Abstract
Purpose
Tigertriever is a novel operator-adjustable clot retriever designed to enhance the operator's options to control the interaction of retriever and clot. The aim of this study was to assess the feasibility, safety and efficacy of the Tigertriever device system.
Methods
Prospective multi-center registry study at three comprehensive stroke centers in Switzerland from 2017 to 2019 of patients with acute ischemic stroke (AIS) and large vessel occlusion (LVO) using Tigertriever as a first-line device.
Results
30 AIS patients (median age 72.5 years (IQR 64–79), 50% women) with a median NIHSS on admission of 11 (IQR 6-13) and a median ASPECT score of 9 (IQR 7–10) were treated with the new Tigertriever and included in this study. The first-pass effect was 24% (n = 7). A good recanalization (eTICI 2 b/2c/3) was achieved in 94% of the cases. Median mRS at 90 days was 1 (IQR 1–2).
Conclusion
This study demonstrated feasibility, safety and effectiveness of the Tigertriever in AIS patients with LVO with a high reperfusion rate.
Keywords: Stroke, large vessel occlusion, mechanical thrombectomy, Tigertriever
Introduction
Several large randomized controlled trials (RCT) have proven the efficacy and safety of endovascular treatment (EVT) in acute ischemic stroke (AIS) patients with large vessel occlusions (LVO).1 Since the inception of EVT a rapid change of device design evolved to optimize the recanalization rates. Several different mechanical thrombectomy techniques have been introduced to potentially enhance successful revascularization.2
Tigertriever (Rapid Medical, Yokneam, Israel) is a novel operator-adjustable clot retriever offering the unique feature of an adjustable diameter of the thrombectomy basket, which facilitates the clot retrieval due to incremental control and adjustable radial force (Figure 1).
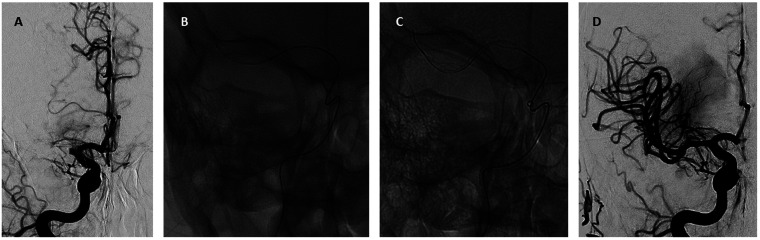
Figure 1.
Illustrative case of Tigertriever use. A 59-years old patient with an acute left-sided facial-brachial hemi-syndrome with multimodal neglect (NIHSS 19; pre-morbid mRS 0) due to proximal M1-segment occlusion of the right middle cerebral artery (a). A Tigertriever (1.5-6mm/32mm) was positioned in the M1 segment, covering the whole thrombus length (b). The diameter of the retrieval basket was manually increased to adapt it to the vessel and to strengthen the radial force (c). After one pass under dual aspiration a complete re-opening of the M1-segment was achieved (eTICI 3) (d). At the 90 days follow-up, the patient presented with a modified Ranking Scale Score (mRS) of 1.
The Tigertriever device (CE marked since 2016) has a braided design consisting of super elastic nitinol wires with a tantalum core and is fully radiopaque. The Tigertriever device family offers three different retrieval basket lengths for the use in different vessel occlusion sides. The Tigertriever (diameter 1.5–6 mm/32 mm) for distal intracranial carotid artery and M1 segment and proximal M2 segment occlusions; Tigertriever 17 (diameter 0.5–3 mm/17 mm) for distal M1 and M2 segment occlusions, as well as the Tigertriever 13 (diameter 0.5–2.5/20.5 mm) for distal vessel occlusions. The Tigertriever is compatible with 0.021 in microcatheters, the Tigertriever 17 with 0.017 in microcatheters as well as the Tigertriever 13 with 0.016 in/0.013 in microcatheters.3
To date, sparse clinical data on this device are available.4,5 The aim was to assess the feasibility, safety and effectiveness of the Tigertriever in AIS patients.
Methods
In this prospective, open-labeled, multicenter registry study three comprehensive stroke centers in Switzerland recruited patients from October 2017 to August 2019. Ethical approval was given by the local ethic committee. All subjects gave written consent to this registry.
Patients
Patients over 18 years (pre-morbid mRS of ≤1) with an AIS due to LVO of the anterior or posterior circulation and with a NIHSS of ≥2 on admission were eligible for EVT with the Tigertriever as first-line device. There was no study specific time window and patients with a wake-up stroke were included. All patients had the regular stroke imaging workup including a CT-scan and CT-angiography. Exclusion criteria were an intracranial hemorrhage, an infarction of relevant size, extreme tortuous vessels and a life expectancy of less then 6 months
Interventional technique
It was at the discretion of the interventionalist, which type of Tigertriever device (Tigertriever standard version, Tigertriever 17) was used. Tigertriever 17 was mostly used for M2 segment occlusions. Each site followed its own thrombectomy protocol. Mostly, a balloon-guided catheter with a distal access catheter was used (Table 1). In all cases, concomitant proximal aspiration during the retrieval maneuver was performed. In most of the cases double aspiration technique combined with the Tigertriever use was performed (n = 22; 73%). Most (n = 24; 80%) of the procedures were performed using a bi-plane angiography system.
Table 1.
Clinical and technical baseline characteristics.
| Clinical and technical characteristics | N = 30 |
|---|---|
| Age in years – median (IQR) | 72.5 (64–79) |
| Women – n. (%) | 15 (50%) |
| Hypertension – n. (%) | 13 (43%) |
| Diabetes mellitus – n. (%) | 2 (7%) |
| Atrial fibrillation – n. (%) | 8 (28%) |
| History of previous ischemic stroke – n. (%) | 2 (6%) |
| NIHSS on admission – median (IQR) | 11 (6–13) |
| Wake-up stroke – n. (%) | 11 (37%) |
| ASPECT Score – median (IQR) | 9 (7–10) |
| MCA-M1 – n. (%) | 13 (43%) |
| MCA-M2 – n. (%) | 12 (40%) |
| Terminal ICA – n. (%) | 1 (3%) |
| Basilar artery – n. (%) | 2 (7%) |
| PCA (P1/P2) – n. (%) | 2 (7%) |
| Intravenous thrombolysis prior to intervention – n. (%) | 14 (47%) |
| General anesthesia – n. (%) | 7 (24%) |
| Tigertriever (net length 32mm, diameter 1.5–6.0mm) – n. (%) | 22 (73%) |
| Tigertriever 17 (net length 23mm, diameter 0.5–3.0mm) – n. (%) | 8 (27%) |
| Balloon-catheter – n. (%) | 24 (82%) |
| Distal-access-catheter – n. (%) | 28 (94%) |
| Aspiration – n. (%) | 30 (100%) |
| Rescue device – n. (%) | 6 (20%) |
| Time from groin-puncture-to-recanalization [6]– median (IQR) | 49 (38–61) |
| Time from symptom-onset-to-recanalization [6]– median (IQR) | 317 (197–575) |
| Dwell time – median (IQR) | 4 (1–5) |
ASPECTS, Alberta stroke program early CT score; ICA, internal carotid artery; IQR, interquartile range; MCA, middle cerebral artery; mRS, modified Ranking Scale; n, number; NIHSS, National Institute of Health Stroke Scale; PCA, posterior cerebral artery.
Outcome and safety measures
Outcome measures were successful reperfusion assessed with the extended Thrombolysis in Cerebral Infarction (eTICI) scale,6 success after first pass defined as eTICI 3 (FPE) and good functional outcome at 90 days measured with the modified Rankin Scale (mRS ≤2). Safety endpoints included symptomatic intracranial hemorrhage (sICH) and mortality rate within the follow-up period of 90 days. Experienced neuro-interventionalists performed the interventions. Stroke neurologists assessed the clinical status. An external independent core laboratory (UCLA, Los Angeles, USA) analyzed the procedural angiography data. Descriptive data analysis was performed using STATA 14.2 (StataCorp, Texas, USA).
Results
Clinical and technical characteristics
30 patients with a median age of 72.5 years (IQR 64–79) and a female:male ratio of 1:1 were enrolled in this trial (Table 1). Thirty-seven percent (n = 11) were wake-up strokes. Median National Institute of Health Stroke Scale (NIHSS) score on admission was 11 (IQR 6–13; minimum-maximum: 4 to 40). Median Alberta Stroke Program Early CT score (ASPECTS) was 9 (IQR 7–10). Most LVO were located in the territory of the middle cerebral artery (n = 25; 83%). Half of the patients received IV thrombolysis prior to the intervention. In two patients (6%) additional intra-arterial tPA was administered. Most cases (n = 23; 77%) were treated under conscious sedation. Median procedural time (groin-puncture-to-recanalization) was 49 minutes (IQR 38–61).
Outcome measures
First Pass Effect (FPE) was 24% (n = 7) (Table 2). Good final reperfusion rate (eTICI 2 b/2c/3) was achieved in 94% with a median number of passes of 1 (IQR 1–2), including the use of a rescue device in 20% (n = 6) of the cases (in 4 cases ERIC retrieval device (Microvention, Aliso Viejo CA, USA), in 1 case TREVO retrieval device (Stryker Neurovascular, Fremont CA, USA), in 1 case SOFIA 5 PLUS (Microvention, Aliso Viejo CA, USA)). Beside one symptomatic intracranial hemorrhage during bailout maneuver performed with another retrieval device, there were no short-term or long-term sequelae related to the device in use. A mRS of ≤2 at 3 months was achieved in 83% of the cases. Three patients (10%) died not related to the device use during the follow-up period. One patient died due to aspiration pneumonia, one due to complication after cardiac intervention, and one died due to blood cancer sequelae diagnosed within the follow-up period.
Table 2.
Outcome measures and safety endpoints.
| Outcome measures and safety endpoints | N = 30 |
|---|---|
| First pass effect – n. (%) | 7 (24%) |
| Number of passes – median (IQR) | 1 (1–2) |
| Good reperfusion rate eTICI2b-3 – n. (%) | 28 (94%) |
| eTICI 3 – n. (%) | 13 (48%) |
| Good functional outcome (mRS ≤2) – n. (%) | 25 (83%) |
| Clinical outcome (mRS) at follow-up – median (IQR) | 1 (1–2) |
| NIHSS 24-hours post-procedural – n. (%) | 2 (1–6) |
| Symptomatic intracranial hemorrhage – n. (%) | 1 (3%)a |
| Asymptomatic intracranial hemorrhage – n. (%) | 2 (6%) |
| Vasospasm – n. (%) | 0 |
| Vessel Dissection – n. (%) | 0 |
| Vessel Perforation – n. (%) | 0 |
| Distal embolic vessel occlusion – n. (%) | 0 |
| New ischemic event within 24-hours – n. (%) | 0 |
| Re-occlusion within 24-hours – n. (%) | 1 (3%) |
| Mortality rate within 24-hours post-procedural – n. (%) | 0 |
| Mortality rate with the follow-up period – n. (%) | 3 (10%)b |
| Other severe adverse events – n. (%) | 1 (3%)b |
eTICI, extended Thrombolysis in Cerebral Infarction scale; IQR, interquartile range; mRS, modified Ranking Scale; n., number; NIHSS, National Institute of Health Stroke Scale.
asICH occurred after bail-out maneuver with an additional device.
bNot device related.
Discussion
This registry showed high efficacy and a good safety profile for the Tigertriever retrieval system in AIS patients with LVO. Since the introduction of EVT in AIS, several different thrombectomy devices have appeared. With the introduction of so-called stent-retriever devices – like the Solitaire, TREVO or ERIC system with its high reperfusion rate – EVT led to a breakthrough in AIS treatment.7–9 However, there is no thrombectomy device on the market that achieves a reperfusion rate of 100%. In fact, the various thrombectomy devices rather complement each other.10
The Tigertriever with its special feature of an operator-adjustable clot retrieval basket allows a case-by case adjustment of the radial force for different thrombus and vessel conditions. Thus, our results demonstrated a comparable FPE to other devices such as the Solitaire stent system of 25.1%.11 Our registry demonstrated a good final reperfusion rate (eTICI 2 b/3) with 94% that is higher than most of the commonly used thrombectomy devices7,8 or latest generation of hybrid-stent retriever such as NeVa (93%)12 or the EmboTrap II (76%, Neuravi/Cerenovus),13 and higher than the results of the recent retrospective Tigertriever study.4 However, in 20% an additional rescue device had to be used. Furthermore, the functional outcome with 83% of the patients having a mRS ≤2 at follow-up demonstrated the efficacy of this device and is similar to others.1
Although this registry has one SAE (3%) and a follow-up mortality rate of 10%, they were not device related. Regarding safety, sICH and mortality rate revealed comparable results to other thrombectomy devices (0-5.3%)1,14 and is in-line with a recent retrospective Tigertriever study.4 Limitations include the relatively small sample size.
Conclusions
This registry study demonstrated the feasibility, safety and effectiveness of the Tigertriever device system in patients with acute ischemic stroke and large vessel occlusion with a high recanalization rate.
Author’s Note
Krassen Nedeltchev and Luca Remonda are also affiliated with University of Berne, Berne, Switzerland.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PG, AH, JW, TK, JB, KN, LR have nothing to disclose relevant to this publication. MD received travel and congress fees from Rapid Medical. DL served as the angiography core lab.
Ethic approval statement
This study was approved by the local ethic committee (EKNZ 2017–00798).
Declaration of conflicting interests
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This post-marketing registry study was funded by Rapid Medical (Yokneam, Israel).
ORCID iDs
Philipp Gruber https://orcid.org/0000-0001-8464-3051
David S Liebeskind https://orcid.org/0000-0002-5109-8736
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 2.Munich SA, Vakharia K, Levy EI. Overview of mechanical thrombectomy techniques. Neurosurgery 2019; 85: S60–S67. [DOI] [PubMed] [Google Scholar]
- 3.Rapid Medical: Tigertriever, www.rapid-medical.com/tigertriever (accessed 16 July 2020).
- 4.Kara B, Selcuk HH, Erbahceci Salik A, et al. Single-center experience with the tigertriever device for the recanalization of large vessel occlusions in acute ischemic stroke. J Neurointerv Surg 2019; 11: 455–459. [DOI] [PubMed] [Google Scholar]
- 5.Kara B, Selcuk HH, Yildiz O, et al. Revascularization of acute basilar artery occlusion using the tigertriever adjustable clot retriever. Clin Neuroradiol 2017; 27: 241–243. [DOI] [PubMed] [Google Scholar]
- 6.Liebeskind DS, Bracard S, Guillemin F, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg 2019; 11: 433–438. [DOI] [PubMed] [Google Scholar]
- 7.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet 2012; 380: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell BC, Hill MD, Rubiera M, et al. Safety and efficacy of solitaire stent thrombectomy: individual patient data meta-analysis of randomized trials. Stroke 2016; 47: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahles T, Garcia-Esperon C, Zeller S, et al. Mechanical thrombectomy using the new ERIC retrieval device is feasible, efficient, and safe in acute ischemic stroke: a Swiss Stroke Center experience. AJNR Am J Neuroradiol 2016; 37: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruber P, Zeller S, Garcia-Esperon C, et al. Embolus retriever with interlinked cages versus other stent retrievers in acute ischemic stroke: an observational comparative study. J NeuroIntervent Surg 2018; 10: e31–e31. [DOI] [PubMed] [Google Scholar]
- 11.Zaidat OO, Castonguay AC, Linfante I, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke 2018; 49: 660–666. [DOI] [PubMed] [Google Scholar]
- 12.Ribo M, Requena M, Macho J, et al. Mechanical thrombectomy with a novel stent retriever with multifunctional zones: initial clinical experience with the NeVa thrombectomy device. J Neuroradiol 2020; 47: 301–305. [DOI] [PubMed] [Google Scholar]
- 13.Valente I, Nappini S, Renieri L, et al. Initial experience with the novel EmboTrap II clot-retrieving device for the treatment of ischaemic stroke. Interv Neuroradiol 2019; 25: 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaidat OO, Bozorgchami H, Ribo M, et al. Primary results of the multicenter ARISE II study (analysis of revascularization in ischemic stroke with EmboTrap). Stroke 2018; 49: 1107–1115. [DOI] [PubMed] [Google Scholar]