Abstract
Physical activity is a keystone of a healthy lifestyle as well as of management of patients with type 1 diabetes. The risk of exercise-induced hypoglycemia, however, is a great challenge for these patients. The glycemic response to exercise depends upon several factors concerning the patient him/herself (eg, therapy, glycemic control, training level) and the characteristics of the exercise performed. Only in-depth knowledge of these factors will allow to develop individualized strategies minimizing the risk of hypoglycemia. The main factors affecting the exercise-induced hypoglycemia in patients with T1D have been analyzed, including the effects of insulin concentration. A model is discussed, which has the potential to become the basis for providing patients with individualized suggestions to keep constant glucose levels on each exercise occasion.
Keywords: carbohydrates, exercise, insulin, hypoglycemia, model, type 1 diabetes
Physical activity should be encouraged in people with type 1 diabetes (T1D),1-3 being a keystone in the management of the illness.4 Indeed, the effects of a healthy lifestyle are well documented,5-8 although the long-term effects are still debated.1,2,9-11
Individuals with T1D, however, have to face a number of exercise-related challenges, among which the risk of hypoglycemia is the strongest one.12-16 Exercise-induced hypoglycemia can be difficult to be identified as the symptoms mimic the feelings due to the exercise itself.17 As a result, a large percentage of patients do not reach the recommended guidelines for exercise,4,18 even so among adolescents.19,20
Personalized patient-oriented strategies should be developed to minimize the risk of exercise-induced hypoglycemia, not only for competitive athletes,2 but also for those who train essentially for well-being.21 To reach this ambitious goal, the main factors affecting the requirements for exercise in people with T1D on each exercise occasion have to be well known.
Effects of Insulin Concentration
In healthy people, blood glucose level is maintained within very narrow limits, insulin concentration being the main messenger that coordinates fuel storage into and fuel mobilization out of the various depots.22 In between meals (ie, in the “fasted” state), insulin concentration is low and the hepatocytes deliver glucose backward to the bloodstream, while the transporter GLUT1 allows for basal glucose uptake in skeletal muscles without the intervention of insulin.23 In the “fed” state, following the ingestion of carbohydrate containing foods, insulin levels are high, opening up peripheral cells for glucose removal to facilitate the return of glycemia to the norm. In skeletal muscles, insulin, as well as muscle contraction (with additive effects), stimulate the translocation to the plasma membrane of the transporter GLUT4.24,25 Glucose trapped within the muscles cannot leave them,23 the stored amount decreasing only if burned for energy production. Glucose enters also into the hepatocytes, through a transporter driven by the gradient between blood and intracellular concentration.23
In a general model of glucose level control, designed as a hydraulic system (Figure 1), insulin concentration is the main parameter managing the control valves governing the glucose fluxes among its depots. The latter are restricted to three main compartments (blood, liver, and muscles), each one with its own starting level, that can either increase or decrease (ie, ΔB, ΔL, ΔM, respectively). The ingestion of carbohydrate containing foods (Fi) increases the amount of glucose in blood, whereas the burning of carbohydrates for energy production (Fo) decreases the muscular stores. The overall equation describing the system is the following:
Figure 1.

Generic model of glucose level control. The main body compartments involved in the glucose fluxes are modeled as a hydraulic system, where insulin acts as main parameter managing control valves for the regulation of the correspondent flux. ΔL, ΔB, and ΔM represent the possible changes in the corresponding compartment starting level, Fi is the amount of glucose entering the system, whereas Fo is the amount of glucose leaving the system. See text for further details.
where Fi, ΔB, and Fo can be rather easily determined, whereas ΔL, ΔM, ΔG can be assessed only through complex and invasive measurements.
The mandatory use of exogenous insulin by people with T1D results in particular conditions compared to healthy individuals:
because of the peripherally delivered insulin, the physiological portal-peripheral insulin gradient, with threefold higher concentrations in the liver, is absent26
to maintain glycemia the most possible in the norm, supraphysiological insulin levels are usually observed in the periphery
insulin level cannot be modified physiologically according to the body needs, for example, as a consequence of exercise, being essentially determined by the last insulin administration in terms of type and dose injected and time elapsed
Insulin Concentrations and Exercise
During aerobic exercise glucose uptake of working muscles can increase up to 50 times. A prompt decrease in insulin concentration occurs in healthy subjects, thus facilitating the release of glucose from the liver to maintain a near normal glycemia.27-29 Independent from insulin signaling, the depletion of glycogen induces the translocation of glucose transporter to the cell membrane,30 so the decrease in circulating insulin does not restrict glucose provision. During predominantly anaerobic activities, insulin concentrations do not decrease so markedly, likely because of the shorter duration of the activity.18
Patients with T1D frequently exercise under supraphysiological insulin levels31 that result in a reduced glucose delivery by the liver because of the inhibitory effect of insulin on glycogen breakdown.32,33 The additive effects of insulin and of the exercise-induced increase in muscle glucose uptake,24,34,35 predispose to hypoglycemia.3 In addition, the high glucose flux into the muscles, due to the high insulin concentration, might induce a greater carbohydrate oxidation rate. Nevertheless, in patients exercising at different time distances from the prebreakfast insulin injection, no differences were observed in the carbohydrate oxidation rate.29,36 Moreover, despite patients showed higher insulin and blood glucose levels, their carbohydrate oxidation rates were not significantly different compared to healthy controls even during incremental37 or prolonged exercise.38 Linear relationships have been proposed to estimate the glucose oxidation rate during exercise (specifically for “active” and “sedentary” subjects) based on the percentage theoretical maximal heart rate.37 Data on healthy people and T1D patients were pooled together39 as well as those of both genders, which effects are still under debate.40
Patients might also exercise at relatively long time distances from the last insulin bolus, when insulin levels might be too low, thus resulting in hyperglycemia because of an excess glucose released from the liver,31,32 not taken up by the working muscles.
Finally, the increased glucose uptake by skeletal muscles for the replenishment of glycogen stores18,41 and the rise in insulin sensitivity after exercise42 maintain elevated the risk of hypoglycemia for several hours after exercise,41,43 with greatest risk during nighttime.
Current Guidelines for Exercise Management in T1D Patients
Individuals with T1D, either trained and untrained, typically require an extra carbohydrate intake and/or an insulin dose reduction before commencing aerobic exercise. Several factors affect the blood glucose responses to exercise in these patients12,18,44 and, consequently, different amounts of carbohydrates and/or insulin dose adjustments should be recommended.18,45 Nevertheless, the variability of the adopted protocols,44 together with the small number of recruited patients or the only observational studies,27 contribute in making it difficult to draw quantitative guidelines for all the possible conditions and, as a result, only rather qualitative guidelines are currently available.18,44
An increased ingestion of carbohydrates is the countermeasure preferred by patients;46 it can be applied also for unplanned exercise,47 thus remaining the mainstay of exercise management.48,49
A frequently suggested strategy is the adjustment of mealtime insulin bolus in anticipation of exercise,50 or of basal insulin infusion in pump therapy.51 A reduced insulin bolus, however, is useful when the exercise is performed early after the meal,49 and does not always exempt patients from extra carbohydrates.52 Moreover, the improved glucose profile is commonly obtained at the cost of hyperglycemia before the effort.53
Recently, the practice of anaerobic exercises has been recommended, since these activities are typically associated with lower declines (or even increases) in glucose levels.18,44,54 An exercise program based on high intensity mode, however, may not be easily administered to all patients10 and there is insufficient evidence of its real advantages.13
Several overlooked factors affect patient’s requirement for exercise. Indeed, an episode of hypoglycemia in the preceding hours, or a preceding exercise, increases the risk of hypoglycemia,55,56 possibly resulting in a vicious cycle with exercise.57 Despite insulin concentration during the effort is recognized as an important factor determining the carbohydrate requirements,27 current guidelines suggest to exercise only during precise narrow time frames58 or consider solely two main conditions, that is, the fasting and the postabsorptive states, respectively.18 Patient’s actual insulin concentration and/or insulin sensitivity at the time of the effort, as well as his or her physical conditioning, have never been included in the guidelines for exercise management so as to tune better the actual needs.
New Methods for Exercise Management in T1D Patients
The development of a tool able to deal with the main factors affecting patients’ need for exercise might overcome the general scarcity of knowledge around exercise management.18 To this aim, however, comprehensive models of insulin action and glucose uptake are needed.59
Closed-loop systems have the potential to ameliorate exercise management in patients with T1D,60 recent algorithms having reduced the exposure to exercise-induced hypoglycemia.61,62 The development of an “exercise smart” artificial pancreas, however, still remains a major hurdle,63 physical activity having been identified as one of the challenges.64,65
In the past years, Perkins and Riddell45 proposed standardized tables reporting recommended amounts of carbohydrates according to the patient’s body mass that included several different types of exercise, in some cases also with varying intensities. Other important factors, such as the patient’s insulin sensitivity, his or her state of physical conditioning, and the timing of the exercise relative to the last insulin bolus31 were, however, not given consideration.
Simplified decision trees have been proposed18 to help patients in determining the best countermeasure; again, however, they hardly include all the influencing factors, thus patients have still difficulty in facing the exercise.
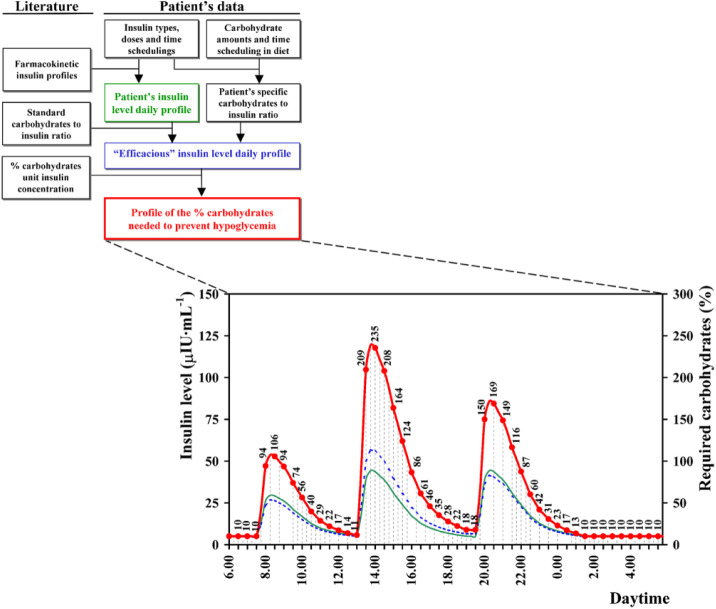
Current knowledge concerning the main physiological effects of insulin concentration has been implemented in an algorithm (called ECRES) recently developed to estimate the glucose supplement required by patients to maintain safe blood glucose levels on each exercise occasion.47 In summary, insulin level throughout the effort is first estimated on the basis of patient’s usual therapy data and on standard pharmacokinetic profiles of the used insulin analogues (Figure 2). The figure clearly shows that rather different insulin concentrations can be observed at the same time distance from a meal (eg, after breakfast or lunch), making too weak the recommendations distinguished only for the fasting and the postabsorptive states.18 The patient’s insulin sensitivity, inferred from his or her dietary carbohydrate-to-insulin ratio, is taken into account to establish the percentage carbohydrates of the overall amount of glucose burned during the exercise, which is linearly related to insulin concentration.47,66 The patient’s insulin sensitivity at the time of the exercise, with the same insulin concentration, affects the required percentage of burned carbohydrates (eg, as in the afternoon compared to the evening in Figure 2); none of the current solutions takes into account this factor. Finally, the carbohydrates supplement required to avoid the exercise induced hypoglycemia is obtained applying the above percentage to the overall amount of glucose burned during the exercise, estimated from patient- and exercise-specific data (ie, age, sex, body mass, training level, duration, and intensity of the exercise).
Figure 2.
Flow diagram of the part of the ECRES algorithm that models the total daily insulin profile (green line), the sensitivity-corrected “efficacious” daily insulin profile (blue line), and the profile of percentage carbohydrates needed to prevent hypoglycemia (red line and dots) (modified from Francescato and Carrato66). The profiles are estimated based on the patient’s individual therapy (ie, insulin types, doses, time scheduling and dietary carbohydrates).
The amount of carbohydrates estimated by the ECRES algorithm enabled 27 patients with T1D to maintain glycemia within the recommended range (ie, 3.9-10 mmol/L) in more than 70% of 1-hour supervised laboratory-based exercises, independent of the time elapsed (1:30, 3:00, and 4:30 hours) from the lunchtime insulin injection.47,66 Similar results were obtained for prolonged exercise (3 hours duration)67 or when patients participated to a stressful event (a 24 × 1-h relay marathon), where each patient ran at a different time throughout the day.46 Finally, a recent study showed that the ECRES algorithm, compared to Perkins’s tabular approach,45 suggested significantly lower amounts of extra carbohydrates for standardized physical activity (14.8 ± 12.0 g vs 23.4 ± 4.7 g, P < .05), while maintaining patients’ blood glucose within safe clinical limits.68
Results of these studies are promising and support the validity of approaches based on the main physiological effects of insulin concentration to obtain personalized systems for the management of exercise in patients with T1D. Future studies should investigate the possibility for further personalization and improvement of the estimation of the actual requirements by using patient’s own collected data (eg, activity tracker, glucose excursions, ingested glucose supplements) and machine learning techniques. Moreover, innovative algorithms for artificial pancreas are needed to enhance glycemic control during and following exercise,69 in particular for the pediatric population,70 likely based also on better estimates of patient’s insulin sensitivity.71,72 Indeed, having an exercise smart system may encourage persons with T1D to increase their physical activity.63
Conclusion
An optimal glucose control remains of paramount relevance in T1D, even during exercise. Clear-cut quantitative approaches to prevent exercise-induced hypoglycemia are needed to allow patients practice regular physical activity and safely enjoy all its beneficial aspects. This paper discussed the main factors affecting patient’s carbohydrate requirement for exercise, focusing in particular on the role of insulin concentration. The promising results obtained by application of a simplified model have been summarized, supporting the validity of personalized approaches. The possibility for further personalization and improvement, however, should be investigated, to reduce even more the risk of exercise-induced hypoglycemia—the most frequent and potentially harmful event during/after exercise.
Footnotes
Abbreviations: Fi, Fo, amounts of glucose entering/leaving the body according to the model; T1D, type 1 diabetes mellitus; ΔL, ΔB, ΔM, changes in the glucose depots of liver, blood, and muscle, respectively; ΔG, change in the rate of gluconeogenesis.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Maria Pia Francescato  https://orcid.org/0000-0002-7892-863X
https://orcid.org/0000-0002-7892-863X
References
- 1. Mozzillo E, Zito E, Maffeis C, et al. Unhealthy lifestyle habits and diabetes-specific health-related quality of life in youths with type 1 diabetes. Acta Diabetol. 2017;54:1073-1080. [DOI] [PubMed] [Google Scholar]
- 2. Horton WB, Subauste JS. Care of the athlete with type 1 diabetes mellitus: a clinical review. Int J Endocrinol Metab. 2016;14:e36091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Codella R, Terruzzi I, Luzi L. Why should people with type 1 diabetes exercise regularly? Acta Diabetol. 2017;54:615-630. [DOI] [PubMed] [Google Scholar]
- 4. Kennedy A, Narendran P, Andrews RC, Daley A, Greenfield SM. Attitudes and barriers to exercise in adults with a recent diagnosis of type 1 diabetes: a qualitative study of participants in the Exercise for Type 1 Diabetes (EXTOD) study. BMJ Open. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tikkanen-Dolenc H, Wadén J, Forsblom C, et al. Physical activity reduces risk of premature mortality in patients with type 1 diabetes with and without kidney disease. Diabetes Care. 2017;40:1727-1732. [DOI] [PubMed] [Google Scholar]
- 6. Bohn B, Herbst A, Pfeifer M, et al. Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: a cross-sectional multicenter study of 18,028 patients. Diabetes Care. 2015;38:1536-1543. [DOI] [PubMed] [Google Scholar]
- 7. Leroux C, Gingras V, Desjardins K, et al. In adult patients with type 1 diabetes healthy lifestyle associates with a better cardiometabolic profile. Nutr Metab Cardiovasc Dis. 2015;25:444-451. [DOI] [PubMed] [Google Scholar]
- 8. Chimen M, Kennedy A, Nirantharakumar K, Pang TT, Andrews R, Narendran P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia. 2012;55:542-551. [DOI] [PubMed] [Google Scholar]
- 9. Häyrinen M, Tarkka IM. Physical activity does not inevitably improve quality of life in young adults with type 1 diabetes. Diabetes Res Clin Pract. 2016;121:99-101. [DOI] [PubMed] [Google Scholar]
- 10. Codella R. Boiling factors in the pot of type 1 diabetes mellitus management: the role of exercise. Med Hypotheses. 2017;105:48. [DOI] [PubMed] [Google Scholar]
- 11. Tikkanen-Dolenc H, Wadén J, Forsblom C, et al. Frequent and intensive physical activity reduces risk of cardiovascular events in type 1 diabetes. Diabetologia. 2017;60:574-580. [DOI] [PubMed] [Google Scholar]
- 12. Yardley JE, Brockman NK, Bracken RM. Could age, sex and physical fitness affect blood glucose responses to exercise in type 1 diabetes? Front Endocrinol. 2018;9:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hasan S, Shaw SM, Gelling LH, Kerr CJ, Meads CA. Exercise modes and their association with hypoglycemia episodes in adults with type 1 diabetes mellitus: a systematic review. BMJ Open Diab Res Care. 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lascar N, Kennedy A, Hancock B, et al. Attitudes and barriers to exercise in adults with type 1 diabetes (T1DM) and how best to address them: a qualitative study. PLOS ONE. 2014;9:e108019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pinsker JE, Kraus A, Gianferante D, et al. Techniques for exercise preparation and management in adults with type 1 diabetes. Can J Diab. 2016;40:503-508. [DOI] [PubMed] [Google Scholar]
- 16. Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care. 2008;31:2108-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill NE, Campbell C, Buchanan P, Knight M, Godsland IF, Oliver NS. Biochemical, physiological and psychological changes during endurance exercise in people with type 1 diabetes. J Diabetes Sci Technol. 2017;11:529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riddell MC, Gallen IW, Smart CE, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;5:377-390. [DOI] [PubMed] [Google Scholar]
- 19. Kummer S, Stahl-Pehe A, Castillo K, et al. Health behaviour in children and adolescents with type 1 diabetes compared to a representative reference population. PLOS ONE. 2014;9:e112083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Lima VA, Mascarenhas LPG, Decimo JP, et al. Physical activity levels of adolescents with type 1 diabetes physical activity in T1D. Pediatr Exerc Sci. 2017;29:213-219. [DOI] [PubMed] [Google Scholar]
- 21. Lasorsa I, D’Antrassi P, Ajčević M, et al. Personalized support for chronic conditions: a novel approach for enhancing self-management and improving lifestyle. Appl Clin Inform. 2016;7:633-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cahill GF. Physiology of insulin in man: the Banting Memorial Lecture 1971. Diabetes. 1971;20:785-799. [DOI] [PubMed] [Google Scholar]
- 23. Adeva-Andany MM, González-Lucán M, Donapetry-García C, Fernández-Fernández C, Ameneiros-Rodríguez E. Glycogen metabolism in humans. BBA Clin. 2016;5:85-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coderre L, Kandror KV, Vallega G, Pilch PF. Identification and characterization of an exercise-sensitive pool of glucose transporters in skeletal muscle. J Biol Chem. 1995;270:27584-27588. [DOI] [PubMed] [Google Scholar]
- 25. Jensen J, Rustad PI, Kolnes AJ, Lai Y-C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol. 2011;2:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Canavan JP, Flecknell PA, New JP, Alberti KGMM, Home PD. The effect of portal and peripheral insulin delivery on carbohydrate and lipid metabolism in a miniature pig model of human IDDM. Diabetologia. 1997;40:1125-1134. [DOI] [PubMed] [Google Scholar]
- 27. García-García F, Kumareswaran K, Hovorka R, Hernando ME. Quantifying the acute changes in glucose with exercise in type 1 diabetes: a systematic review and meta-analysis. Sports Med. 2015;45:587-599. [DOI] [PubMed] [Google Scholar]
- 28. Camacho RC, Galassetti P, Davis SN, Wasserman DH. Glucoregulation during and after exercise in health and insulin-dependent diabetes. Exerc Sport Sci Rev. 2005;33:17-23. [PubMed] [Google Scholar]
- 29. Francescato MP, Geat M, Fusi S, Stupar G, Noacco C, Cattin L. Carbohydrate requirement and insulin concentration during moderate exercise in type 1 diabetic patients. Metabolism. 2004;53:1126-1130. [DOI] [PubMed] [Google Scholar]
- 30. Ploug T, Galbo H, Richter EA. Increased muscle glucose uptake during contractions: no need for insulin. Am J Physiol Endocrinol Metab. 1984;247:E726-E731. [DOI] [PubMed] [Google Scholar]
- 31. Riddell MC, Perkins BA. Type 1 diabetes and vigorous exercise: applications of exercise physiology to patient management. Can J Diab. 2006;30:63-71. [Google Scholar]
- 32. Wallberg-Henriksson H. Acute exercise: fuel homeostasis and glucose transport in insulin-dependent diabetes mellitus. Med Sci Sports Exerc. 1989;21:356-361. [PubMed] [Google Scholar]
- 33. Brugnara L, Vinaixa M, Murillo S, et al. Metabolomics approach for analyzing the effects of exercise in subjects with type 1 diabetes mellitus. PLOS ONE. 2012;7:e40600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci USA. 1995;92:5817-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lai Y-C, Zarrinpashneh E, Jensen J. Additive effect of contraction and insulin on glucose uptake and glycogen synthase in muscle with different glycogen contents. J Appl Physiol. 2010;108:1106-1115. [DOI] [PubMed] [Google Scholar]
- 36. Chokkalingam K, Tsintzas K, Snaar JEM, et al. Hyperinsulinaemia during exercise does not suppress hepatic glycogen concentrations in patients with type 1 diabetes: a magnetic resonance spectroscopy study. Diabetologia. 2007;50:1921-1929. [DOI] [PubMed] [Google Scholar]
- 37. Francescato MP, Cattin L, Geat M, Noacco C, di Prampero PE. Glucose pulse: a simple method to estimate the amount of glucose oxidized during exercise in type 1 diabetic patients. Diabetes Care. 2005;28:2028-2030. [DOI] [PubMed] [Google Scholar]
- 38. Geat M, Stel G, Poser S, Driussi C, Stenner E, Francescato MP. Whole-body glucose oxidation rate during prolonged exercise in type 1 diabetic patients under usual life conditions. Metabolism. 2013;62:836-844. [DOI] [PubMed] [Google Scholar]
- 39. Francescato MP, Zanier M, Gaggioli F. Prediction of glucose oxidation rate during exercise. Int J Sports Med. 2008;29:706-712. [DOI] [PubMed] [Google Scholar]
- 40. Brockman NK, Yardley JE. Sex-related differences in fuel utilization and hormonal response to exercise: implications for individuals with type 1 diabetes. Appl Physiol Nutr Metab. 2018;43:541-552. [DOI] [PubMed] [Google Scholar]
- 41. McMahon SK, Ferreira LD, Ratnam N, et al. Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J Clin Endocr Metab. 2007;92:963-968. [DOI] [PubMed] [Google Scholar]
- 42. Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. Int J Sports Med. 2000;21:1-12. [DOI] [PubMed] [Google Scholar]
- 43. Davey RJ, Howe W, Paramalingam N, et al. The effect of midday moderate-intensity exercise on postexercise hypoglycemia risk in individuals with type 1 diabetes. J Clin Endocr Metab. 2013;98:2908-2914. [DOI] [PubMed] [Google Scholar]
- 44. Farinha JB, Krause M, Rodrigues-Krause J, Reischak-Oliveira A. Exercise for type 1 diabetes mellitus management: general considerations and new directions. Med Hypotheses. 2017;104:147-153. [DOI] [PubMed] [Google Scholar]
- 45. Perkins BA, Riddell MC. Type 1 diabetes and exercise: using the insulin pump to maximum advantage. Can J Diab. 2006;30:72-79. [Google Scholar]
- 46. Buoite Stella A, Assaloni R, Tonutti L, et al. Strategies used by patients with type 1 diabetes to avoid hypoglycaemia in a 24x1-h marathon. Comparison with the amounts of carbohydrates estimated by a customisable algorithm. Can J Diab. 2016;41:184-189. [DOI] [PubMed] [Google Scholar]
- 47. Francescato MP, Geat M, Accardo A, Blokar M, Cattin L, Noacco C. Exercise and glycemic imbalances: a situation-specific estimate of glucose supplement. Med Sci Sports Exerc. 2011;43:2-11. [DOI] [PubMed] [Google Scholar]
- 48. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gallen I. Hypoglycemia associated with exercise in people with type 1 diabetes. Diabet Hypoglycemia. 2014;7:3-10. [Google Scholar]
- 50. Chiang JL, Kirkman MS, Laffel LMB, Peters AL. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37:2034-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Franc S, Daoudi A, Pochat A, et al. Insulin-based strategies to prevent hypoglycaemia during and after exercise in adult patients with type 1 diabetes on pump therapy: the DIABRASPORT randomized study. Diabetes Obes Metab. 2015;17:1150-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. West DJ, Stephens JW, Bain SC, et al. A combined insulin reduction and carbohydrate feeding strategy 30 min before running best preserves blood glucose concentration after exercise through improved fuel oxidation in type 1 diabetes mellitus. J Sports Sci. 2011;29:279-289. [DOI] [PubMed] [Google Scholar]
- 53. Campbell MD, Walker M, Trenell M, et al. Metabolic implications when employing heavy pre- and post-exercise rapid-acting insulin reductions to prevent hypoglycaemia in type 1 diabetes patients: a randomised clinical trial. PLOS ONE. 2014;9:e97143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yardley JE, Sigal RJ. Exercise strategies for hypoglycemia prevention in individuals with type 1 diabetes. Diabetes Spectr. 2015;28:32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Galassetti P, Mann S, Tate D, Neill RA, Wasserman DH, Davis SN. Effect of morning exercise on counterregulatory responses to subsequent, afternoon exercise. J Appl Physiol. 2001;91:91-99. [DOI] [PubMed] [Google Scholar]
- 56. Galassetti P, Tate D, Neill RA, Richardson A, Leu SY, Davis SN. Effect of differing antecedent hypoglycemia on counterregulatory responses to exercise in type 1 diabetes. Am J Physiol Endocrinol Metab. 2006;290:E1109-E1117. [DOI] [PubMed] [Google Scholar]
- 57. Ertl AC, Davis SN. Evidence for a vicious cycle of exercise and hypoglycemia in type 1 diabetes mellitus. Diabetes Metab Res Rev. 2004;20:124-130. [DOI] [PubMed] [Google Scholar]
- 58. Chacko E. Preventing exercise-induced hypoglycaemia in insulin-dependent diabetes. Diabetologia. 2016;59:2487-2488. [DOI] [PubMed] [Google Scholar]
- 59. Kudva YC, Carter RE, Cobelli C, Basu R, Basu A. Closed-loop artificial pancreas systems: physiological input to enhance next-generation devices. Diabetes Care. 2014;37:1184-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bally L, Thabit H. Closing the loop on exercise in type 1 diabetes. Curr Diabetes Rev. 2018;14:257-265. [DOI] [PubMed] [Google Scholar]
- 61. Huyett LM, Ly TT, Forlenza GP, et al. Outpatient closed-loop control with unannounced moderate exercise in adolescents using zone model predictive control. Diabetes Technol Ther. 2017;19:331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Breton MD, Cherñavvsky DR, Forlenza GP, et al. Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care. 2017;40:1644-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Riddell MC, Zaharieva DP, Yavelberg L, Cinar A, Jamnik VK. Exercise and the development of the artificial pancreas: one of the more difficult series of hurdles. J Diabetes Sci Technol. 2015;9:1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jayawardene D, McAuley S, Horsburgh J, et al. Closed-loop insulin delivery for adults with type 1 diabetes undertaking high-intensity interval exercise versus moderate-intensity exercise: a randomized, crossover study. Diabetes Technol Ther. 2017;19:340-348. [DOI] [PubMed] [Google Scholar]
- 65. van Bon AC, Verbitskiy E, von Basum G, Hoekstra JBL, DeVries JH. Exercise in closed-loop control: a major hurdle. J Diabetes Sci Technol. 2011;5:1337-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Francescato MP, Carrato S. Management of exercise-induced glycemic imbalances in type 1 diabetes. Curr Diabetes Rev. 2011;7:253-263. [DOI] [PubMed] [Google Scholar]
- 67. Francescato MP, Stel G, Stenner E, Geat M. Prolonged exercise in type 1 diabetes: performance of a customizable algorithm to estimate the carbohydrate supplements to minimize glycemic imbalances. PLOS ONE. 2015;10:e0125220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ajcevic M, Francescato MP, Geat M, Accardo A. Comparison of ECRES algorithm with classical method in management of diabetes type 1 exercise-related imbalances. IFMBE Proceedings. 2019;38:803-806. [Google Scholar]
- 69. Colberg SR, Laan R, Dassau E, Kerr D. Physical activity and type 1 diabetes: time for a rewire? J Diabetes Sci Technol. 2015;9:609-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Esposito S, Santi E, Mancini G, et al. Efficacy and safety of the artificial pancreas in the paediatric population with type 1 diabetes. J Transl Med. 2018;16:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Toffanin C, Zisser H, Doyle FJ, III, Dassau E. Dynamic insulin on board: incorporation of circadian insulin sensitivity variation. J Diabetes Sci Technol. 2013;7:928-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schiavon M, Dalla Man C, Cobelli C. Insulin sensitivity index-based optimization of insulin to carbohydrate ratio: in silico study shows efficacious protection against hypoglycemic events caused by suboptimal therapy. Diabetes Technol Ther. 2018;20:98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]