Abstract
Background:
Continuous glucose monitoring (CGM) is a powerful tool to be considered both in clinical practice and clinical trials. However, CGM has been criticized for being inaccurate for many reasons including a physiological delay. This study sought to investigate the current delay issue and propose a simple post-processing procedure.
Method:
More than a million hours of the Dexcom G4 CGM from 472 subjects investigated in a state-of-the-art clinical trial were analyzed by time shifting the CGM measurements and comparing them to plasma glucose (PG) measurements. The resultant CGM measurements were then assessed in relation to real-world clinical research endpoints.
Results:
A CGM time shift of −9 minutes was optimal and reduced mean absolute relative difference (MARD) statistically significantly with 1.0% point. The MARD reduction resulted in better clinical research endpoints of hypoglycemia and postprandial glucose increments.
Conclusions:
The delay in CGM is still an issue. The delay in this study was identified to be 9 minutes compared to PG. With a simple post-processing approach of time shifting the CGM measurements with −9 minutes, it was possible to obtain a statistically significantly lower MARD and subsequently obtain clinical research endpoints of improved validity.
Keywords: diabetes, continuous glucose monitoring, delay, endpoints, hypoglycemia, clinical trials
Continuous glucose monitoring (CGM) has for many years been a promising and utilized tool for both people with diabetes mellitus (DM) and health care physicians responsible for the diabetes treatment.1-3 Simultaneously, a recurrent topic has been the accuracy of the devices.4,5 The preferred site for glucose sensing continuous to be the subcutaneous interstitial fluid, but changes in this glucose concentration will always be delayed with respect to changes in plasma glucose (PG) causing a so-called physiological delay.6,7 Also, a device-related filter routine delay is typically observed, and the sensors based on glucose oxidase often have a nonspecific offset current.8,9 The resulting inaccuracy has led to criticism and questioning of the fundamental assumption that interstitial fluid glucose and PG can be made identical.10 On the other hand, many experts and studies have concluded that CGM devices can be deemed acceptable, and that the inaccuracy was related to older devices.4,5,11 In the beginning of the third millennium, mean absolute relative difference (MARD) of CGM devices was reported to be around 20% with delays exceeding 10-15 minutes,8,12,13 but with the advent of new algorithms for updated noise reduction and modified calibration, devices have improved a lot with MARD going toward 10% and delays of less than 10 minutes.6,7,14 These improvements have paved the way for a more positive attitude toward CGM devices.4
In February 2017, an international panel of experts in CGM technology at the Advanced Technologies & Treatments for Diabetes Congress reached consensus about the use of CGM.2 Not only was the conclusion that the advanced CGM metrics should be considered as parameters that complement HbA1c in clinical practice, they should also be recognized by governing bodies as valuable and meaningful in clinical trials of new drugs and devices for diabetes treatment. Moreover, hypoglycemia was mentioned as an important endpoint in clinical trials. To correctly detect episodes of hypoglycemia, the CGM accuracy is very important, and especially during conditions with fast declining PG resulting in iatrogenic hypoglycemia, low detection rates have been observed.15,16 This is unfortunate when hypoglycemia endpoints have high impact in clinical trials. Another important research endpoint in clinical trials investigating fast-acting insulins is the postprandial glucose levels.17 The research endpoints will typically be calculated based on the first 0.5-1 hour and then a delay of around 10 minutes can be particular important. Device-related inaccuracies, such as, the filter routine delay and offset current might be possible to reduce, but the fundamental physiological delay, when using the interstitial fluid measuring site, cannot be removed.
This study sought to investigate (1) the delay issue of Dexcom G4 CGM measurements compared to PG in a clinical trial investigating the ultra-fast acting insulin Fiasp® and (2) how simple post-processing can be utilized to improve the associated clinical research endpoints.
Methods
Data Material
Data from a recent clinical trial conducted by Novo Nordisk A/S, to investigate the efficacy and safety of continuous subcutaneous insulin infusion of Fiasp® compared to NovoRapid® in 472 people with type 1 diabetes (T1D), were available for analysis. During three periods of 14 days, the subjects wore the CGM device Dexcom® G4 Platinum (DG4P, Dexcom, San Diego, CA, USA). They were instructed to calibrate the device according to manufacturer’s instructions. During two of the periods, the subjects underwent a standardized liquid meal test where six blood samples (–2, 30, 60, 120, 180 and 240 minutes after start) were drawn an analyzed by a central laboratory. Furthermore, they were instructed to perform 4 self-measured blood glucose (SMBG) measurements per day throughout the conduct of the trial and so-called 7-7-9 SMBG profiles (three consecutive days where SMBG was performed 7, 7, and 9 times, respectively) three times during the trial. Information about treatment was not used in this study.
Time Shifting CGM Measurements
Due to the physiological delay between interstitial glucose and PG, a negative time shift of the CGM measurements was proposed as an ultra-simple and quick solution to optimize the clinical research endpoints derived from the CGM measurements. The CGM measurements was time shifted for all subjects from −30 to 10 minutes by each minute to find the optimal time shift. The CGM measurements were then compared to the PG laboratory values and to SMBG measurements by the mean absolute difference (MAD).
Statistical Analyses
Subject characteristics are presented with means and standard deviations or percentages. Amount of glucose readings are presented as durations or counts. Number of hypoglycemic episodes are presented as the number of PG readings below or equal to 70 mg/dL. Symptomatic PG unconfirmed hypoglycemic episodes are not included in the number.
To find the most optimal time shift of the CGM measurements, MAD was calculated as the absolute difference between each CGM and PG measurement and between each CGM and SMBG measurement, which was then averaged by subject. The CGM measurements were interpolated linearly to get pairs of CGM and PG measurements and pairs of CGM and SMBG measurements. An interpolation was only carried out if the distance between the two adjacent CGM measurements were 5 minutes or less. The presented MAD was then the grand mean of the MADs calculated per subject. The procedure was done similarly for MARD with the addition that the absolute CGM-PG difference was divided by the PG value and the absolute CGM-SMBG difference was divided by SMBG value. To test the difference between MARD before and after time shifting, a paired t-test was performed.
Two typical clinical research endpoints are presented before and after time shifting the CGM measurements: (1) number of hypoglycemic episodes and (2) postprandial glucose increments (after meal tests).17 For hypoglycemia, the sensitivity and specificity of the CGM device’ ability to detect Level 1 (PG ≤ 70 mg/dL) and Level 2 (PG < 54 mg/dL) hypoglycemic episodes18 are presented. MAD between CGM and PG of postprandial glucose increments following the meal test is presented at the four postprandial timepoints for blood samples, 30, 60, 120, and 240 minutes.
The descriptive and inferential analyses were conducted in R version 2.15.2. The significance level was set at a P value of less than .05 for two-sided testing.
Results
Subject characteristics and glucose data are shown in Table 1. Slightly more males were enrolled and the subjects had an average age of 44 and were on average preobese as defined by WHO.19
Table 1.
Subject Characteristics at Randomization and Amount of Glucose Data Available.
| Variable | |
|---|---|
| n | 472 |
| Age, mean ± SD | 44 ± 15 |
| Sex (%) | |
| Female | 57 |
| Male | 43 |
| Body mass index at baseline (kg/m2), mean ± SD | 26 ± 4 |
| HbA1c at baseline (%), mean ± SD | 7.5 ± 0.5 |
| Diabetes duration, mean ± SD | 24 ± 12 |
| CGM duration (hours) | 1 005 425 |
| Number of PG measurementsa | 13 059 |
| Number of SMBG measurements | 38 182 |
| Number of hypoglycemic episodesb | 561 |
Blood samples analyzed by a central laboratory. bCount of PG measurements below or equal to 70 mg/dL.
In Figure 1, MAD between CGM and PG/SMBG measurements is shown as a function of time shift of CGM measurements. As can be observed there is a nadir at −9/−10 minutes, and the drop in MAD is most pronounced for SMBG.
Figure 1.
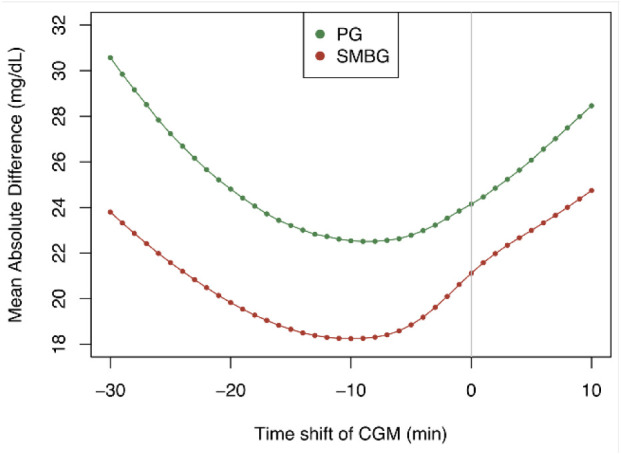
Mean absolute difference between CGM and PG and CGM and SMBG as a function time shifted CGM measurements. The nadir is at −9 minutes for PG and −10 minutes for SMBG.
Based on the above investigation of the time shift of CGM leading to lowest MAD, MARD for CGM and PG is shown in Table 2 where CGMshift denotes CGM measurements shifted −9 minutes and -10 minutes. As is evident from the table, shifting the CGM measurements leads to a statistically significant 1.0%-points lower MARD with PG as reference and 1.8%-point lower MARD with SMBG as reference.
Table 2.
Change in Mean Absolute Relative Difference (MARD) After Shift of the CGM Measurements.
| CGM | CGMshift | P * | |
|---|---|---|---|
| MARDa (%), mean ± SD | 13.7 ± 7.2 | 12.7 ± 7.5 | <.0001 |
| MARDb (%), mean ± SD | 13.1 ± 5.5 | 11.3 ± 5.8 | <.0001 |
The Reference to CGM is PG and SMBG, Respectively. aPG is reference and CGM is shifted −9 minutes. bSMBG is reference and CGM is shifted −10 minutes. *Test was applied on MARDs per subject.
Clinical research endpoints for CGM and CGMshift with PG as reference are shown in Table 3. For all endpoints, time shifting the CGM measurements leads to an improvement. The specificity of Level 2 hypoglycemia is unchanged to first decimal point though. The improvement is in terms of a higher sensitivity and specificity in the hypoglycemic endpoints. The sensitivity of Level 2 hypoglycemia is increased with more than 11%-points. For the postprandial glucose endpoints, the improvement is in terms of reductions in MAD. The improvement is most pronounced 30 minutes after the meal intake.
Table 3.
Changes in Clinical Research Endpoints After −9 minutes Shift of the CGM Measurements.
| Research endpoint | CGM | CGMshift | Changeb |
|---|---|---|---|
| Level 1 hypoglycemia (PG ≤ 70 mg/dL) | |||
| Sensitivity | 35.7% | 41.6% | 5.9% c |
| Specificity | 97.5% | 98.1% | 0.6% c |
| Level 2 hypoglycemia (PG < 54 mg/dL) | |||
| Sensitivity | 44.8% | 56.2% | 11.4% c |
| Specificity | 99.4% | 99.4% | +0.0% c |
| Postprandial glucose (mg/dL) | |||
| 30 min MADa | 27.9 | 23.0 | –4.9 |
| 60 min MADa | 30.8 | 27.6 | –3.2 |
| 120 min MADa | 32.3 | 29.2 | –3.1 |
| 180 min MADa | 26.7 | 23.8 | –2.9 |
The Reference to CGM is the PG Measurements from Blood Samples. aMean absolute difference between CGM and PG postprandial glucose increments. Notice that the assumption is that the PG value and timing is the truth. bAll changes marked with bold are improvements. C%-point change.
Discussion
From Figure 1 it is evident that a delay of CGM measurements of 9-10 minutes compared to PG still exist for a newer CGM device used in a state-of-the-art clinical trial. From a direct measurement using a microdialysis catheter in the abdominal subcutaneous space, the physiological delay is, according to Basu et al,20 7-8 minutes in fasting patients with T1D. With the 9-10 minutes delay found in this study, the filter-routine delay is therefore no more than 1-3 minutes in this study of the Dexcom G4 CGM. The 9-10 minutes delay is in line with a mean delay of 9.5 minutes of the raw signal from a CGM sensor compared to SMBG reported by Schmelzeisen-Redeker et al.6 In another study by Sinha et al,7 the delay of a DG4P sensor was found to be 5.6 minutes. However, the population was healthy subjects, and due to the rapid changes in PG seen in people with diabetes, it is anticipated that a larger delay will be seen in people with diabetes compared to people without. In a study by Kuroda et al,21 the delay during a hyperglycemic clamp was investigated. They showed that the delay of CGM measurements to reach maximum glucose value was 27 minutes longer compared to blood glucose measurements, which indicates that during rapid glucose changes, the delay of CGM becomes larger, and at some point, the delay causes CGM to completely fail to resemble blood glucose. The bad performance during rapid changes is confirmed by Pleus et al.16 This property is especially important to keep in mind for clinical trials investigating bolus insulins where rapid blood glucose changes are often seen. The delay of −9 minutes found in this study will not be the same for other devices, which mean that different delays should be identified and used for post-processing of CGM measurements from other devices.
The observed MARD of approximately 14% with PG as reference in this study is similar to studies investigating MARD of the DG4P.14,16 However, during rapid PG changes (<−3 mg/dL/min and >3 mg/dL/min) Pleus et al16 observed an increase of MARD to 25%, and the performance of the CGM sensor in this study is thus deemed good. Nevertheless, it was possible to obtain a 1.0%-point decrease in MARD, simply by shifting the CGM measurements −9 minutes post hoc. The effect of this time shift on the clinical research endpoints is significant with a more than 11%-point increase in the CGM detection of Level 2 hypoglycemia. The time shift did not only improve research endpoints on glucose excursions, but also improved postprandial glucose estimation for all blood sample time points. Better results might have been obtained with a personalized time shift of the CGM measurements as proposed by Schmelzeisen-Redeker et al,6 but to keep simplicity as focal point, we choose to suggest a general time shift approach.
A limitation of this study is that the CGM measurements are being post-processed to mimic blood glucose values. One could argue that interstitial glucose values themselves could have a stronger association to clinical outcomes, for example, late-diabetic complications. However, more evidence about such associations is needed. Another limitation is that measures of exercise were not obtained in the Onset® 5 trial. Exercise and movement have been shown to affect the performance of the CGM devices.22 However, since the participants wore the CGM devices at home, it is expected that the devices have been exposed to exercise in different everyday situations.
We acknowledge CGM as a tool for assessing glucose variability and for detection of glucose excursions where SMBG is inappropriate, for example, to detect nocturnal hypoglycemia. However, with the current low detection rate of hypoglycemia (sensitivity<50%, assuming that PG is the truth, value- and time-wise) and poor estimates of postprandial glucose increments, we recommend to use and interpret these research endpoints in clinical trials with care.
Conclusion
The delay in CGM is still an issue in a state-of-the-art clinical trial investigating an ultra-fast-acting bolus insulin in 472 subjects with T1D. The delay in this study was identified to be 9 minutes compared to PG. With a simple post-processing approach of time shifting the CGM measurements with −9 minutes, it was possible to obtain a statistically significantly lower MARD and subsequently obtain clinical research endpoints of improved validity.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; DM, diabetes mellitus; DG4P, Dexcom G4 Platinum; MAD, mean absolute difference; MARD, mean absolute relative difference; PG, plasma glucose; SMBG, self-measured blood glucose; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CD is employed at Novo Nordisk A/S. OH is member of Committee for Education, Danish Diabetes Academy funded by Novo Nordisk Foundation. PV has travel grants and unrestricted grants from MSD, Amgen, Eli Lilly, Norvartis, Servier. PV has research collaboration with MSD and Kyowa Kirin.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Morten Hasselstrøm Jensen  https://orcid.org/0000-0002-6649-8644
https://orcid.org/0000-0002-6649-8644
References
- 1. Rodbard D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther. 2017;19(suppl 3):S25-S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391:1367-1377. [DOI] [PubMed] [Google Scholar]
- 4. Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol. 2015;9:209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petrie JR, Peters AL, Bergenstal RM, Holl RW, Fleming GA, Heinemann L. Improving the clinical value and utility of CGM systems: issues and recommendations a joint statement of the European association for the study of diabetes and the American Diabetes Association diabetes technology working group. Diabetes Care. 2017;40:1614-1622. [DOI] [PubMed] [Google Scholar]
- 6. Schmelzeisen-Redeker G, Schoemaker M, Kirchsteiger H, Freckmann G, Heinemann L, Del Re L. Time delay of CGM sensors: relevance, causes, and countermeasures. J. Diabetes Sci Technol. 2015;9:1006-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinha M, McKeon KM, Parker S, et al. A comparison of time delay in three continuous glucose monitors for adolescents and adults. J Diabetes Sci Technol. 2017;11:1132-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rebrin K, Sheppard NF, Steil GM. Use of subcutaneous interstitial fluid glucose to estimate blood glucose: revisiting delay and sensor offset. J Diabetes Sci Technol. 2010;4:1087-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heinemann L, Stuhr A. Self-measurement of blood glucose and continuous glucose monitoring: is there only one future? Eur Endocrinol. 2018;14:24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mazze RS, Strock E, Borgman S, Wesley D, Stout P, Racchini J. Evaluating the accuracy, reliability, and clinical applicability of continuous glucose monitoring (CGM): is CGM ready for real time? Diabetes Technol Ther. 2009;11: 11-18. [DOI] [PubMed] [Google Scholar]
- 11. Christiansen MP, Garg SK, Brazg R, et al. Accuracy of a fourth-generation subcutaneous continuous glucose sensor. Diabetes Technol Ther. 2017;19:446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The Diabetes Research in Children Network (DirecNet) Study Group. The accuracy of the GlucoWatch G2 biographer in children with type 1 diabetes: results of the Diabetes Research in Children Network (DirecNet) accuracy study. Diabetes Technol Ther. 2003;5:791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. FDA. Summary of safety and effectiveness data. CDRH Document. 2006;8-27. [Google Scholar]
- 14. Boscari F, Galasso S, Facchinetti A, et al. FreeStyle Libre and Dexcom G4 Platinum sensors: accuracy comparisons during two weeks of home use and use during experimentally induced glucose excursions. Nutr Metab Cardiovasc Dis. 2018;28:180-186. [DOI] [PubMed] [Google Scholar]
- 15. Jensen MH, Christensen TF, Tarnow L, Mahmoudi Z, Johansen MD, Hejlesen OK. Professional continuous glucose monitoring in subjects with type 1 diabetes: retrospective hypoglycemia detection. J Diabetes Sci Technol. 2013;7:135-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pleus S, Schoemaker M, Morgenstern K, et al. Rate-of-change dependence of the performance of two CGM systems during induced glucose swings. J Diabetes Sci Technol. 2015;9:801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Russell-Jones D, Bode BW, De Block C, et al. Fast-acting insulin aspart improves glycemic control in basal-bolus treatment for type 1 diabetes: results of a 26-week multicenter, active-controlled, treat-to-target, randomized, parallel-group trial (onset 1). Diabetes Care. 2017;40:943-950. [DOI] [PubMed] [Google Scholar]
- 18. International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/l (54 mg/dl) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2017;40:155-157. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization. Body mass index, 2019. Available at: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
- 20. Basu A, Dube S, Slama M, et al. Time lag of glucose from intravascular to interstitial compartment in type 1 diabetes. J Diabetes Sci Technol. 2015;9:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuroda A, Taniguchi S, Akehi Y, et al. Accuracy and time delay of glucose measurements of continuous glucose monitoring and bedside artificial pancreas during hyperglycemic and euglycemic hyperinsulinemic glucose clamp study. J Diabetes Sci Technol. 2017;11:1096-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Biagi L, Bertachi A, Quirós C, et al. Accuracy of continuous glucose monitoring before, during, and after aerobic and anaerobic exercise in patients with type 1 diabetes mellitus. Biosensors. 2018;8:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


