Abstract
Background
Vascular angioplasty and stenting of middle cerebral artery (MCA) and basilar artery (BA) stenoses are associated with poor clinical outcomes and high mortality rates thought to be related to the abundance of perforating arteries in those segments. This study explores the use of Solitaire AB as an off-label vascular stent to treat stenoses in the MCA and BA.
Methods
Solitaire AB stents were placed during angioplasty and stenting of MCA and BA stenoses in patients at our department between January 2015 and May 2017 with 6-36 months follow-up. Operative results were assessed by follow-up angiography and transcranial doppler after the procedure. Neurologic status was evaluated before and after treatment according to the modified Ranking Scale (mRS).
Results
A total of 32 patients were included in the study. Seventeen (53.12%) patients presented with MCA stenosis and 15 (46.87%) with BA stenosis. The 30-day rate of procedure-related complications was 3.1% (1/32). Post-stenting residual stenosis degrees ranged from 0% to 40% (mean 13.44% ± 10.66%). Mean degree of residual stenosis in 26 patients followed up by DSA was 8.64% ± 9.67%. The mRS 0-2 was achieved in all (100%) patients at 6-12 months post-procedure.
Conclusions
Our study indicates the off-label use of Solitaire AB for stenting is effective and safe for MCA and BA stenoses with high technical success and low complications. We recommend that lesion-specific therapy with an anatomically fitted stent design enables optimal treatment for intracranial stenosis.
Keywords: Solitaire, stent, cerebral middle artery, basilar artery, perforator
Introduction
In light of the SAMMPRIS trial results,1 the guidelines issued by the American Heart Association and American Stroke Association (AHA/ASA) for the prevention of stroke in patients with stroke and transient ischemic attack (TIA) state that the Wingspan stent system is not recommended as an initial treatment of intracranial atherosclerotic disease, and also recommend that the usefulness of other stents is unknown and is considered investigational.2 Perforating artery strokes, distal emboli, delayed stent thrombosis, and subarachnoid or intraparenchymal hemorrhage were reported as causes of strokes by the SAMMPRIS.3,4 Different segments of the intracranial vasculature were associated with different levels of complication risk.3 The middle cerebral artery (MCA) and basilar artery (BA) have the highest complication risk, because the abundance of perforating arteries in those segments poses a high chance of occlusions upon stent placement.
Solitaire AB stent is an assistant stent originally used for treating wide-necked cerebral aneurysm,5 while its high porosity (less strut coverage to obstruct perforating arteries) and high radial force (strong scaffolding) could potentially result in better performance than on-label vascular stents for MCA and BA stenoses,6 although it remains to be proven. Therefore, we investigate using Solitaire AB stent, an unconventional intracranial device, to treat these specific vascular segments and evaluate clinical outcomes in comparison with the Wingspan system.
To the author’s knowledge, this is the first study to assess Solitaire AB stenting for intracranial stenoses in perforator rich segments. The clinical and angiographic outcomes were analyzed retrospectively.
Methods
Patients selection
Thirty-two consecutive patients with intracranial atherosclerosis stenosis (ICAS) in the MCA and BA were treated by Solitaire AB stenting (Medtronic, Irvine, California, USA) in our department between January 2015 to May 2017. In accordance with the guidelines on intracranial angioplasty and stenting for cerebral atherosclerosis by the American Society of Interventional and Therapeutic Neuroradiology (ASITN), Society of Interventional Radiology (SIR), and American Society of Neuroradiology (ASNR), all symptomatic patients with >50% intracranial artery stenosis who failed medical therapy were considered for endovascular treatment.7 Sixteen out of seventeen cases with recent strokes received treatment with Solitaire stent three to six weeks after their last stroke, while one case with acute infarction was performed within one week after failing medical therapy. Fifteen cases with TIA received immediate Solitaire stenting treatment upon qualified response testing for antiplatelet drugs.8 All procedures were determined after discussion between the interventional neuroradiologists and stroke neurologists.
The following seven features were defined as high-risk criteria:3,9 (1) stenosis with fenestration; (2) tortuous access; (3) stenosis with dissection; (4) basilar tip stenosis; (5) MCA bifurcation stenosis; (6) MCA M2 segment stenosis; and (7) stenosis with acute infarction. The study was approved by the ethics committee of our hospital. Written informed consent was obtained from the patients or their legally authorized representatives.
Endovascular procedure
Angiographies were performed under local anesthesia by a consultant neurointerventionalist. Using transfemoral access, a 4 F catheter angiogram was performed for all patients. Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) were also performed in most patients. All patients received dual antiplatelet therapy (aspirin 100 mg daily and clopidogrel 75 mg daily) for at least four days before the procedure.10 The TEG-platelet mapping was used as response testing for antiplatelet drugs. In all patients, the inhibition rate of arachidonic acid (AA) was higher than 50%, and the inhibition rate of adenosine diphosphate (ADP) was higher than 30%. Lipid-lowering drugs (Atorvastatin 40 mg daily or Rosuvastatin 20 mg daily) were administered for at least one week before the procedure, with LDL-C < 100 mg in all patients.
Angioplasties were performed under general anesthesia by consultant neurointerventionalists (performing more than 100 intracranial endovascular procedures per year). A single wall puncture of the femoral artery was performed, and a 6 F short sheath was inserted into the femoral artery. A bolus of heparin 3000 to 5000 IU was intravenously administered immediately after insertion of a 6 F guiding catheter Envoy (Cordis Neurovascular, Miami, Florida, USA) to the cervical internal carotid artery (ICA) or vertebral artery (VA). Diameters were measured at sites of the greatest stenosis and the normal artery to estimate percent stenosis according to the Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) protocol.11 A Prowler14 microcatheter (Cordis Neurovascular, Miami, Florida, USA) was manipulated across the stenotic segment using a 0.014″ (200 cm) Synchro microwire (Stryker Neurovascular, Fremont, California, USA). The Synchro microwire was then exchanged for a 0.014″ (300 cm) X-Celerator microwire (Medtronic, Irvine, California, USA). A Gateway balloon (Stryker Neurovascular, Freemont, California, USA) was placed over the exchange X-Celerator microwire and inflated slowly at 6 to 8 atmospheric pressure with a 50% mixture of iodinated contrast (Omnipaque 300 or visipaque 320 (GE Healthcare, Princeton, NJ, USA)) and saline. The inflation and deflation were performed for more than 60 seconds to minimize intimal injury.12 The balloon length was selected to match lesion length, and the diameter of the balloon was selected to match the distal artery diameter. Following angioplasty, the balloon was removed, and conventional angiography was repeated to confirm the effectiveness of angioplasty. A Rebar-18 microcatheter (Medtronic, Irvine, California, USA) was placed through the X-Celerator microwire, which was then followed by the removal of the 300 cm long microwire. A Solitaire AB stent was deployed through the Rebar-18 microcatheter. Before and after detaching stent, angiography was used to determine the deployment position. VasoCT evaluated the postprocedural residual stenosis.13
Clopidogrel and Aspirin were maintained for at least six months after the operation. If follow-up angiography confirmed no in-stent restenosis, the Clopidogrel was discontinued. Lifelong Aspirin 100 mg daily was recommended.14
Follow-up
Neurological examinations were performed prior to the operation and at 6-12 months post-stent placement respectively to assign modified Rankin Scale (mRS) scores. Good neurologic outcome was defined as mRS of 0-2 and mRS of 3-5 was considered poor neurologic outcome. In the case of clinical worsening, MRI was performed. After six months, patients were asked to arrange appointments for angiography and clinical examination. If the patient refused angiography, transcranial doppler imaging was used as an alternative to assess residual stenosis.15 Follow-up information on clinical outcome was reviewed via face-to-face or telephone interview by two trained doctors who were blinded to the treatment strategies. The angiography data were interpreted by two neuroradiologists with consensus.
Statistical analysis
Frequencies and percentages were calculated for categorical variables. Means, standard deviations, medians, and ranges are determined for continuous variables. Statistical analyses were performed using the SAS v9.4 software (SAS institute, Cary, North Carolina, USA).
Results
Patient baseline
Patient baseline characteristics are summarized in Table 1. The study in accordance with SAMMPRIS inclusion criteria enrolled 32 patients (25 male and 7 female) with a mean age of 57.34 ± 8.47 years (ranging 37–70).1 Seventeen patients (53.12%) presented with ischemic stroke, including eight patients with basal ganglia perforator stroke, eight patients with pontine and mesencephalon perforator stroke which included one progressive stroke with severe BA stenosis (Figure 1), and one patient with cortex stroke. The remaining fifteen patients (46.87%) presented with TIA. Of them, five patients still had ischemic event after maximal medical therapy, and the others did not have a second ischemic event before selection for treatment. The mRS scores of 31 patients were lower than 2, and one patient with a progressive stroke in the territory of BA stenosis had a mRS score of 3.16
Table 1.
Clinical baseline of the 32 patients.
| Age (mean ± SD), years | 37-70 (57.34 ± 8.47) |
| Male (n = 32), n (%) | 25 (78.12) |
| Symptom | |
| TIA, n (%) | 15 (46.87) |
| Stroke, n (%) | 17 (53.12) |
| Type 1: haemodynamics, n | 0 |
| Type 2: vessel-to-vessel emboli, n | 6 |
| Type 3: perforator, n | 11 |
| Hypertension (n = 32), n (%) | 22 (68.75) |
| Diabetes mellitus (n = 32), n (%) | 13 (40.62) |
| Smoking (n = 32), n (%) | 14 (43.75) |
| Hyperlipidemia (n = 32), n (%) | 6 (18.75) |
| Coronary artery disease (n = 32), n (%) | 4 (12.5) |
| Modified Rankin Scale Score | |
| 0 | 28 |
| 1 | 2 |
| 2 | 1 |
| 3 | 1 |
Standard Deviation (SD).
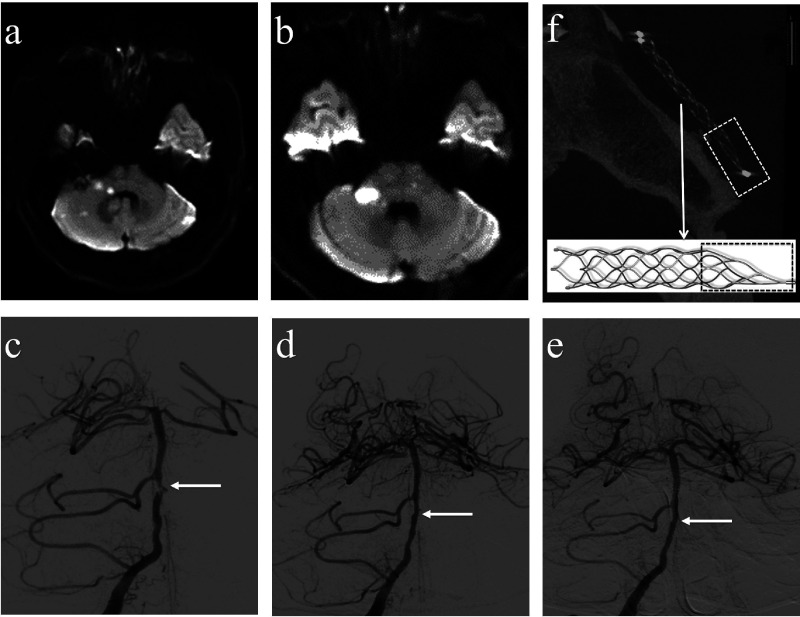
Figure 1.
A 47-year-old man with symptomatic BA stenosis. (a) Initial diffusion-weighted images reveal infarctions in the pons and cerebella. (b) Symptoms progressed despite dual antiplatelet medication and tirofiban; therefore, the five-day follow-up diffusion-weighted images showed that infarctions in the pons and cerebella increased. (c) The right vertebral angiography disclosed a severe degree of stenosis (white arrow) in the BA. (d) The patients underwent angioplasty and solitaire stent placement, with the image demonstrating post-procedural residual stenosis ≤10% (white arrow). (e) The five-months follow-up angiogram showed a successfully stented portion (white arrow). (f) The VasoCT scan showed the morphology of the stent after placement. The dashed rectangles indicate the 13 mm long tail at the end of Solitaire AB stent (reproduced with permission of Medtronic).
DSA findings
DSA characteristics are summarized in Table 2. Lesion locations were divided into MCA M1, MCA M2 (Figure 2) and BA. We used the WASID criteria to measure the degree of stenosis. Seventeen patients (53.13%) were defined as high-risk cases by arterial morphology, as summarized in Table 2. The remaining 15 patients were simple cases located in the MCA and BA.
Table 2.
DSA characteristics of the 32 patients.
| Lesion location (n = 32), n (%) | |
| MCA M1 | 15 (46.88) |
| MCA M2 | 2 (6.25) |
| BA | 15 (46.88) |
| Degree of stenosis (n = 32), n (%) | |
| 70-79 | 2 (6.25) |
| 80-89 | 12 (37.50) |
| 90-99 | 18 (56.25) |
| Lesion length (mm), mean±SD | 6.41 ± 2.23 |
| Stenosis with high risk (n = 32), n (%) | 17 (53.13) |
| Stenosis with fenestration | 3 |
| Tortuous access | 4 |
| Stenosis with dissection | 3 |
| Basilar tip stenosis | 1 |
| MCA bifurcation stenosis | 2 |
| MCA M2 segment stenosis | 2 |
| Basilar stenosis and acute infarction | 2 |
Middle Cerebral Artery (MCA); Basilar Artery (BA); Standard Deviation (SD).
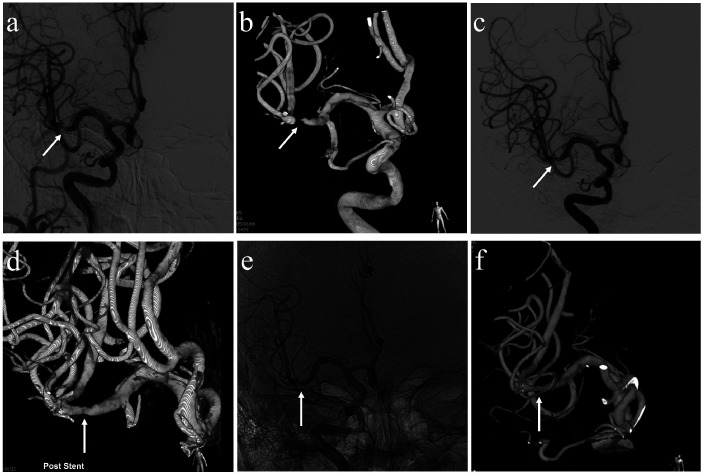
Figure 2.
A 45-year-old man with symptomatic cerebral middle artery M2 segment stenosis. (a,b) The right internal carotid angiography and 3 D angiography disclosed a severe degree of stenosis in the MCA M2 segment (white arrows). (c,d) Post-procedural residual stenosis ≤20% (white arrows). (e–f) An eightmonths follow-up angiogram showed a successfully stented portion (white arrow).
Outcomes and complications
Table 3 summarizes the data of outcomes and complications. The technical success rate was 100%. The post-stenting residual stenosis of 28 patients was ≤20% and 4 patients ranged 20–40% with mean residual stenosis of 13.44% ± 10.66%. There were three procedural complications, including one long-term morbidity where one patient with basilar terminus stenosis had a permanent infarction in the mesencephalon caused by occlusion of a basilar terminus perforator branch. Intra-procedural in-stent thrombosis occurred to two patients with MCA stenosis. Tirofiban was delivered via microcatheter intraprocedurally,17,18 resulting in dissolving of the clots and patients recovering without deficit.
Table 3.
Outcomes and complications of the 32 patients.
| Treatment result, n(%) | |
|---|---|
| Technical success | 32 (100%) |
| Stenosis degree (post-treatment) | |
| 0–20% | 28 (87.50) |
| 20–40% | 4 (12.50) |
| Complication | 3 (9.35%) |
| Ischemic stroke with symptom at 30–day | 1 (3.1%) |
| Intra-procedural in-stent thrombosis | 2 (6.25%) |
Follow-up results
Thirty-two patients had mean follow-up of 24.1 months (ranging 6–36 months). The symptomatic stroke rate was 3.1%, and the death rate was 0%. New mRS scores were assessed for each patient 6–12 months later. The mRS score for 31 patients was 0, and the patient with basilar terminus perforator stroke had an mRS score of 2. Twenty-six patients (81.25%) consented to the follow-up angiography and other six patients (18.75%) were followed by transcranial doppler. Follow-up DSA showed the residual stenosis of 26 patients ranged from 0% to 40% (mean 8.64 ± 9.67%). Two asymptomatic patients (6.25%) with MCA stenosis had in-stent restenosis, with the stenosis lower than 40%.
Discussion
It is tempting to classify intracranial stenosis simply by the vessel involved; however, the interplay between the central nervous system and angioarchitecture has significance.15 In clinical practice, stenting for different portions of intracranial arteries represents different levels of risk. In the SAMMPRIS trial, the percutaneous transluminal angioplasty and stenting group included 224 patients, which included ICA 45 (21.4%), VA 38 (17%), MCA 92 (41.1%), and BA 49 (21.9%). In the analysis of SAMMPRIS trial reported by Colin et al.,3 complications of stenting for intracranial atherosclerotic disease encompassed perforator infarction, delayed intraparenchymal hemorrhage, and wire perforation. Lesion locations of the 34 patients who experienced complications in SAMMPRIS were ICA in 3 patients, VA in 3, MCA in 14, BA in 14. It suggests that complication rates for ICA and intracranial VA were 6.67% and 7.89% respectively, which were approximately equal to the medical-management group. Complications of MCA (15.2%) and BA (28.6%) were significantly higher because of wire perforations and perforator infarcts; therefore, it is reasonable to consider MCA and BA stenoses as a separate category with a higher-risk profile, requiring specialized treatment.
According to SAMMPRIS trial, the generic percutaneous transluminal angioplasty and stenting of intracranial arteries may hold a high risk for poor outcome when performed with the Wingspan stent. However, the recent WEAVE trial (Wingspan Stent System Post Market Surveillance) indicated poor clinical results in the SAMMPRIS trial were not primarily associated with the Wingspan stent itself, but more likely from factors such as inexperienced interventionalists, poor patient selection, and underdeveloped standards of practice.8 Please note WEAVE trial is a registry while SAMMPRIS is an RCT study. In our study, more than half of the enrolled patients (seventeen) featured high-risk criteria, representing the operation difficulty. Nevertheless, we experienced low rates of residual stenosis, stroke, and mortality when using Solitaire AB in perforator rich segments of the MCA and BA. The research reveals that stent design or selection of appropriate stents, especially when used at a high-risk portion of intracranial arteries, is important to enhance clinical outcomes.
In recent years, Solitaire AB has been permanently implanted to restore distal flow after failure of mechanical thrombectomy, indicating it may be a safer device for intracranial stenosis.14,19,20 Radial force and porosity are two important technical parameters of stent.21,22 Solitaire AB has the best radial force to reduce residual stenosis in comparison to the currently available stents (i.e., Enterprise and Neuroform) with indications for intracranial placement, second to the Wingspan stent.23 The closed-cell and high porosity of Solitaire AB stent can avoid stent struts crossing over the ostia of the abundant perforating arteries in the MCA and BA to alleviate perforator infarcts. Furthermore, its delivery is relatively simple, requiring a Rebar (or similar) microcatheter across the stenosis with fewer catheter exchanges than the Wingspans system.24,25 Use of Solitaire AB in this study, instead of the Wingspan stent, presented a complication rate lower than the medical-management group of the SAMMPRIS trial.
Another major complication of intracranial stents is in-stent restenosis. Restenosis rates in studies using the Wingspan stent ranged from 4.3% to 29.7%.26,27 Comparatively, the restenosis rate (6.25%) obtained for Solitaire AB in this study falls within the lower range for that of the Wingspan stent. The high porosity of Solitaire AB presumably makes it difficult to cause intimal hyperplasia.28,29
The major limitation of this study is its single-center, single-arm, retrospective design with a small patient cohort, limiting generalizability of the results. Also, Solitaire AB is not globally available. One potential issue of Solitaire AB is the 13 mm long tail (Figure 1(f)), which may be thrombogenic. Customized intracranial stents for intracranial stenoses and perforator rich segments may further promote clinical outcome.
Conclusion
Our retrospective study has shown that Solitaire AB may be a safe and effective option for stenting of perforator rich intracranial segments, such as the MCA and BA. This finding indicated the importance of treating intracranial stenosis at perforator rich segments with artery-specific stents.
Authors’ contributions
XC and FH performed literature review, and drafted the manuscript. JW, CT, ZD, XL contributed in angiography and operation. BL, HS were responsible for patient’s management and follow-up. SY, XC contributed in revising the manuscript. All authors read and approved the final manuscript.
Ethical approval
The study was approved by the ethics committee of the PLA General Hospital. Written informed consent was obtained from the patients or their legally authorized representatives.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Key Research and Development Program (2017YFC1307701).
ORCID iDs
Xiangyu Cao https://orcid.org/0000-0002-4591-3220
Chenglin Tian https://orcid.org/0000-0002-2455-6930
Xing Chen https://orcid.org/0000-0001-7084-3052
References
- 1.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014; 383: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 2160–2236. [DOI] [PubMed] [Google Scholar]
- 3.Derdeyn CP, Fiorella D, Lynn MJ, et al. Mechanisms of stroke after intracranial angioplasty and stenting in the SAMMPRIS trial. Neurosurgery 2013; 72: 777–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silber T, Ziemann U, Ernemann U, et al. Analysis of periinterventional complications of intracranial angioplasty and stenting: a single center experience. Eur J Radiol 2014; 83: 2190–2195. [DOI] [PubMed] [Google Scholar]
- 5.Duan G, Feng Z, Zhang L, et al. Solitaire stents for the treatment of complex symptomatic intracranial stenosis after antithrombotic failure: safety and efficacy evaluation. J Neurointerv Surg 2016; 8: 680–684. [DOI] [PubMed] [Google Scholar]
- 6.Krischek Ö, Miloslavski E, Fischer S, et al. A comparison of functional and physical properties of self-expanding intracranial stents (Neuroform3, wingspan, solitaire, leo(+), enterprise). Minim Invasive Neurosurg 2011; 54: 21–24. [DOI] [PubMed] [Google Scholar]
- 7.Higashida RT, Meyers PM, Connors JJ, et al. Intracranial angioplasty and stenting for cerebral atherosclerosis: a position statement of the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, and the American Society of Neuroradiology. J Vasc Interv Radiol 2009; 20: S312–S316. [DOI] [PubMed] [Google Scholar]
- 8.Alexander MJ, Zauner A, Chaloupka JC, et al. WEAVE trial final results in 152 on-label patients. Stroke 2019; 50: 889–894. [DOI] [PubMed] [Google Scholar]
- 9.Mori T, Fukuoka M, Kazita K, et al. Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol 1998; 19: 1525–1533. [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang WJ, Yu W, Du B, et al. Wingspan experience at Beijing Tiantan hospital: new insights into the mechanisms of procedural complication from viewing intraoperative transient ischemic attacks during awake stenting for vertebrobasilar stenosis. J Neurointerv Surg 2010; 2: 99–103. [DOI] [PubMed] [Google Scholar]
- 11.The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) study group. Prognosis of patients with symptomatic vertebral or basilar artery stenosis. The Warfarin-Aspirin symptomatic intracranial disease (WASID) study group. Stroke 1998; 29: 1389–1392. [DOI] [PubMed] [Google Scholar]
- 12.Gao P, Wang D, Zhao Z, et al. Multicenter prospective trial of stent placement in patients with symptomatic high-grade intracranial stenosis. AJNR Am J Neuroradiol 2016; 37: 1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon YH, Yoon W, Jung MY, et al. Outcome of mechanical thrombectomy with solitaire stent as first-line intra-arterial treatment in intracranial internal carotid artery occlusion. Neuroradiology 2013; 55: 999–1005. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed SU, Mann J, Houde J, et al. Permanent implantation of the solitaire device as a bailout technique for large vessel intracranial occlusions. J Neurointerv Surg 2019; 11: 133–136. [DOI] [PubMed] [Google Scholar]
- 15.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011; 365: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Zhao ZW, Gao GD, et al. Wingspan stent for high-grade symptomatic vertebrobasilar artery atherosclerotic stenosis. Cardiovasc Intervent Radiol 2012; 35: 268–278. [DOI] [PubMed] [Google Scholar]
- 17.Siebler M, Hennerici MG, Schneider D, et al. Safety of tirofiban in acute ischemic stroke: the SaTIS trial. Stroke 2011; 42: 2388–2392. [DOI] [PubMed] [Google Scholar]
- 18.Straub S, Junghans U, Jovanovic V, et al. Systemic thrombolysis with recombinant tissue plasminogen activator and tirofiban in acute middle cerebral artery occlusion. Stroke 2004; 35: 705–709. [DOI] [PubMed] [Google Scholar]
- 19.Li DD, Huang H, Fang JH, et al. Solitaire stent permanent implantation as an effective rescue treatment for emergency large artery occlusion. World Neurosurg 2019; 124: E533–E539. [DOI] [PubMed] [Google Scholar]
- 20.Nappini S, Limbucci N, Leone G, et al. Bail-out intracranial stenting with solitaire AB device after unsuccessful thrombectomy in acute ischemic stroke of anterior circulation. J Neuroradiol 2019; 46: 141–147. [DOI] [PubMed] [Google Scholar]
- 21.Bae YJ, Jung C, Kim JH, et al. Potential for the use of the solitaire stent for recanalization of middle cerebral artery occlusion without a susceptibility vessel sign. AJNR Am J Neuroradiol 2014; 35: 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jahan R. Solitaire flow-restoration device for treatment of acute ischemic stroke: safety and recanalization efficacy study in a swine vessel occlusion model. AJNR Am J Neuroradiol 2010; 31: 1938–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stampfl S, Hartmann M, Ringleb PA, et al. Stent placement for flow restoration in acute ischemic stroke: a single-center experience with the solitaire stent system. AJNR Am J Neuroradiol 2011; 32: 1245–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mourand I, Machi P, Milhaud D, et al. Mechanical thrombectomy with the solitaire device in acute basilar artery occlusion. J Neurointerv Surg 2014; 6: 200–204. [DOI] [PubMed] [Google Scholar]
- 25.Zaidat OO, Castonguay AC, Gupta R, et al. North American solitaire stent retriever acute stroke registry: post-marketing revascularization and clinical outcome results. J Neurointerv Surg 2014; 6: 584–588. [DOI] [PubMed] [Google Scholar]
- 26.Bai WX, Gao BL, Li TX, et al. Wingspan stenting can effectively prevent long-term strokes for patients with severe symptomatic atherosclerotic basilar stenosis. Interv Neuroradiol 2016; 22: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy EI, Turk AS, Albuquerque FC, et al. Wingspan in-stent restenosis and thrombosis: incidence, clinical presentation, and management. Neurosurgery 2007; 61: 644–651. [DOI] [PubMed] [Google Scholar]
- 28.Castanõ C, Dorado L, Guerrero C, et al. Mechanical thrombectomy with the solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke 2010; 41: 1836–1840. [DOI] [PubMed] [Google Scholar]
- 29.Leung TW, Yu SC, Lam WW, et al. Would self-expanding stent occlude middle cerebral artery perforators. Stroke 2009; 40: 1910–1912. [DOI] [PubMed] [Google Scholar]