Abstract
As a biomaterial, silk presents unique features with a combination of excellent mechanical properties, biocompatibility and biodegradability. The biodegradability aspects of silk biomaterials, especially with options to control the rate from short (days) to long (years) time frames in vivo, make this protein-based biopolymer a good candidate for developing biodegradable devices used for tissue repairs and tissue engineering, as well as medical device implants. Silk materials, including native silk fibers and a broad spectrum of regenerated silk materials have been investigated in vitro and in vivo to demonstrate degradation by proteolytic enzymes. In this mini review, we summarize the findings on these studies on the enzymatic degradation of Bombyx mori (B. mori) silk materials. We also present a discussion on the factors that dictate the degradation properties of silk materials. Finally, in future perspectives, we highlight some key challenges and potential directions toward the future study of the degradation of silk materials.
Graphical Abstract

1. Introduction
The combination of excellent mechanical properties, biocompatibility and biodegradability makes silk different than other biomaterials.1, 2 Silk is a protein-based biopolymer where amino acids serve as fundamental building blocks (e.g., monomers) to determine the functions of the protein through the specific primary sequence, diverse secondary structures, and higher-order structures (structural hierarchy).3, 4 The unique repetitive sequences found in silk proteins allows the formation of a variety of secondary and tertiary structures, and these structures are further able to form into semi-crystalline structures via self-assembly, leading to outstanding mechanical properties, such as high toughness and strength depending on the specific silk type.3, 4 In addition, as a protein, silk is biocompatible and degrades in vitro and in vivo in response to proteolytic enzymes, which makes it a good biomaterial option for developing medical devices.1, 5,6–11
One well-known silk-based medical device is the silk suture, commercialized for decades. Early silk sutures were made from native silkworm silk fibers, but these formulations do not show significant degradation over relatively short time periods (~60 days). Thus, these traditional silk sutures widely used throughout the world are defined by the United States Pharmacopeia as a non-degradable biomaterial due to the negligible loss in tensile strength in vivo over time.1 This lack of proteolytic digestion is due to the use of waxes and other treatments for the sutures, which negate access by the enzymes to the proteins to initiate the digestion process. In recent years, with an improved understanding of the fundamental structures and properties of silk, along with options to improve the purification of the native fiber structural core (fibroin) without residual contaminating proteins (e.g., sericin), degradable silk biomaterials have been generated which are also biocompatible. In particular, the degradation properties, as well as advanced processing techniques have been elucidated for silk to support the engineering of new forms of silk materials. These new forms include regenerated silk materials to achieve controllable or tunable properties (e.g., mechanics, degradation rates).3, 11, 12 Some engineered silk materials have higher rates of biodegradability than raw silk fibers in vitro and in vivo, while others are slower.5, 13–15 In this mini-review, we summarize studies on enzymatic degradation of silk materials and discuss the factors influencing this process, with an aim to stimulate more fundamental research on the topic. Since silk fibers produced by the domestic silkworm (Bombyx mori) are the abundant and well-studied raw material available world-wide for biomedical applications, while also providing a proven clinical track record in suture format, this source of silk is the focus of this review and “silk materials” refers to B. mori silk materials in this review exclusively. Other silks (e.g., other robust structural silks, such as other silkworm silks, spider silks involved in orb web construction, some bioengineered silks) would generally follow similar themes as discussed herein for silkworm silk fibroin, with some variations in rates based on differences in primary sequences and overall degree and nature of crystallinity. First, we briefly describe the structures of native silk fibers and regenerated silk materials. Then we review studies on the in vitro enzymatic degradation of both native and regenerated silk materials and discuss degradation-related factors. Subsequently, a brief discussion of the in vivo degradation of silk materials is presented. We also discuss some key challenges and potential directions toward the future study of the degradation of silk materials.
2. Structures of silk materials
Natural B. mori silk fibers are composed of two major protein components, fibroin and sericin, forming a core-shell structure. The silk fibroin is responsible for the mechanical properties (e.g., load-bearing capacity) through self-assembly into a semi-crystalline hierarchical core structure, and the sericin functions as glues to form a shell to hold two fibroin fibers together.16 For biomedical applications, sericin is removed from the native fibers via a degumming process (usually warm water with salts) to obtain purified silk fibroin for further processing; sericin has potential to result in inflammatory responses in vivo.17 In this review, we use the term silk fibroin to refer to degummed B. mori silk unless otherwise stated.
Silk fibroin consists of a heavy (H) chain of ~390 kDa and a light (L) chain of ~26 kDa, connected by a disulfide bond at the C-terminus, forming a H-L complex.18 This H-L complex also binds a glycoprotein, named P25 (30 kDa), in a ratio of 6:1 via hydrophobic interactions to form an elementary micellar unit.19 In the H-chain, the C-terminal and N-terminal capping sequences are nonrepetitive. However, the rest of the silk fibroin sequence contains 11 short hydrophilic regions and 12 hydrophobic domains that account for over 90% of the silk H-chain. The hydrophilic domains assemble into random coils and/or helical structures, and the hydrophobic domains transition into β-sheet structures. In the hydrophobic domains, glycine-X (GX) repeats predominate, where X is alanine, serine or tyrosine (Figure 1A).18 This highly repetitive sequence forms into antiparallel β-sheet structures and constitutes the bulk of the crystalline/semi-crystalline domains in native silk fibroin fibers.20–22 Furthermore, the silk fibroin fibers possess a hierarchical structure where highly oriented β-sheets nanocrystallites are embeded in an amorphous matrix, which are then organized in nanofibrils or fibrillar entities, responsible for the excellent strength of the fibers.22, 23
Figure 1.

(A) Illustration of B. mori silk fibroin where some repetitive motifs (e.g. GAGAGS) are presented in the heavy chain of the fibroin. (B) In the native B. mori silkworm gland, liquid silk fibroin is presented in silk I structures prior to spinning. Upon spinning, the silk I structure converts to silk II structure, forming native B. mori silk fibers with β-sheet nanocrystalline structures.
Silk I and silk II are reported as dimorphic structures of B. mori silk fibroin where silk I is defined as the structure of silk fibroin found in the native middle silk gland prior to natural spinning and silk II is defined as the structure of silk fibroin in native silk fibers (Figure 1B). Besides native silk, silk I and silk II are also commonly utilized for the structural analysis of regenerated silk materials. Therefore, it is necessary to briefly introduce these two types of structures to inform the latter discussion on structure-degradation relationships in a more comprehensive fashion. The structures of silk fibroin in silk I and silk II have been studied extensively with a variety of techniques including X-ray diffraction, solid-state NMR spectroscopy, and vibrational spectroscopy.3, 22, 24, 25 These structural characterization techniques have illustrated that in silk II, the GAGAGS motifs and the GAGAGY motifs flanking the GAGAGS repetitive sequences form into the β-sheet structures and are further organized to form β-sheet nanocrystallites.20 Other non-repetitive motifs constitute the amorphous domains. In silk I, silk fibroin possesses predominantly conformations of random coil, turn, and helical structures where GAGAGX (X=S, Y, V) motifs were hypothesized to be in type II β-turn structures in silk I.26
Natural silk fibers can be reverse engineered back to a solution of protein chains, followed by formulation to generate a variety of silk-based material formats (e.g., regenerated silk materials) including films, sponges, hydrogels, particles, and scaffolds.3, 27 In general, the regeneration process starts with degumming and dissolution via chemical processes, e.g. by boiling natural silk fibers in sodium bicarbonate aqueous solution for 5–60 min to remove the sericin with some degradation of silk fibroin chains, to obtain degummed silk. During the degumming, the molecular weight of silk protein decreases due to cleavage of the disulfide bond between the fibroin H-chain and L-chain and fragmentation of the amorphous silk sequences in the fibroin.28 The degummed silk fibers can be dissolved into a concentrated chaotropic agent to dissemble the higher order silk structure. The resulting silk fibroin solution (usually a few weight percent) is then dialyzed to yield an aqueous silk solution that is stable at 4°C for several weeks depending on the conditions. Compared to native silk fibroin (e.g., spinning dope from the gland of the silkworm), this regenerated silk fibroin preserves the primary amino acid sequence and can form into the expected secondary structures to those found in native silk fibroin, but has different rheological properties.29 The regenerated silk materials (e.g., films, sponges) with silk I structure can be prepared from regenerated silk solution.22, 30 Furthermore, the silk I structure in liquid or solid regenerated silk materials can be converted to the silk II structure by a range of chemical and physical methods (e.g., methanol treatment, water annealing, sonication, autoclaving). For example, early studies have shown that the regenerated silk materials can be prepared with control of crystallinity and content of β-sheet structures by monitoring the water vapor annealing temperature and time, which offers a simple, sustainable approach to regulate the structure of regenerated silk materials.30, 31 With increasing the water vapor annealing temperature or time, a higher content of β-sheet structures (maximum ~60%) can be achieved (Figure 2). It is known that the structures of silk materials strongly dictate the enzymatic degradability of the materials. Therefore, different enzymatic degradation properties can be achieved for silk materials or devices via structural control during materials processing or post-treatment. In the following section, we focus on the structure-degradability relationships and discuss the factors influencing the enzymatic degradation of the silk materials.
Figure 2.
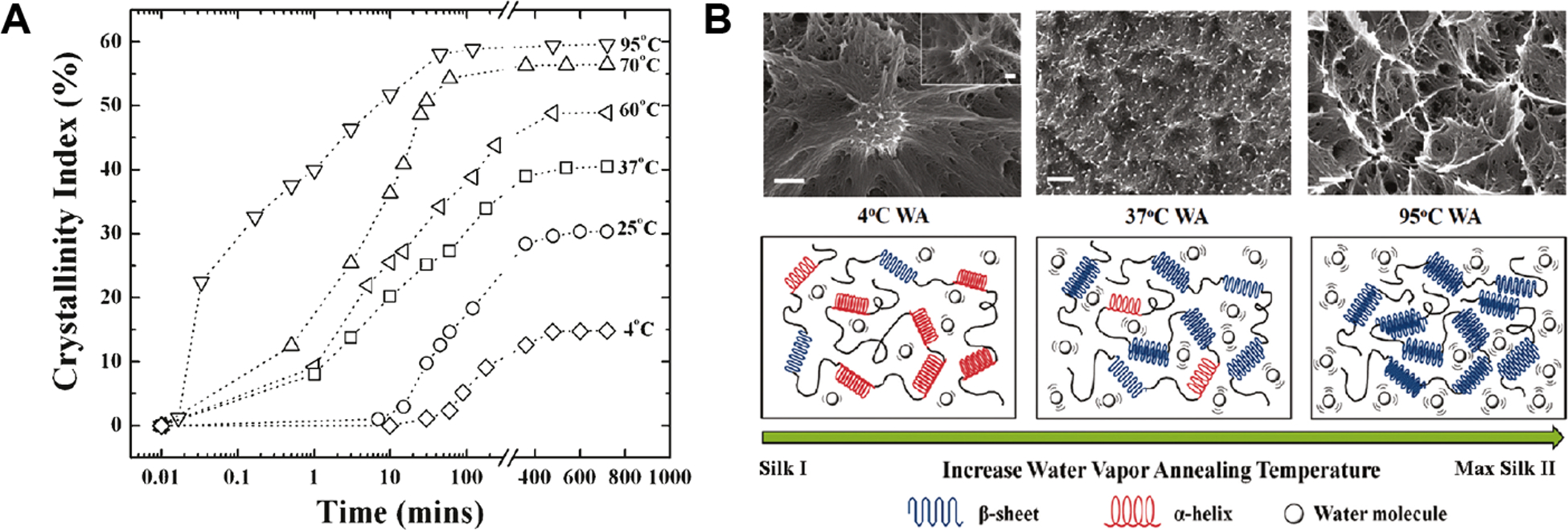
(A) Crystallinity index (β-sheet content) as a function of water vapor annealing time at different temperatures. (B) Mechanisms of temperature-controlled water vapor annealing to silk fibroin protein. Through the variation of water vapor temperature, the structure of regenerated silk materials can be fully controlled from a helical/turn/random coil dominated silk I structure to the β-sheet enriched silk II structure. Scale bars in SEM images are 200 nm. Reprinted with permission from ref31 Copyright 2011 American Chemical Society.
3. In vitro enzymatic degradation of silk materials
As a protein-based biomaterial, silk fibroin degrades in vitro and in vivo in response to proteolytic enzymes (e.g., protease XIV, α-chymotrypsin, proteinase K, papain, collagenase, etc.). Enzymatic degradation leads to the breakdown of the silk fibroin into smaller polypeptides and eventually amino acids, which are easily absorbed or metabolized in vivo. A number of proteolytic enzymes degrade silk fibroin as summarized in Table 1. Some of these enzymes, such as protease XIV and α-chymotrypsin, have numerous cleavage sites along the silk fibroin chains, which supports more efficient degradation.32 However, α-chymotrypsin has a more limited degradation effect on degummed silk fibers.14, 33 In addition, papain, a cysteine protease has limited cleavage sites (~40) along the silk fibroin chain yet results in significant degradation of silk nanoparticles over 3 weeks.33 Thus, predicting silk fibroin degradation requires consideration of both the cleavage sites as well as the material structure. A generally accepted model suggests that the enzymatic degradation of silk starts with the hydrophilic amorphous domains, including the C-termini, N-termini, linker segments in the heavy chain, and the light chain. Subsequently, the more crystalline domains with packed structures are degraded. From studies of a broad spectra of silk material formats, protease XIV is the most efficient proteolytic enzyme reported to date for degrading silk fibroin in almost any material format, including regenerated silk fibers, films, sponges, and bulk materials.5, 12–14, 32–37 Protease XIV is a nonmammalian enzyme cocktail that is used for studying silk degradation in vitro with activity towards degrading β-sheet crystalline structures.
Table 1.
| Enzyme | Cleavage Sites | No. of cleavage sites in silk fibroin |
|---|---|---|
| Protease XIV | Tyr, Phe, Trp, His, Lys, Arg | ~390 |
| α-chymotrypsin | Tyr, Phe, Trp, Val, Ile, Leu | ~520 |
| Proteinase K | His, Phe, Trp, Tyr, Ala, Ile, Leu, Pro, Val, Met | ~2200 |
| Papain | Lys, Arg | ~40 |
| Matrix metalloproteinases-1 | Gly-Ile, Gly-Leu | <10 |
| Matrix metalloproteinases-2 | Gly-Ile, Gly-Leu, Gly-Val, Gly-Phe, Gly-Asn, Gly-Ser | ~600 |
| Collagenase | X-Gly-Pro | ~15 |
Natural silk fibers were used to fabricate bioabsorbable sutures with a longer time period for proteolytic degradation in vitro and in vivo.1, 34, 38 Previous studies had shown that silk fibroin in natural silk fibers was proteolytically degraded with predictable rates of decrease in fiber diameter, loss of mechanical properties (failure strength), reduction in mechanical cycles to failure, and mass loss when incubated at 37°C in 1.0 mg/mL protease XIV solution (compared to controls incubated in phosphate buffered saline solution).34 In addition, increased fragmentation of silk fibroin was observed over time during enzymatic degradation with increasing time of exposure to the protease XIV. Gel electrophoresis further indicated a decreased amount of the 25 Da fibroin light chain and a shift in the molecular weight of the fibroin heavy chain with increasing incubation time in protease.34 However, the degradation behavior of natural fibers was different from regenerated silk materials, where the degradation rate of the natural fibers was much slower,14 primarily due to the compact semi-crystalline structure with highly orientated fibrous structure.
The in vitro proteolytic degradation of regenerated silk materials has been broadly studied with fibers, films, sheets, hydrogels, sponges, and blocks. In Table 2, representative in vitro proteolytic degradation studies on these different regenerated silk materials are summarized.28, 36, 37 As mentioned earlier, among the enzymes used, protease XIV was the most efficient for degrading the regenerated silk fibroin across the different processing methods and material formats in general. Some other enzymes also showed degradation efficiency on specific samples. For example, proteinase K showed better degradation efficiency on silk films than protease XIV.32 α-Chymotrypsin was effective for degrading silk hydrogels and porous sheets but showed no significant degradation of silk nanoparticles prepared via nanoprecipitation, while papain degraded the silk nanoparticles efficiently.13, 32, 33 Regardless of enzyme preference, the processing methods used to make regenerated silk materials dictates the degradation properties of the products, since the structures and morphologies of these silk materials vary based on the processing method utilized. Water vapor annealing can be used to control the crystallinity of the silk materials and thus achieve different degradation properties. Silk materials processed with a higher water vapor annealing temperature showed higher crystallinity and thus slower degradation (Figure 3).31 In addition, for silk blocks, the thermal processing produced materials with tunable degradation rates comparable to solvent-based methods.37, 39
Table 2.
Summary of in vitro proteolytic degradation studies for different regenerated silk materials.
| Material format | Processing method | Mw | Proteolytic degradation profiles | Refs |
|---|---|---|---|---|
| Fiber | Wet spinning 10 wt% silk in HFIP |
<100 kDa | 37.2% mass loss after 30 days in 5 U/mL actinomyces enzyme solution | 40 |
| Electrospinning | N/A | 65% mass loss after 24 days in 1 U/mL protease XIV solution | 41 | |
| Film | Solution casting Water annealing for 1 hours |
~200 kDa | 47% mass loss after 120 hours in 1.8 U/mL proteinase K solution 9% mass loss after 120 hours in 0.1 U/mL protease XIV solution 9% mass loss after 120 hours in 12 U/mL α-chymotrypsin solution 7% mass loss after 120 hours in 75 U/mL collagenase solution 4% mass loss after 120 hours in 8750 U/mL MMP-1 solution 4% mass loss after 120 hours in 313 U/mL MMP-2 solution |
32 |
| Solution casting Water annealing for 24 hours |
N/A | ~20% mass loss after 24 hours in 5.6 U/mL protease XIV solution ~100% mass loss after 14 days in 5.6 U/mL protease XIV solution |
30 | |
| Solution casting Water annealing for 4 hours |
N/A | ~40% mass loss after 24 hours in 2.3 U/mL protease XIV solution | 36 | |
| Solution casting Slow drying over 3 days |
N/A | ~40% mass loss after 24 hours in 2.3 U/mL protease XIV solution | 36 | |
| Solution casting with glycerol as additive, then stretching to 250 % | N/A | ~80% mass loss after 24 hours in 2.3 U/mL protease XIV solution | 36 | |
| Solution casting Methanol/Water-90/10 treated |
N/A | ~20% mass loss after 24 hours in 5.6 U/mL protease XIV solution ~80% mass loss after 14 days in 5.6 U/mL protease XIV solution |
30 | |
| Solution casting Methanol/Water-50/50 treated |
~120 kDa | ~2.6% mass loss after 24 hours with collagenase-to-silk ratio of 1:20 ~6.0% mass loss after 24 hours with α-chymotrypsin-to-silk ratio of 1:20 ~18.2% mass loss after 24 hours with protease XIV-to-silk ratio of 1:20 |
14 | |
| Hydrogel | Sonication | ~200 kDa | 42% mass loss after 120 hours in 1.8 U/mL proteinase K solution 42% mass loss after 120 hours in 0.1 U/mL protease XIV solution 11% mass loss after 120 hours in 12 U/mL α-chymotrypsin solution 16% mass loss after 120 hours in 75 U/mL collagenase solution 4% mass loss after 120 hours in 8750 U/mL MMP-1 solution 3% mass loss after 120 hours in 313 U/mL MMP-2 solution |
32 |
| Sponges | Aqueous salt-leaching | N/A | >80% mass loss after 24 hours in 2 U/mL protease XIV solution 24% area decrease after 56 days of in vitro osteogenic culture |
42 |
| HFIP-based salt-leaching | N/A | No significant mass loss was observed after 24 hours in 2 U/mL protease XIV solution | 42 | |
| Freeze drying Autoclaving |
>90 kDa | ~60 % mass loss after 24 hours in 3.5 U/mL protease XIV solution | 28 | |
| Porous sheet | Freeze drying | 20–70 kDa | 32 % mass loss after 15 days in 1 U/mL α-chymotrypsin solutions 52 % mass loss after 15 days in 1 U/mL collagenase IA; 70 % mass loss after 15 days in 1 U/mL protease XIV |
13 |
| Nanoparticles | Nanoprecipitation | N/A | ~60 % mass loss after 24 hours in 3.5 U/mL protease XIV solution ~50 % mass loss after 5 days in 3.5 U/mL papain solution No significant mass loss was observed after 20 days in 3.5 U/mL α-chymotrypsin solution |
33 |
| Blocks | Solvent-based process | N/A | ~17 % mass loss after 8 weeks in 5 U/mL protease XIV solution | 39 |
| Thermal processing | ~200 kDa | 10–65 % mass loss after 30 days in 5 U/mL protease XIV solution 5–40 % mass loss after 30 days in 40 U/mL α-chymotrypsin solution |
37 |
Notes: HFIP, hexafluoro-2- propanol; MMP, matrix metalloproteinase; all degradation studies carried out at 37°C.
Figure 3.

(A) Three-day protease XIV enzymatic degradation study of silk films prepared using 12 h of water vapor annealing at different temperatures. (B) SEM images showing the morphologies of 70°C water-annealed samples at different degradation times: 0; 4; 24; 75 h. Scale bars are 200 nm. Reprinted with permission from ref31 Copyright 2011 American Chemical Society.
Factors dictating the enzymatic degradation of regenerated silk materials can be categorized into two main groups: (a) structure-related and (b) morphology-related. Structure-related factors include molecular weight, protein secondary structures, crystallinity, and hierarchical structure. Morphology-related factors include material format, porosity and surface morphology. All these factors have to be considered when assessing the degradation properties of regenerated silk materials.
The molecular weight of silk fibroin and degree of crystallinity play critical roles in determining degradation properties, as with degradable synthetic polymers. In general, higher molecular weight and higher crystallinity result in slower degradation. Manipulating these two parameters allows control of degradation properties of the silk materials, and these two parameters are dependent on processing conditions (e.g., degumming, water vapor annealing, etc.). Harsher degumming conditions and longer degumming times lead to lower molecular weight since silk fibroin is susceptible to slow hydrolysis due to the alkaline conditions utilized during the process. Thus, the protein structures of regenerated silk fibroin change compared to the native fiber features, which leads to changes in susceptibility to enzymatic degradation.43
Crystallinity is more complicated since this factor is correlated with molecular weight but also determined by the ‘curing’ related to the formation of protein secondary structures. Obtaining accurate degrees of crystallinity for silk materials remains a challenge since different experimental approaches and analytical methods including X-ray diffraction (XRD), vibrational spectroscopy, and differential scanning calorimetry (DSC) may lead to different values for crystallinity even using the same silk materials.25, 44–46 Vibrational spectroscopy such as Fourier-transform infrared spectroscopy (FTIR) and Raman spectroscopy serves as primary tools in determining the content of β-sheet structures in silk materials. Since the β-sheet structures contribute significantly to the crystallinity, the content of β-sheet structures and crystallinity were used interchangeable in some studies.31, 46 In contrast, XRD relies on the deconvolution of the diffraction profiles of silk materials where some variations were found between different data analysis methods.47 According to the literature, silk fibroin presents in a semi-crystalline structure (silk II) in the native state (fibers), with ~46 % crystalline domains composed primarily of antiparallel β-sheet structures.25 When silk fibroin is dissolved, the β-sheet structures are disrupted and then undergo refolding and intermolecular re-self-assembly during the regeneration process, where amorphous structures or semi-crystalline structures can be produced depending on the specific conditions. Generally, reconstitution of β-sheet structures in regenerated silk materials contributes to the crystallinity, while in some process such as slow drying, silk I structure with a limited amount of β-sheet structures form into the crystalline phase.48 Based on these facts, regenerated silk materials with similar silk fibroin molecular weights and degrees of crystallinity may have different enzymatic degradation properties due to the differences in content of the various molecular structures. Silk materials with amorphous structures commonly have faster proteolytic degradation rates compared to those with semi-crystalline structures, since the amorphous structures are more loosely packed and more accessible to the proteolytic enzymes. Hence, the amorphous structures are firstly degraded, followed by the degradation of crystalline structures. This was demonstrated in some reported studies where the crystallinity of regenerated silk materials increased at the initial stages of enzymatic degradation.13, 14, 36 Furthermore, some fundamental understanding on the enzymatic degradation of different molecular structures, including β-sheet nanocrystalline structures and semi-crystalline silk I structures has been achieved. Anti-parallel β-sheet nanocrystalline structures collapse into nanofibrils and subsequently to nanofilaments and soluble fragments when degraded by protease XIV. In contrast, α-chymotrypsin cannot digest the β-sheet nanocrystalline structures as it can only degrade the loosely packed amorphous structures (Figure 4).35 Degradation of semi-crystalline silk I structures by protease XIV was performed on silk films prepared from slow drying the aqueous silk solution. In this case, nanostructured globules were identified in the silk films where both nanocrystalline domains and amorphous domains were present.48 The amorphous domains degraded first, followed by release of the nanocrystalline structures into solution.36 In addition, the hierarchical structure of silk materials is another factor influencing the degradation property. Natural silk fibers possess a well-organized hierarchical structure with highly ordered nanofibrils. Such complex structures makes the natural silk fibers robust and protected from enzymatic degradation compared with regenerated silk materials, thus they degrade more slowly.14, 40
Figure 4.
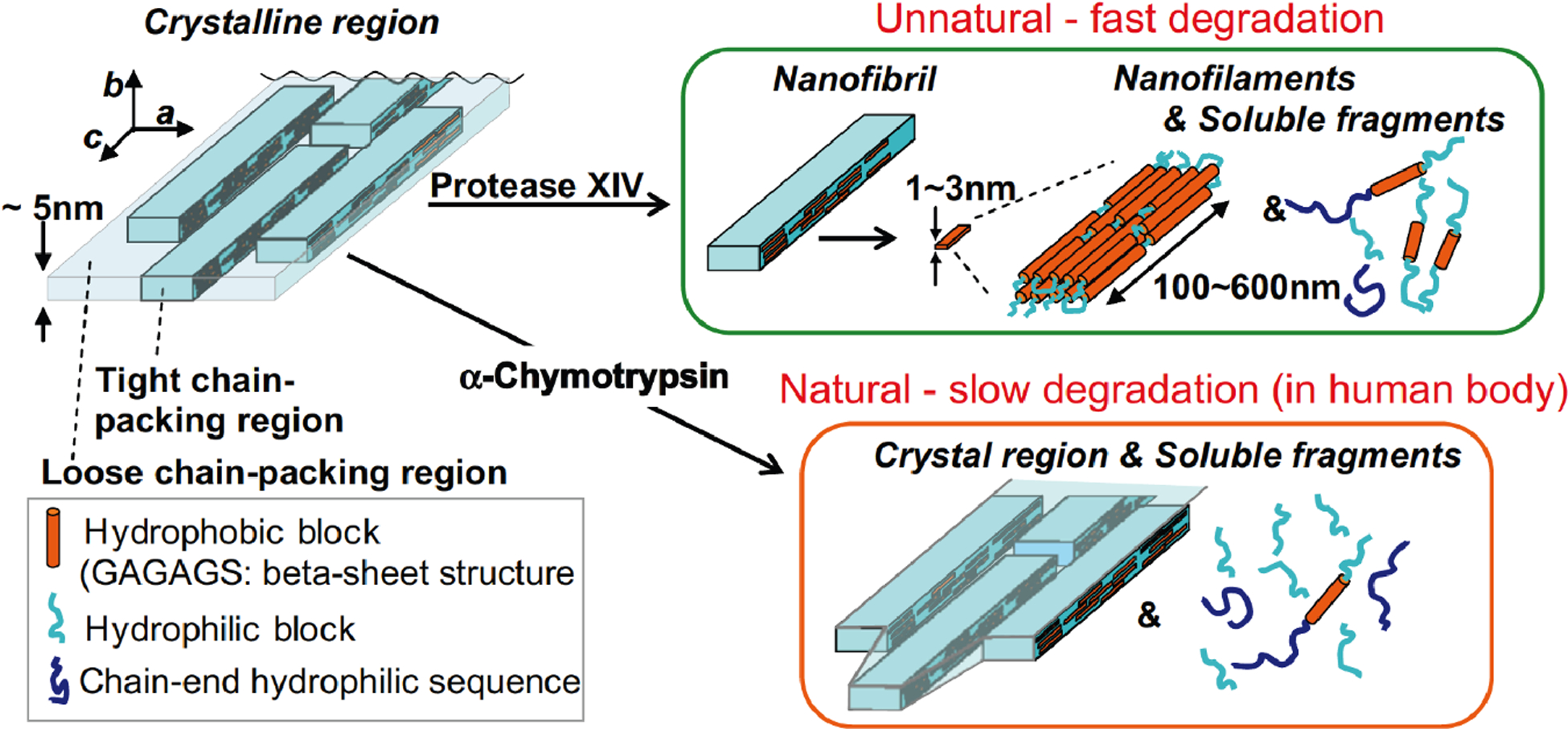
Model of enzymatic degradation of β-sheet crystalline regions of silk fibroin. Both tight and loosely chain-packing regions coexist in the crystalline region. Upon enzymatic degradation by protease XIV, the loosely chain-packing regions are degraded first, followed by degradation of tightly chain-packing structures into nanofilaments and soluble fragments containing β-sheet structures. Upon enzymatic degradation by α-chymotrypsin, the edges and ends of loosely chain-packed regions are degraded; crystalline domains and soluble fragments without β-sheet structure are presented after degradation. Reprinted from ref.35 Copyright 2010 Elsevier Ltd.
With respect to morphology-related factors, material format plays a critical role since it dictates physical properties such as porosity and surface morphology. Generally, material formats with denser structures and lower porosity, such as silk fibers, films or blocks have longer degradation times due to limited enzyme accessibility and the surface erosion features of the enzymes. When designing silk-based materials to meet different applications, the porosity or surface features can be engineered to achieve predictable degradation profiles.49–51
Cell-mediated in vitro degradation studies have also been conducted in contrast to the bulk of the in vitro studies which focus on enzymatic, cell-free approaches. This study indicated that osteoblasts and osteoclasts were capable of degrading silk films in vitro compared to human mesenchymal stem cells (hMSCs) and film controls without cells, forming degradation pits on the surface of silk films (Figure 5).52 In addition, the involvement of specific matrix metalloproteinases (MMPs) and integrin signaling in the degradation process was determined. The osteoclasts generated the highest level of MMPs 1 and 2, and upregulated integrins α2 and β1. The osteoblasts upregulated integrins α5 and β1. These studies not only provide direct evidence that silk materials can be degraded via cell-mediated pathways, but also help us better understand the role of different cell types in silk degradation relating to overall regenerative outcomes.
Figure 5.
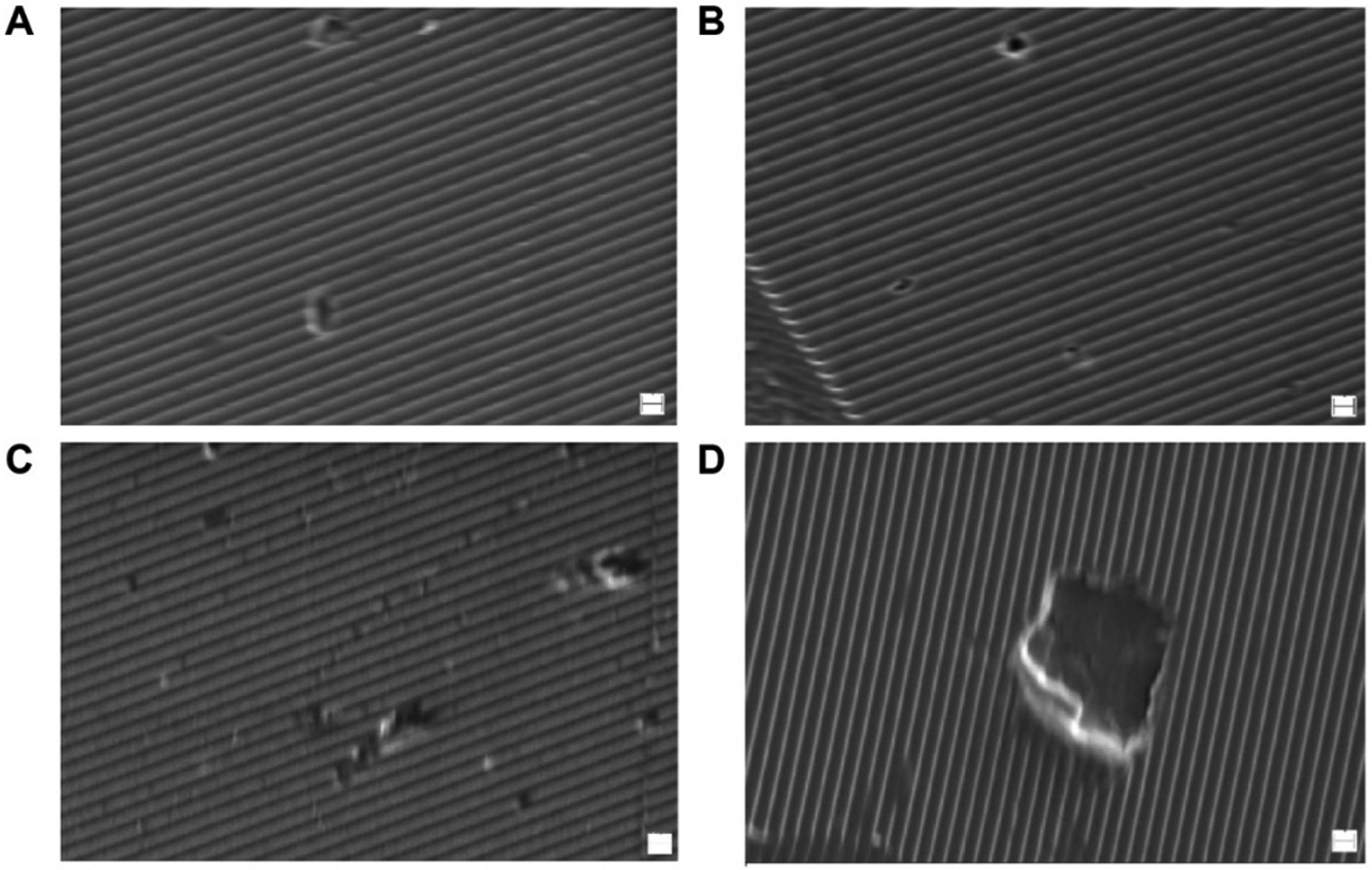
SEM images of degradation pits on patterned silk films caused by osteoblasts (A, B) and osteoclasts (C, D). Scale bars are 1 μm. Reprinted with permission from ref52 Copyright 2010 American Chemical Society.
4. In vivo degradation of silk materials
The in vivo degradation of silk materials relates to the host immune response where immune cells, specifically macrophages and foreign-body giant cells (FBGCs), play an important role in the degradation process for silk materials.15, 49 Regarding the degradation mechanisms, silk fibroin is degraded in vivo via immune cell-mediated pathways: phagocytosis by macrophages and/or foreign body giant cells, and extracellular degradation is mediated by proteolytic enzymes secreted by the cells.11 For silk scaffolds implanted in rats, regions accessible to macrophages showed visible evidence of degradation, unlike the cell-free regions.52 In addition, the degradation rate of in vivo can be tuned from minutes to years by controlling the structure and morphology of the implanted silk materials, similar to in vitro degradation of silk. Generally, silk materials with a lower β-sheet content degrade faster, partially due to the dissolution of the amorphous phase of silk.49 Porous silk scaffolds showed higher degradation rates than densely compacted silk bulk materials since cellular infiltration and enzyme diffusion was faster for silk materials with higher porosity.49, 53 Compared to in vitro degradation studies of silk materials, very limited fundamental work has been reported for the in vivo situation, in order to understand the degradation pathway of silk materials including the characterization of macrophage phenotype activation, locally released cytokines, time frames of host response to silk implants and implantation site (e.g., tissue location)-dependent degradation behavior. Although some silk-based devices, such as SERI scaffolds, have demonstrated degradability and have been approved by the FDA,38, 54–56 a comprehensive understanding of the degradation mechanism remains a need.
5. Future perspectives
The proteolytic degradation of silk materials is complicated since it is related to a wide range of structural, morphological and biological factors. Over the past a few years, some progress has been made to achieve an understanding of the proteolytic degradation of silk materials with proteolytic enzymes in vitro and animal studies in vivo. However, more effort and investigations are needed. For example, more thorough studies on the degradation mechanisms of different silk structures, such as the silk I crystalline structure are necessary. In addition, how the structures of silk materials change during in vitro enzymatic degradation require further investigation. For in vivo responses of silk materials, degradation mechanisms are still lacking, including tissue location differences, the roles of various cell types, and the mass balance of residues and degradation products in vivo. Additionally, analysis of metabolites during silk degradation and metabolism using fluorescently or isotopically labelled silk is needed for understanding the life cycle of silk materials in vivo. Toward practical applications, to achieve optimized structure-property-function designs is critical, where degradability is a central focus. Advanced experimental and computational tools are required to achieve this goal. A recent study on in vivo degradation of recombinant spider silk proteins was assessed by integrating experimental approaches and multiscale molecular dynamic simulations to predict outcomes, in terms of degradation in vivo. The results showed that higher exposure of less ordered structures (e.g., random coil) and reduced exposure of ordered structures (e.g., β-sheet and helix) at the surface of the self-assembled recombinant proteins led to higher degradation rates. This study demonstrated the feasibility and opportunities of using computational modelling to investigate the structure-degradation relationship for silk-based biomaterials as well as other polymer systems.57 Some of the novel features of silk biomaterials, including tunable degradation rates from days to years, the ability to sequester and activate digestive enzymes from within, the options to tailor surface chemistry to control interfacial responses, control of molecular weight and crystalline content, and the ability to exploit the remarkably robust mechanical features of silks to cover soft (e.g., adipose, brain) to hard (e.g., bone and cartilage) tissues, all point to a positive future for degradable medical devices based on silk protein.
Acknowledgements
We thank the many students and collaborators who have worked with us on the various aspects of silk degradation. Further, we thank the NIH (R01EB021264, P41EB002520, R01AR068048, R01NS094218, U01EB014976, R01AR070975) and the AFOSR (FA9550-17-1-0333) for funding support.
References
- 1.Altman GH; Diaz F; Jakuba C; Calabro T; Horan RL; Chen J; Lu H; Richmond J; Kaplan DL, Silk-based biomaterials. Biomaterials 2003, 24, 16. [DOI] [PubMed] [Google Scholar]
- 2.Vepari C; Kaplan DL, Silk as a Biomaterial. Prog. Polym. Sci 2007, 32 (8–9), 991–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koh L-D; Cheng Y; Teng C-P; Khin Y-W; Loh X-J; Tee S-Y; Low M; Ye E; Yu H-D; Zhang Y-W; Han M-Y, Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci 2015, 46, 86–110. [Google Scholar]
- 4.Yarger JL; Cherry B,R; Vaart A. v. d., Uncovering the structure–function relationship in spider silk. Nat. Rev. Mater 2018, 3, 11. [Google Scholar]
- 5.Cao Y; Wang B, Biodegradation of silk biomaterials. Int. J. Mol. Sci 2009, 10 (4), 1514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawker MJ; Guo C; Omenetto FG; Kaplan DL, Solvent-Free Strategy To Encapsulate Degradable, Implantable Metals in Silk Fibroin. ACS Appl. Bio Mater 2018, 1 (5), 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mu X; Wang Y; Guo C; Li Y; Ling S; Huang W; Cebe P; Hsu HH; De Ferrari F; Jiang X; Xu Q; Balduini A; Omenetto FG; Kaplan DL, 3D Printing of Silk Protein Structures by Aqueous Solvent-Directed Molecular Assembly. Macromol. Biosci 2020, 20 (1), e1900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roh TT; Chen Y; Paul HT; Guo C; Kaplan DL, 3D bioengineered tissue model of the large intestine to study inflammatory bowel disease. Biomaterials 2019, 225, 119517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urie R; Guo C; Ghosh D; Thelakkaden M; Wong V; Lee JK; Kilbourne J; Yarger J; Rege K, Rapid Soft Tissue Approximation and Repair Using Laser-Activated Silk Nanosealants. Adv. Funct. Mater 2018, 28 (42). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao S; Guo C; Kumarasena A; Omenetto FG; Kaplan DL, 3D Printing of Functional Microalgal Silk Structures for Environmental Applications. ACS Biomater. Sci. Eng 2019, 5 (9), 4808–4816. [DOI] [PubMed] [Google Scholar]
- 11.Li C; Guo C; Fitzpatrick V; Ibrahim A; Zwierstra MJ; Hanna P; Lechtig A; Nazarian A; Lin SJ; Kaplan DL, Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater 2020, 5, 61–81. [Google Scholar]
- 12.Holland C; Numata K; Rnjak-Kovacina J; Seib FP, The biomedical use of silk: past, present, future. Adv. Healthc. Mater 2019, 8, 26. [DOI] [PubMed] [Google Scholar]
- 13.Li M; Ogiso M; Minoura N, Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials 2003, 24, 9. [DOI] [PubMed] [Google Scholar]
- 14.Arai T; Freddi G; Innocenti R; Tsukada M, Biodegradation of Bombyx mori silk fibroin fibers and films. J. Appl. Polym. Sci 2004, 91, 8. [Google Scholar]
- 15.Thurber AE; Omenetto FG; Kaplan DL, In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao Z; Vollrath F, Surprising strength of silkworm silk. Nature 2002, 418 (6899), 741. [DOI] [PubMed] [Google Scholar]
- 17.Panilaitis B; Altman GH; Chen J; Jin H-J; Karageorgiou V; Kaplan DL, Macrophage responses to silk. Biomaterials 2003, 24 (18), 3079–3085. [DOI] [PubMed] [Google Scholar]
- 18.Zhou CZ; Confalonieri F; Jacquet M; Perasso R; Li ZG; Janin J, Silk fibroin: structural implications of a remarkable amino acid sequence. Proteins 2001, 44 (2), 119–22. [DOI] [PubMed] [Google Scholar]
- 19.Nony P; Prudhomme JC; Couble P, Regulation of the P25 gene transcription in the silk gland of Bombyx. Biol. Cell 1995, 84 (1–2), 43–52. [DOI] [PubMed] [Google Scholar]
- 20.Asakura T; Yao J; Yamane T; Umemura K; Ulrich AS, Heterogeneous structure of silk fibers from Bombyx mori resolved by 13C solid-state NMR spectroscopy. J. Am. Chem. Soc 2002, 124 (30), 8794–5. [DOI] [PubMed] [Google Scholar]
- 21.Asakura T; Suzuki Y; Nakazawa Y; Yazawa K; Holland GP; Yarger JL, Silk structure studied with nuclear magnetic resonance. Prog. Nucl. Magn. Reson. Spectrosc 2013, 69, 23–68. [DOI] [PubMed] [Google Scholar]
- 22.Asakura T; Okushita K; Williamson MP, Analysis of the Structure ofBombyx moriSilk Fibroin by NMR. Macromolecules 2015, 48 (8), 2345–2357. [Google Scholar]
- 23.Guo C; Zhang J; Wang X; Nguyen AT; Liu XY; Kaplan DL, Comparative Study of Strain-Dependent Structural Changes of Silkworm Silks: Insight into the Structural Origin of Strain-Stiffening. Small 2017, 13 (47). [DOI] [PubMed] [Google Scholar]
- 24.Lefevre T; Paquet-Mercier F; Rioux-Dube JF; Pezolet M, Review structure of silk by raman spectromicroscopy: from the spinning glands to the fibers. Biopolymers 2012, 97 (6), 322–36. [DOI] [PubMed] [Google Scholar]
- 25.Guo C; Zhang J; Jordan JS; Wang X; Henning RW; Yarger JL, Structural Comparison of Various Silkworm Silks: An Insight into the Structure-Property Relationship. Biomacromolecules 2018, 19 (3), 906–917. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y; Yamazaki T; Aoki A; Shindo H; Asakura T, NMR study of the structures of repeated sequences, GAGXGA (X = S, Y, V), in Bombyx mori liquid silk. Biomacromolecules 2014, 15 (1), 104–12. [DOI] [PubMed] [Google Scholar]
- 27.Rockwood DN; Preda RC; Yucel T; Wang X; Lovett ML; Kaplan DL, Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc 2011, 6 (10), 1612–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rnjak-Kovacina J; Wray LS; Burke KA; Torregrosa T; Golinski JM; Huang W; Kaplan DL, Lyophilized Silk Sponges: A Versatile Biomaterial Platform for Soft Tissue Engineering. ACS Biomater. Sci. Eng 2015, 1 (4), 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holland C; Terry AE; Porter D; Vollrath F, Natural and unnatural silks. Polym. Degrad. Stab 2007, 48, 5. [Google Scholar]
- 30.Jin HJ; Park J; Karageorgiou V; Kim UJ; Valluzzi R; Cebe P; Kaplan DL, Water-Stable silk films with reduced β-Sheet content. Adv. Funct. Mater 2005, 15 (8), 1241–1247. [Google Scholar]
- 31.Hu X; Shmelev K; Sun L; Gil ES; Park SH; Cebe P; Kaplan DL, Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules 2011, 12 (5), 1686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown J; Lu CL; Coburn J; Kaplan DL, Impact of silk biomaterial structure on proteolysis. Acta Biomater. 2015, 11, 212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wongpinyochit T; Johnston BF; Seib FP, Degradation Behavior of Silk Nanoparticles—Enzyme Responsiveness. ACS Biomater. Sci. Eng 2018, 4 (3), 942–951. [DOI] [PubMed] [Google Scholar]
- 34.Horan RL; Antle K; Collette AL; Wang Y; Huang J; Moreau JE; Volloch V; Kaplan DL; Altman GH, In vitro degradation of silk fibroin. Biomaterials 2005, 26 (17), 3385–93. [DOI] [PubMed] [Google Scholar]
- 35.Numata K; Cebe P; Kaplan DL, Mechanism of enzymatic degradation of bets-sheet crystals. Biomaterials 2010, 31, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Q; Zhang B; Li M; Zuo B; Kaplan DL; Huang Y; Zhu H, Degradation mechanism and control of silk fibroin. Biomacromolecules 2011, 12 (4), 1080–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo C; Li C; Vu HV; Hanna P; Lechtig A; Qiu Y; Mu X; Ling S; Nazarian A; Lin SJ; Kaplan DL, Thermoplastic moulding of regenerated silk. Nat. Mater 2020, 19, 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fine NA; Lehfeldt M; Gross JE; Downey S; Kind GM; Duda G; Kulber D; Horan R; Ippolito J; Jewell M, SERI surgical scaffold, prospective clinical trial of a silk-derived biological scaffold in two-stage breast reconstruction: 1-year data. Plast. Reconstr. Surg 2015, 135 (2), 339–51. [DOI] [PubMed] [Google Scholar]
- 39.Perrone GS; Leisk GG; Lo TJ; Moreau JE; Haas DS; Papenburg BJ; Golden EB; Partlow BP; Fox SE; Ibrahim AM; Lin SJ; Kaplan DL, The use of silk-based devices for fracture fixation. Nat. Commun 2014, 5, 3385. [DOI] [PubMed] [Google Scholar]
- 40.Zuo B; Dai L; Wu Z, Analysis of structure and properties of biodegradable regenerated silk fibroin fibers. J. Mater. Sci 2006, 41 (11), 3357–3361. [Google Scholar]
- 41.Zhou J; Cao C; Ma X; Hu L; Chen L; Wang C, In vitro and in vivo degradation behavior of aqueous-derived electrospun silk fibroin scaffolds. Polym. Degrad. Stab 2010, 95 (9), 1679–1685. [Google Scholar]
- 42.Park SH; Gil ES; Kim HJ; Lee K; Kaplan DL, Relationships between degradability of silk scaffolds and osteogenesis. Biomaterials 2010, 31 (24), 6162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandal BB; Kundu SC, Biospinning by silkworms: silk fiber matrices for tissue engineering applications. Acta Biomater. 2010, 6 (2), 360–71. [DOI] [PubMed] [Google Scholar]
- 44.Ling S; Qi Z; Knight DP; Shao Z; Chen X, Synchrotron FTIR microspectroscopy of single natural silk fibers. Biomacromolecules 2011, 12 (9), 3344–9. [DOI] [PubMed] [Google Scholar]
- 45.Drummy LF; Farmer BL; Naik RR, Correlation of the β-sheet crystal size in silk fibers with the protein amino acid sequence. Soft Matter 2007, 3 (7), 877–882. [DOI] [PubMed] [Google Scholar]
- 46.Hu X; Kaplan D; Cebe P, Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules 2006, 39 (18), 6161–6170. [Google Scholar]
- 47.Plaza GR; Perez-Rigueiro J; Riekel C; Perea GB; Agullo-Rueda F; Burghammer M; Guinea GV; Elices M, Relationship between microstructure and mechanical properties in spider silk fibers: identification of two regimes in the microstructural changes. Soft Matter 2012, 8 (22), 6015–6026. [Google Scholar]
- 48.Lu Q; Hu X; Wang X; Kluge JA; Lu S; Cebe P; Kaplan DL, Water-insoluble silk films with silk I structure. Acta Biomater. 2010, 6 (4), 1380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y; Rudym DD; Walsh A; Abrahamsen L; Kim HJ; Kim HS; Kirker-Head C; Kaplan DL, In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008, 29 (24–25), 3415–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo Y; Chen Z; Wen J; Jia M; Shao Z; Zhao X, A simple semi-quantitative approach studying the in vivo degradation of regenerated silk fibroin scaffolds with different pore sizes. Mater. Sci. Eng. C Mater 2017, 79, 161–167. [DOI] [PubMed] [Google Scholar]
- 51.Numata K; Ifuku N; Isogai A, Silk Composite with a Fluoropolymer as a Water-Resistant Protein-Based Material. Polymers 2018, 10 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sengupta S; Park S-H; Seok GE; Patel A; Numata K; Lu C-L; Kaplan DL, Quantifying osteogenic cell degradation of silk biomaterials. Biomacromolecules 2010, 11, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu K; Shi Z; Zhang S; Zhou Z; Sun L; Xu T; Zhang Y; Zhang G; Li X; Chen L; Mao Y; Tao TH, A Silk Cranial Fixation System for Neurosurgery. Adv. Healthc. Mater 2018, 7 (6), e1701359. [DOI] [PubMed] [Google Scholar]
- 54.Gross JE, Use of SERI Surgical Scaffold for Soft-tissue Support in a Massive Weight Loss Patient. Plast. Reconstr. Surg. Glob. Open 2013, 1 (9), e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clemens MW; Downey S; Agullo F; Lehfeldt MR; Kind GM; Palladino H; Marshall D; Jewell ML; Mathur AB; Bengtson BP, Clinical application of a silk fibroin protein biologic scaffold for abdominal wall fascial reinforcement. Plast. Reconstr. Surg. Glob. Open 2014, 2 (11), e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gross JE; Horan RL; Gaylord M; Olsen RE; McGill LD; Garcia-Lopez JM; Biber K; Barnico K; Toponarski I; Altman G, An evaluation of SERI surgical scaffold for soft-tissue support and repair in an ovine model of two-stage breast reconstruction. Plast. Reconstr. Surg 2014, 134 (5), 700e–704e. [DOI] [PubMed] [Google Scholar]
- 57.Dinjaski N; Ebrahimi D; Qin Z; Giordano JEM; Ling S; Buehler MJ; Kaplan DL, Predicting rates of in vivo degradation of recombinant spider silk proteins. J. Tissue Eng. Regen. Med 2018, 12 (1), e97–e105. [DOI] [PubMed] [Google Scholar]


