Abstract
We report a case of an unruptured, symptomatic, large right cavernous internal carotid artery aneurysm successfully treated with a new balloon-expandable flow diverter – Xcalibur Aneurysm Occlusion Device (AOD). Follow up imaging performed at six months demonstrated complete exclusion of the aneurysm and regression in dimensions, resulting in resolution of mass effect and clinical improvement.
Keywords: Cavernous segment aneurysm, flow diverter, new device
Background
Multiple treatment options are available for symptomatic cavernous segment aneurysm, like surgical ligation of the cervical internal carotid artery (ICA), trapping of the aneurysm with bypass, surgical clipping, detachable balloons with an intent of parent vessel occlusion (PVO), aneurysmal coiling with or without stent/balloon assistance and flow diversion.1 We report the first case of cavernous segment symptomatic aneurysm successfully treated with a new balloon- mounted device- Xcalibur AOD (Merlin MD, Singapore) (Figures 1 and 2).
Figure 1.
Illustrative images of Xcalibur Aneurysm Occlusive Device. (a and b) Stainless steel construct with ultra-thin polymer covered membrane. (c) Device mounted on a balloon catheter.
Figure 2.
Cross sectional construct of the device. The stainless steel frame of the device is sandwiched between the ultra-thin polymer membrane on the inner and outer sides. The inner layer of the membrane is straight and hydrophobic, while the outer layer is undulating and hydrophilic.
Case presentation
A 58-year-old lady presented at our institute with an acute onset of the right third nerve palsy and mild chemosis (right eye) for one-week. She had no comorbidities, like diabetes and hypertension. History of trauma was conspicuously absent. Her visual acuity and visual field were unaffected, and no other cranial nerve deficits were present.
Investigation
Non-contrast computed tomography (NCCT) of the head showed a hyperdense lesion involving the right cavernous sinus (Figure 3). Magnetic resonance imaging (MRI) showed a large partially thrombosed aneurysm afflicting the cavernous segment of the right ICA, measuring 18 × 20 mm (Figure 4). A diagnostic cerebral angiogram was performed, which revealed a laterally directed aneurysm with a neck of 7 mm, that was arising from the posterior aspect of the horizontal part of the cavernous segment of the right ICA (C4 segment of Fischer classification) (Figure 5).
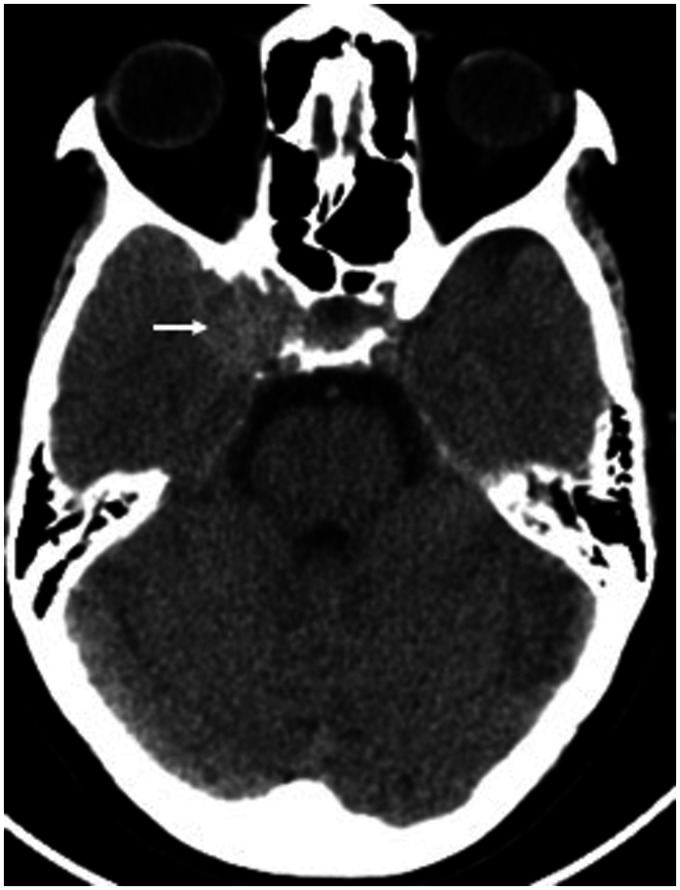
Figure 3.
NCCT head axial section showing the right cavernous hyperdense mass lesion (white arrow).
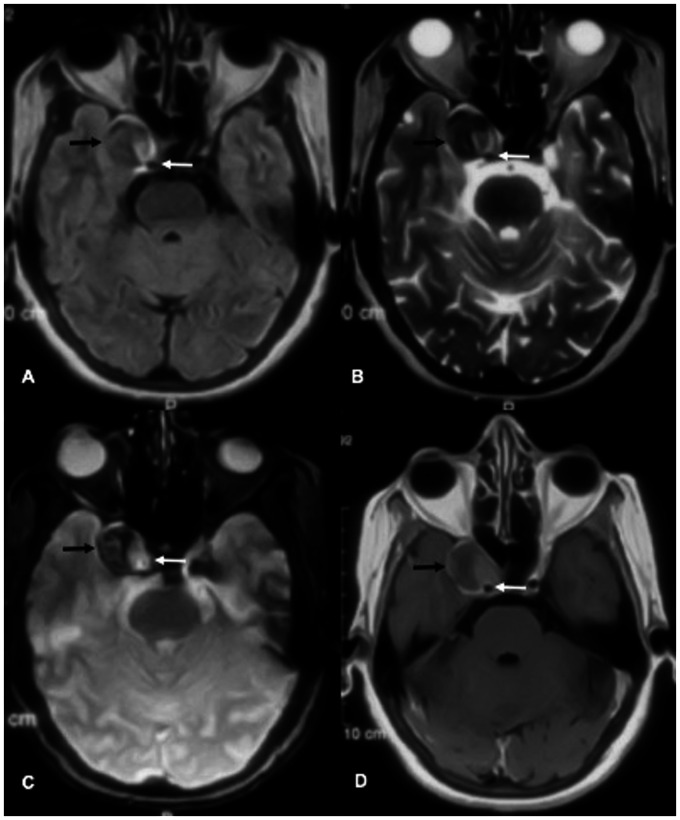
Figure 4.
MRI brain axial section: (a) FLAIR, (b) T2w, (c) T2*w and (d) post contrast T1w images show partially thrombosed right cavernous segment aneurysm (black arrow). White arrow shows the right cavernous segment ICA.
Figure 5.
Diagnostic angiogram of right ICA: (a) anteroposterior (AP) and (b) lateral view showing cavernous segment aneurysm (black arrow).
Treatment
After careful consideration, it was decided to deploy a recently launched balloon mounted flow diverter – Xcalibur Aneurysm Occlusion Device (AOD) across the aneurysm’s neck to reconstruct the parent artery. The patient was pre-medicated with a daily dose of oral Aspirin 150 mg and Clopidogrel 75 mg for five days.
The procedure was done under general anesthesia. The right femoral artery was accessed by 7F long sheath [Cook medical] with continuous heparinized saline infusion attached to it throughout the procedure. After securing the arterial access, 5000 units of IV heparin was administered as a bolus. The bi-axial system was used consisting of 7F long sheath - 6F Neuron [Penumbra, Inc. California], which was parked in the right cervical and petrous ICA, respectively. The distal vessel was accessed using Traxcess 014 micro-guide wire (Microvention Inc.) through 6F Neuron; the distal tip of the microwire was placed in the M3 segment of the right MCA. Xcalibur AOD, measuring 4 × 20 mm, was tracked over the Traxcess microwire (Rapid exchange system). After positioning the device optimally across the aneurysm’s neck, it was cautiously deployed by gradual balloon inflation (Figure 6). The post-deployment angiogram revealed a complete device opening and optimal wall approximation. A very sluggish intra aneurysmal flow in the form of an eclipse was identified– OKM grade – B3 (Figure 7). All catheters and sheath were removed at the end of the procedure.
Figure 6.
(a and b) Pre-deployment AP and Lateral images; (c and d) post deployment of Xcalibur AOD AP and lateral images. Deployment balloon over which flow diverter is mounted has proximal and distal markers (Thin black arrows). Flow diverter’s proximal and distal markers are shown by thin white arrows. (*) and thick white arrows indicate position of neuron guiding catheter and 7F long sheath, respectively. Traxcess microwire is placed in MCA with the distal end in the M4 segment (thick black arrow). Inset pictures are magnified images of the device.
Figure 7.
Immediate angiogram after deploying Xcalibur AOD. Lateral view angiogram: (a) arterial phase, (b) and (c) venous phase. Anterior view angiogram: (d) arterial phase, (e) and (f) venous phase. Significant stasis in the aneurysm is evident with eclipse formation (black arrow).
Outcome and follow up
The patient remained stable following the procedure and was discharged in a couple of days. Subsequent clinical follow up at three months showed complete resolution of chemosis and right third nerve palsy. Intravenous contrast XperCT and MRI follow up at six months demonstrated total exclusion of the aneurysm (Figure 8).
Figure 8.
Six months follow up imaging. (a) IV Contrast XperCT shows optimal wall approximation of the device with no obvious in- stent stenosis. (b) T2WI shows complete disappearance of the aneurysm (Black arrow). (c) TOF MRA axial image and (d) TOF MRA MIP 3D reconstructed images show no residual aneurysm. There is signal loss/susceptibility artefact (*) due to flow diverter in the cavernous segment of the right ICA.
Device details – Xcalibur aneurysm occlusive device (AOD)
Xcalibur Aneurysm Occlusive Device (AOD) is a balloon mounted flow diverter. The supporting metal frame of the device is made up of stainless steel tubing, which is laser-cut into a strut and ring pattern. Two radio-opaque markers are embedded on either end of the device to assist precise deployment. The supporting metal frame is completely sandwiched between bilayer, ultra-thin, microporous polymer. The luminal surface of the device is smooth and hydrophobic, while the abluminal surface is undulating and hydrophilic (Figures 1 and 2). This design attributes to its low porosity of 30-35 percent which is much lower than self-expanding flow diverters.2
The device is available in lengths of 15 mm, 20 mm, and 25 mm, with diameters of 3.25 mm to 4.5 mm, with 0.25 mm increments in the diametric dimension. The device diameter is selected based on the distal vessel caliber (aneurysmal outflow zone) with an oversizing of 0.25 to 0.5 mm. If there is a mismatch of more than 0.5 mm between proximal and distal diameters of the vessel, mid and proximal portions of the device are selectively dilated using NChant post- dilatation balloon (Merlin MD, Singapore) to achieve optimal device-wall apposition. NChant balloon is available in 7 mm length with diametrical dimensions of 3.75, 4.25 and 4.75 mm. The proximal end of the smallest (3.25 mm) device can be safely dilated up to 4.75 mm (Figure 9). The purchase in radial dimension is associated with non-linear diminution in the longitudinal dimension, leading to foreshortening (5–25%) of the device depending on the degree of dilatation.
Figure 9.
Illustration showing deployment technique of the device in case of significant mismatch between the size of distal and proximal vessel diameter. In this example, the distal vessel diameter is 3.25 mm and the proximal vessel diameter is 4.5 mm. The device size is selected based on the distal vessel diameter. (a) The device is deployed via its own balloon catheter. The distal end of the device is anchored first. Due to mismatch in the size of the vessel diameter, the proximal end of the device is not well opposed to the vessel wall and shows endo-leak. (b and c) Sequential post dilatation is done only in the mid and proximal segments of the device by bigger diameter (4.5 mm) N-chant PDB (Post-Dilatation Balloon) catheter.
This balloon mounted rapid exchange system tracks over a 0.014-inch microwire. It requires a guiding conduit having a minimum of 0.070-inches internal diameter. The indeflator is used to inflate the balloon, which releases the device. The nominal pressure of the balloon for the device deployment is 6 atmospheres.
The device being balloon mounted, possess minimal shape memory, hence is not well suited for the extracranial cervical ICA deployment. Furthermore, the least available device dimension is 3.25 mm. It is not advisable to use the device in vessels with a diameter lesser than 2.75 mm because of the safety concern related to oversizing the device by more than 0.5mm relative to the vessel.
Discussion
Carotid cavernous aneurysms constitute 2-9 percent of intracranial aneurysms and have female predilection.1 Though there are few studies on the natural course of cavernous segment aneurysms, the risk of rupture and subarachnoid hemorrhage (SAH) is minimal. One of the studies had reported the rate of SAH at 0.19% per patient-year.3 Therefore, small asymptomatic cavernous segment aneurysms (<10 mm) and those who remain stable on interval evaluation can be conservatively followed up, though no consensus protocol exists.4,5 Cavernous segment aneurysms manifest with headache and/or cranial neuropathies. Occasionally, thromboembolic complications could announce its presence. Rupture commonly results in direct carotico-cavernous fistula (CCF), although rare, more grave manifestation could be epistaxis secondary to sphenoid sinus erosion.1,6,7 There are multiple treatment options available for symptomatic cavernous segment aneurysm aiming to alleviate the mass effect. Surgical PVO with or without bypass, endovascular PVO, coiling with and without assistance, and flow diverters are the few management alternatives.1,6,8,9 As compared to surgical ligation of ICA, endovascular PVO is advantageous because juxta-aneurysmal occlusion can be achieved. This precludes the development of a long column of intraluminal thrombus and avoids impending thromboembolic complications.10 Furthermore, the clinical improvement ensuing mass effect reduction and aneurysmal occlusion with PVO and flow diversion are temporally more stable as compared to coiling/stent-assisted coiling. But a destructive procedure, such as PVO, is associated with risks of significant neurological complications. Thus, flow diversion has emerged as a safe and effective management alternative for symptomatic cavernous segment aneurysms (Table 1). Aneurysmal exclusion apart, flow diverters also remodel adjacent dysplastic vessel.6–14
Table 1.
Comparative results of endovascular therapy for CCAs.
| Author (Year) |
Complete/near complete occlusion, % |
Recanalization/retreatment, % |
Favorable clinical outcome, % |
Complications, % |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PVO | Coiling + SAC | FD | PVO | Coiling + SAC | FD | PVO | Coiling + SAC | FD | PVO | Coiling + SAC | FD | |
| van Rooij 14 (2012) | 68 | 87.1 | – | 0 | 19.35 | – | 92 | 100 | – | 8 | 0 | – |
| Puffer et al.11 (2014) | – | – | 65.9 | – | – | 0 | – | – | 90 | – | – | 18.18 |
| Starke et al.7 (2014) | 100 | 89.55 | 100 | 14.3 | 47.06 | 0 | 85.7 | 98.36 | 100 | 20 | 7.46 | 0 |
| Zanaty et al.8 (2014) | 86.67% | 77.42% | 89.83% | 13.33% | 21.50% | 5.08% | 89.83% | 50.65% | 92.16% | 13.33% | 7.53% | 3.39% |
| Miyachi et al.9 (2017) | 100 | 100 | 100 | 0 | 22.22 | 0 | 80.95 | 72.22 | 100 | 36.36 | 27.78 | 44.44 |
In the index case with a wide neck aneurysm, the endovascular treatment options were stent-assisted coiling and flow diversion. As flow diversion scores over stent-assisted coiling with higher aneurysm occlusion rates and resolution of mass effect related symptoms, flow diverter emerged as a logical choice. The Xcalibur AOD was preferred because of its higher flow diversion capability, and having been used to treat direct CCF at our institute.15 In the present case, Xcalibur AOD has shown results comparable to the other flow diverters.5,8,9,11 In our index case there was total aneurysm occlusion with complete resolution of the mass effect related symptoms at 6 months follow up.
The device shows future promise to be used in similar cases, though it needs to be validated with more extensive studies.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:Nihar Vijay Kathrani and Arun Kumar Gupta are Proctors of Xcalibur AOD, Merlin, MD.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Richa Singh Chauhan https://orcid.org/0000-0003-0919-1811
Nihar Vijay Kathrani https://orcid.org/0000-0002-6537-2273
Karthik Kulanthaivelu https://orcid.org/0000-0002-1585-8769
References
- 1.Ambekar S, Madhugiri V, Sharma M, et al. Evolution of management strategies for cavernous carotid aneurysms: a review. World Neurosurg 2014; 82: 1077–1085. [DOI] [PubMed] [Google Scholar]
- 2.Maragkos GA, Dmytriw AA, Salem MM, et al. Overview of different flow diverters and flow dynamics. Neurosurgery 2020; 86: S21–S34. [DOI] [PubMed] [Google Scholar]
- 3.Kupersmith MJ, Stiebel-Kalish H, Huna-Baron R, et al. Cavernous carotid aneurysms rarely cause subarachnoid hemorrhage or major neurologic morbidity. J Stroke Cerebrovasc Dis 2002; 11: 9–14. [DOI] [PubMed] [Google Scholar]
- 4.Brugge KG. Cavernous sinus segment internal carotid artery aneurysms: whether and how to treat. Am J Neuroradiol 2012; 33: 327–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanweer O, Raz E, Brunswick A, et al. Cavernous carotid aneurysms in the era of flow diversion: a need to revisit treatment paradigms. Am J Neuroradiol 2014; 35: 2334–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan N, Mu S, Wang L, et al. Endovascular treatment of 147 cases of cavernous carotid aneurysms: a single-center experience. J Stroke Cerebrovasc Dis 2016; 25: 1929–1935. [DOI] [PubMed] [Google Scholar]
- 7.Starke RM, Chalouhi N, Ali MS, et al. Endovascular treatment of carotid cavernous aneurysms: complications, outcomes and comparison of interventional strategies. J Clin Neurosci 2014; 21: 40–46. [DOI] [PubMed] [Google Scholar]
- 8.Zanaty M, Chalouhi N, Starke RM, et al. Flow diversion versus conventional treatment for carotid cavernous aneurysms. Stroke 2014; 45: 2656–2661. [DOI] [PubMed] [Google Scholar]
- 9.Miyachi S, Ohnishi H, Hiramatsu R, et al. Innovations in endovascular treatment strategies for large carotid cavernous aneurysms—the safety and efficacy of a flow diverter. J Stroke Cerebrovasc Dis 2017; 26: 1071–1080. [DOI] [PubMed] [Google Scholar]
- 10.Elhammady MS, Wolfe SQ, Farhat H, et al. Carotid artery sacrifice for unclippable and uncoilable aneurysms: endovascular occlusion vs common carotid artery ligation. Neurosurgery 2010; 67: 1431–1437. [DOI] [PubMed] [Google Scholar]
- 11.Puffer RC, Piano M, Lanzino G, et al. Treatment of cavernous sinus aneurysms with flow diversion: results in 44 patients. Am J Neuroradiol 2014; 35: 948–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandra P, Gaikwad S, Garg A, et al. Monitored gradual occlusion of the internal carotid artery followed by ligation for giant internal carotid artery aneurysms. Neurol India 2012; 60: 174. [DOI] [PubMed] [Google Scholar]
- 13.Roski RA, Spetzler RF, Nulsen FE. Late complications of carotid ligation in the treatment of intracranial aneurysms. J Neurosurg 1981; 54: 583–587. [DOI] [PubMed] [Google Scholar]
- 14.van Rooij WJ. Endovascular treatment of cavernous sinus aneurysms. Am J Neuroradiol 2012; 33: 323–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswas S, Kathrani NV, Jitender S, et al. XCalibur aneurysm occlusion device for the treatment of direct carotid cavernous fistula: expansion of armamentarium. BMJ Case Rep 2019; 12: e014475. [DOI] [PMC free article] [PubMed] [Google Scholar]