Alexandra L. Migdal  https://orcid.org/0000-0003-0058-8353
https://orcid.org/0000-0003-0058-8353
Elias K. Spanakis  https://orcid.org/0000-0002-9352-7172
https://orcid.org/0000-0002-9352-7172
Rodolfo J. Galindo  https://orcid.org/0000-0002-9295-3225
https://orcid.org/0000-0002-9295-3225
Francisco J. Pasquel  https://orcid.org/0000-0002-3845-6703
https://orcid.org/0000-0002-3845-6703
Saumeth Cardona  https://orcid.org/0000-0003-1295-1754
https://orcid.org/0000-0003-1295-1754
References
- 1. Miller KM, Hermann J, Foster N, et al. Longitudinal changes in continuous glucose monitoring use among individuals with type 1 diabetes: international comparison in the German and Austrian DPV and U.S. T1D exchange registries. Diabetes Care. 2020;43(1):e1-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dexcom. Safety information 2020. https://www.dexcom.com/safety-information. Accessed April 1, 2020.
- 3. Umpierrez GE, Klonoff DC. Diabetes technology update: use of insulin pumps and continuous glucose monitoring in the hospital. Diabetes Care. 2018;41(8):1579-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Umpierrez GE, Klonoff DC. Response to comment on Umpierrez and Klonoff. Diabetes technology update: use of insulin pumps and continuous glucose monitoring in the hospital. Diabetes Care. 2018;41:1579-1589. Diabetes Care. 2019;42(4):e66-e67. [DOI] [PubMed] [Google Scholar]
- 5. Pasquel FJ, Umpierrez GE. Individualizing inpatient diabetes management during the COVID-19 pandemic. J Diabetes Sci Technol. 2020;14(4):705-707. doi: 10.1177/1932296820923045. [DOI] [PMC free article] [PubMed] [Google Scholar]